Abstract
Foramen magnum meningioma poses a challenge for neurosurgeons. Prognosis has generally improved with diagnostic and surgical advances over the past two decades; however, it may ultimately depend more on the surgeon's ability to tailor the approach and interpret intraoperative risks in single cases. The series comprised 64 patients operated on for ventral and ventrolateral foramen magnum meningioma. All patients underwent preoperative magnetic resonance imaging and received surgery via the dorsolateral route, rendering the series homogeneous in neuroradiological workup and surgical treatment. Particular to this series was that the majority of patients were of advanced age (n = 29; age, >65 years), had serious functional impairment (n = 30, Karnofski score <70), and large tumors (mean diameter, 3.5 cm). Total tumor removal was achieved in 52 (81 %) patients; operative mortality was nil. Early outcome varied depending on difficulties encountered at surgery (cranial nerve position and type of involvement in particular) and type of preoperative dysfunction. Long-tract signs and cerebellar deficits improved in 74 and 77 % of cases, respectively, but only 27 % of cranial nerve deficits did so. Surgical complications most often involved the cranial nerves: cranial nerve impairment, especially of the 9th through the 12th cranial nerves, due to stretching or encasement was noted in 44 cases. At final outcome assessment, two thirds of the cranial nerve deficits cleared, and all but two patients returned to a normal productive life. One patient was reoperated on during the follow-up period. Foramen magnum meningiomas behave like clival or spinal tumors depending on their prevalent extension. A dorsolateral approach tailored to tumor position and extension and meticulous surgical technique allow for definitive control of surgical complications. Scrupulous postoperative care may prevent dysphagia, a major persistent complication of surgery. Long-term observation of indolent tumor behavior at follow-up suggests that incomplete resection may be a viable surgical treatment option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Foramen magnum meningiomas account for 2.5 % of all intracranial meningiomas and 4 % of those found in the posterior fossa; 90 % are located ventrally and ventrolaterally [13, 14, 20, 60–62]. Meningiomas arising in the foramen magnum are classified as a separate subgroup according to the first and still undisputed reference scheme devised by Castellano and Ruggiero in 1953 [18]. With continuing advances in imaging technology in the last decades, tumor involvement of the skull base area can now be more precisely delineated. The foramen magnum extends anteriorly from the junction of the lower and middle third of the clivus to the upper edge of the C2 body, laterally from the jugular tubercle to the upper aspect of the C2 lamina, and posteriorly from the anterior edge of the occipital bone to the C2 spinous process. Since foramen magnum meningiomas involve the clival area, they share several of the surgical difficulties associated with this location [6, 11, 25, 37, 38, 50, 52–54, 61].
Published surgical series addressed diagnostic problems before the 1970s and technical surgical refinements thereafter [5, 16, 17, 19, 21, 31, 39, 59]. Development of the dorsolateral approach and its later refinements provided better tumor exposure and enhanced safety in tumor removal. Nevertheless, controversy surrounds management in individual cases as regards tumor variation and range of surgical options in a multiple-step approach. The focus of this article is to draw attention to uncertainties and difficulties in the surgical management of foramen magnum meningioma.
Case material
The series included 64 patients (48 females and 16 males; average age, 59 years; range, 27–82) operated on for new tumors between January 1990 and September 2010 at the Department of Neurosurgery, Verona University Hospital, and retrospectively analyzed. The series included 29 patients of advanced age (>65 years). The mean duration of disease was 33 months (range, 1 month to 17 years). Symptoms and neurological signs are listed in Tables 1 and 2. The predominant signs at admission were quadriparesis, gait ataxia, sensory disturbance, and 9th-10th cranial nerve deficits, resulting in severe disability as assessed by the Karnofski score: 34 patients were able to carry out activities of daily living (>70), 11 only self-care (70–60), and 19 required total assistance (<60).
Neuroradiological workup included contrast-enhanced magnetic resonance imaging (MRI) in all 64 patients, computed tomography (CT) in 56 (plus bone algorithm in 52), and bilateral vertebral angiography in 18 (Figs. 1, 2, and 3). The tumor position at the foramen magnum was: midline ventral in 24 cases and ventrolateral in 40, according to their extension, whether it was symmetric or not, often beyond the midline in both cases. The mean diameter of the mass measured along the sagittal plane was 35 mm. The extent of foramen magnum occupancy reflects the severity of brain stem compression, which in this series was <50 % in 11 cases, 50–70 % in 20, and >70 % in 33, leaving only a thin slice of brain stem and creating an enormous challenge for the surgeon and a considerable risk for the patient. Twelve patients presented with hydrocephalus accompanied in seven by signs of intracranial hypertension. The meningioma was supplied by an arterial feeder in 13 out of 18 patients. In our practice, angiography was carried out for exclusively diagnostic purposes early in this patient series. In selected cases, when MRI features suggested high vascularity, angiography was scheduled, and endovascular embolization was prepared. Embolization was performed in hypervascularized tumors with feeders from the external carotid artery or from branches of the vertebral artery. Clinical and radiological assessments were performed at different time points during the postoperative follow-up period (mean, 138 and 92 months, respectively) in all patients.
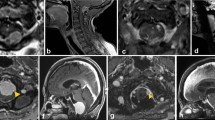
Exemplifying ventrolateral foramen magnum meningiomas with prevalent clival growth. Preoperative axial (a), sagittal (b), and coronal (c) T1-weighted MR images showing a right large enhancing mass exerting a considerable mass effect on the lower brain stem. Postoperative axial (d), sagittal (e), and coronal (f) T1-weighted enhanced MR images showing no residual tumor or brainstem re-expansion
Exemplifying ventrolateral foramen magnum meningiomas with prevalent spinal growth. Preoperative axial (a) T1-weighted MR image showing a small left enhancing mass compressing and displacing the brain stem. The tumor leaves a comparably small corridor to the ventral rim of the foramen, requiring maximal bone resection medially to the sigmoid sinus and at the atlo-occipital junction, as demonstrated on the 3D CT scan (b). Intraoperative view at the dural opening showing wide exposure after bone resection (c). Postoperative axial (d) T1-weighted enhanced MR image showing no residual tumor or brain stem re-expansion
Results
Surgical approach
The patients were placed in a semi-sitting position. The patient's head was fixed in a Mayfield headrest, then cautiously flexed and tilted to the opposite side. The incision was made in a hockey-stick fashion. After downward reflection of the suboccipital muscles, the posterior arch of the atlas was resected from the midline to the vertebral groove, and the course of the artery between C0 and C1 was identified. Low suboccipital craniectomy was performed. The medial rim of the sigmoid sinus was unroofed up to the jugular bulb, and the foramen magnum was opened. The next steps were performed under an operating microscope. While protecting the underlying vertebral artery with a spatula, the posteromedial half of the occipital condyle and a lesser portion of the superior lateral mass and facet of C1 were drilled in just over half of the cases (n = 35). Then, 1–2 cm of the dura anterior to the entrance of the vertebral artery was exposed. Drilling was continued intracranially and rostrally until the jugular tubercle was flattened (n = 21). The hypoglossal nerve canal running below it was identified extradurally.
The dura was opened posterior to the sigmoid sinus, and the incision was continued downward and posterior to the entrance of the vertebral artery in a curvilinear fashion, thus allowing lateral mobilization of the vertebral artery when the dura was reflected and tented to the margins of the craniectomy. This maneuver also permitted retraction of the sigmoid sinus anteriorly. Transection of the dentate ligament may further enhance the working space by relaxing the brain stem, whose exposure is not required, leaving the dura covering it.
Operative findings
Presentation of the tumor and its relation to neural and vascular structures differed from case to case depending on the site of dural attachment and rostrocaudal extension. Several recurrent patterns could be identified: midline ventrally located tumors pushed the 7th through the 12th cranial nerves backwards; ventrolateral tumors pushed the ninth–tenth and possibly the 7th-8th cranial nerves upwards; the 11th was pushed backwards; and the 12th was often encased by the tumor. The vertebral artery was identified at its dural entry in most cases, but in 31 (48 %), it was encased by the tumor in this area, where it was ventrolateral in 23 cases and ventral in 8. In the remaining 33 cases, it was displaced posteriorly. In the cases of large tumors, the posterior inferior cerebral artery (PICA) was usually displaced medially and the anterior inferior cerebral artery (AICA) and basilar artery displaced superiorly. Tumor encasement of these structures created surgical difficulties (Table 3), compounded by excessive bleeding (n = 2, 3 %), fibrous and dense tumor consistency (n = 28, 44 %), and heavy calcification (n = 7, 11 %). The tumor showed aggressive behavior with invasion of the dura in 5 (8 %) cases, and absence of the arachnoid plane was noted in 21 (33 %) cases, involving the vertebral artery in nine, the basilar artery in five, and the brain stem in seven.
Surgical results
Radical removal was achieved in 52 (81 %) patients; in the remaining 12, remnants were left attached to the perforators originating from the bottom of the basilar artery (n = 3) or to the vertebral artery (n = 10); in four of these ten patients, a heavy calcification closely adhering to the brain stem was left untreated. Only one elderly patient developed nonsurgery-related complications (bronchopneumonia). Operative mortality was nil. To minimize surgical risk, total removal was not attempted in some elderly patients. Long-tract signs (quadriparesis, hemiparesis, sensory deficit) and cerebellar deficits improved remarkably during the postoperative course in 74 and 77 % of cases, respectively; pre-existing cranial nerve deficits improved in 27 % of cases during the perioperative period (Table 2). New cranial nerve dysfunction (n = 23) developed, attributable to the 9th through the 12th cranial nerves in particular. All patients with dysphagia were treated with conservative management, and none underwent permanent ancillary surgery or vocal cord augmentation. Clinical oropharyngeal assessment and endoscopic evaluation of laryngeal function were performed as soon as the patient was able to cooperate, before and immediately after extubation. The aims are prevention of aspiration, adequate feeding, and rehabilitation. The first issue is addressed by keeping the patient in the supine position, preferably contralateral to the impaired side, encouraging self-aspiration and maximizing respiratory physiotherapy. Intensive and meticulous rehabilitation included orofacial exercise and swallowing therapy. The majority (71 %) were able to swallow a sufficient oral diet within 1 month after surgery, meanwhile requiring an endonasal feeding tube, and the remaining patients were independent within 3 months after surgery and required temporary gastrostomy.
Table 2 reports the pre- and post-treatment clinical characteristics. Functional recovery (Karnofski score >70) was achieved in all but the six patients who had presented with severe disability before surgery. All but one patient with total tumor removal showed no residual disease. This patient had an aggressive meningioma and was reoperated on with subtotal removal 8 years later; this is the only reoperation in this series. Three of the 13 patients with subtotal removal received gamma knife radiosurgery soon after the operation. All are presently under observation (mean, 88 months), and none has shown evidence of tumor regrowth.
Discussion
The prognosis of foramen magnum meningiomas has improved with continuing advances in surgical technique and approach. An initial turning point in the treatment of foramen magnum meningioma came with the advent of the surgical microscope and CT in the mid-1970s, followed by the development of lateral approaches and the advent of MRI in the mid-1980s [1, 29, 34, 47, 57, 58]. In 1978, Seeger was the first to briefly describe an extreme-lateral approach for craniospinal lesions, including suboccipital craniectomy and drilling of the occipital condyle and of the jugular tubercle [51]. In 1987, Gilsbach et al. reported their results in 12 patients operated on at the same institution [26]. The dorsolateral approach introduced by Koos et al. in 1985 and later described by Perneczski in 1986, a member of the same school [35, 44], resembles the lateral suboccipital approach first described by Heros in 1985 [30]. Both approaches improved the exposure of the lower clivus and the upper cervical canal for removal of midline lesions, with minimal retraction of the cerebellum and complete sparing of the brain stem. Since then, posterolateral approaches, however named (dorsolateral transcondylar or far lateral), have become a flexible corridor encompassing different degrees of craniocaudal and mediolateral exposure [7, 12, 22, 23, 46, 55]. Mastoidectomy, condylectomy, extent of cervical laminectomy, and vertebral artery mobilization may all be performed and tailored to the individual case [10, 56]. In their study, Salas et al. distinguished six possible variations of the dorsolateral approach: transfacetal, retrocondylar, partial transcondylar, complete transcondylar, extreme-lateral transjugular, and transtubercular, each specific to different pathologies and locations, reserving the partial transcondylar (n = 14), the retrocondylar (n = 5), and the extreme-lateral transjugular (n = 4) for meningioma [48]. The most controversial step in the technique involves mobilization of the vertebral artery, occasionally applied to increase anterior exposure in intradural tumors [2, 4, 7, 8].
The choice of patient position (supine, park bench, semi-sitting) will usually depend on the surgeon's preference and may influence the extent of bone resection. In our experience, the semi-sitting position remains the single most important step in the procedure. The semi-sitting position offers a view from below, thus reducing the need for lateral exposure while permitting tumor debulking from below and delivered downward, away from the lower cranial nerves [40]. With the patient so positioned, anteriorly located tumors require tilting of the head in order to lower and shift the contralateral anterior rim of the foramen magnum forward to bring it within the surgeon's visual field. We fully agree with other operators that overuse of a novel surgical approach may result in unnecessary additional risks for the patient.
Results of tumor resection
The main difficulties encountered in the surgical treatment of foramen magnum meningioma have been investigated in detail by operators highly experienced in treating skull base tumors. Table 4 compares three variables in surgical tumor removal (case series size, tumor resection, and mortality rates) gleaned from studies published between 1978 and 2010. The discrepancies closely reflect differences in population characteristics, since tumor recurrence, extradural growth, vascular encasement, and arachnoid scarring are all widely accepted as factors arguing against radical excision [4, 24, 49, 54]. In our series, for example, tumor fragments were left attached to the arteries and the brain stem because of the absence of an arachnoidal plane in some cases.
Difficulties may be related to involvement of vascular components or the cranial nerves. Vascular dissection may be problematic in hard tumors encasing vessels and when arachnoid scarring occurs. In surgical planning, MRI imaging is useful for predicting vascular encasement, although it can slightly overestimate this feature and yields no information on cleavage. The vertebral artery carries the highest risk of injury, being involved during different stages of the procedure: exposure, drilling the condyle, mobilization, and debulking [9, 41, 60]. We did not mobilize the vertebral artery, leaving the peripheral venous plexus intact, in agreement with most operators who consider it a useless maneuver [13, 33, 40, 60]. A few surgeons may still use it in selected cases presenting with complications [4, 53]. Encasement of the PICA and the basilar artery also carries a higher risk of neurological sequelae from dividing the perforators. In meningiomas with vertebrobasilar artery encasement, extradural encasement and reoperation are associated with an elevated risk of vessel rupture and incomplete removal which occur in 41 and 51 % of cases, respectively [53, 60]. In our series, there were no cases of vascular injury.
Surgical technique is key to the management of tumor removal from neural structures. According to Sekhar et al., the brain stem, together with the cavernous sinus, was a recurrent site of remnants in meningiomas involving the clivus, with incomplete removal in 22 % of cases [52, 53]. The expectation of finding the lower cranial nerves in a certain position according to tumor size and position gives the surgeon the mental comfort indispensable for carrying out the operation. This is particularly true in cases where neural encasement is expected, as in large tumors. In ventrolateral tumors, the 11th cranial nerve is pushed posteriorly, the 9th–10th nerves elevated and stretched, and the 12th nerve encased or pushed downwards. Although cranial nerve position is fairly predictable in ventral tumors, these tumors still pose particular challenges for the surgeon. Only George et al. gave comparable importance to the position of cranial nerves classified according to anteroposterior tumor location [14, 24].
We found 65 cranial nerves embedded in tumors which were then dissected and freed along the arachnoidal plane, without causing intraoperative rupture. In their study on a larger series of clival meningiomas, Sekhar et al. reported similar results [52].
Clinical outcome
Perioperative mortality in recent surgical series was reportedly low or absent (Table 4). During the postoperative course, neurological impairment was mainly attributable to cranial nerve dysfunction. Dysphagia remains the most serious complication and is additionally burdened by risks linked to aspiration. Samii et al. reported general complications in 30 % of cases, with aspiration pneumonia the most serious complication (10 %). According to multiple regression analysis, tumor recurrence, arachnoid scarring, prevalent cranial extension, and absence of preoperative lower cranial nerve dysfunction were all found to be significantly associated with aspiration pneumonia [49]. Some studies reported 9th-10th cranial nerve impairment as the most common complication occurring in 43 to 60 % of cases [4, 33, 60], while two others found 12th cranial nerve deficit to be the most frequent complication (6 and 28 %, respectively) [40, 48]. In addition to lower cranial nerve dysfunction, Samii et al. mentioned transient 7th cranial nerve deficits (10 %), gait ataxia (15 %), and persistent paraparesis after anterior spinal artery injury in one case [49].
Overall neurological morbidity can be classified as transient or permanent. The ratio between the two is considered superior to global morbidity as an indicator of outcome quality (range, 27–62 %) [32, 45]. In two relatively recent studies, the prevalent permanent morbidity was 25 and 18 %, and the transient morbidity was 6 and 9 %, respectively [32, 45], while five other studies reported higher transient than permanent morbidity: 12 and 8, 35 and 22, 38 and 19, 50 and 5, and 61 and 2 %, respectively [9, 33, 40, 49, 60]. As a CSF fistula is often mentioned in the various series, it should be considered the most threatening local complication [3, 4, 9, 13, 32, 40, 42, 60] (Table 5).
Our findings confirm previous observations that postoperative impairment is due to cranial nerve dysfunction, especially of the 9th–10th and 12th cranial nerves (44 and 33 %, respectively), leading to life-threatening disturbance of respiration or impaired protection of the tracheobronchial tree, particularly during the early postoperative period. The difficulty in predicting this complication, either by preoperative compensation or clinical postoperative assessment, warrants careful management [60]. We prefer physiotherapist care rather than ancillary surgery if disability is not severe [32, 60]. With this strategy, mild aspiration occurred in only two cases with otherwise very successful results in our series. During the early postoperative course, 74 and 77 % of neurological signs associated with long-tract dysfunction or cerebellar deficits improved, whereas only 27 % of cranial nerve deficits did. Taken together, these data summarize the peculiarity of foramen magnum meningiomas: they may behave as either clival tumors or spinal tumors depending on the type and outcome of neurological dysfunction [33]. This observation is in agreement with other studies which classified patient outcome by tumor location and extension [13, 33, 49, 60]. This, together with a favorable transient/permanent neurological morbidity ratio, explains why the final outcome compared favorably between our and other series, despite severe clinical presentation and advanced age: 91 % of patients resumed a normal productive life within a few months after surgery, and only one patient was reoperated on during the 7-year follow-up period [9, 33]. We also concur with other operators on conservative management in the removal of fibrous tumors intimately adherent to cranial nerves or vessels or both, especially if the surgeon is less experienced, where gamma knife radiosurgery may be a valid ancillary treatment [32, 42, 45].
Conclusions
In this series, observed in the MRI era, mortality was nil, morbidity was very low, and outcome after surgery for ventral and ventrolateral foramen magnum meningioma was good. All patients underwent surgery in the semi-sitting position via the dorsolateral approach. A surgical approach optimized by tailoring the procedure to the single case and knowledge of the position of neurovascular structures can aid in the attempt to reach the gold standard: radical removal at the first operation. Nevertheless, incomplete resection may lead to a long steady state which warrants careful assessment of intraoperative risks. In most cases, functional outcome after long-tract and cerebellar decompression was very good. Transient morbidity was mainly attributable to lower cranial nerve deficits, which need to be adequately managed to prevent major complications.
References
Akalan N, Seckin H, Kilic C, Ozgen (1994) Benign extramedullary tumors in the foramen magnum region. Clinical Neurol Neurosurg 96:284–289
Al-Mefty O, Borba L, Aoki N, Angtuaco E, Pait TG (1996) The transcondylar approach to extradural noneoplastic lesion of the craniovertebral junction. J Neurosurg 84:1–6
Aring CD (1994) Lesions about the junction of medulla and spinal cord. JAMA 229:1879
Arnautovic KI, Al-Mefty O, Husain M (2000) Ventral foramen magnum meningiomas. J Neurosurg: Spine 92(1):71–80
Aydin Y, Ozden B, Barlas O, Turker K, Izgi N (1982) Urgent total removal of a lower clival meningioma. Surg Neurol 18:50–53
Ayeni SA, Ohata K, Tanaka K, Hakuba A (1995) The microsurgical anatomy of the jugular foramen. J Neurosurg 83:903–909
Babu RP, Sekhar LN, Wright DC (1994) Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg 81:49–59
Baldwin HZ, Miller CG, van Loveren HR, Keller JT, Daspit CP, Spetzler RF (1994) The far lateral/combined supra- and infratentorial approach. A human cadaveric prosection model for routes of access to petroclival region and ventral brain stem. J Neurosurg 81:60–68
Bassiouni H, Ntoukas V, Asgari S, Sandalcioglu EI, Stolke D, Seifert V (2006) Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery 59:1177–1185
Bertalanffy H, Gilsbach J, Mayfrank L, Kein HM, Kawase T, Seeger W (1996) Microsurgical management of ventral and ventrolateral foramen magnum meningiomas. Acta Neurochir Suppl 65:82–85
Bertalanffy H, Gilsbach J, Seeger W, Toya S (1994) Surgical anatomy and clinical application of the transcondylar approach to the lower clivus. In: Samii M (ed) Skull base surgery. Karger, Basel, pp 1045–1048
Bertalanffy H, Seeger W (1991) The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery 29:815–821
Borba LA, de Oliveira JG, Giudicissi-Filho M, Colli BO (2009) Surgical management of foramen magnum meningiomas. Neurosurg Rev 32:49–58
Bruneau M, George B (2008) Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisière Hospital and review of the literature. Neurosurg Rev 31(1):19–32
Bruneau M, George B (2010) Classification system of foramen magnum meningiomas. J Craniovertebr Junction Spine 1(1):10–17
Bull J (1974) Missed foramen magnum tumors. Lancet 1:91
Campbell E, Whitfield R (1948) Posterior fossa meningiomas. J Neurosurg 5:131–153
Castellano F, Ruggiero G (1953) Meningiomas of the posterior fossa. Acta Radiol 104:1–157
Cohen L, Macrae D (1962) Tumors in the region of the foramen magnum. J Neurosurg 19:462–469
Dany A, Delcour J, Laine E (1963) Les meningiomes du clivus. Neurochirurgie 9:249–277
Dodge HW, Love JG, Gottlieb CM (1956) Benign tumors at the foramen magnum. Surgical considerations. J Neurosurg 13:603–617
George B, Dematons C, Cophigon J (1988) Lateral approach to the anterior portion of the foramen magnum. Surg Neurol 29:484–490
George B, Lot G (1995) Foramen magnum meningiomas: a review from personal experience of 37 cases and from a cooperative study of 106 cases. Neurosurgery Quarterly 5:149–167
George B, Lot G, Boissonet H (1997) Meningioma of the foramen magnum: a series of 40 cases. Surg Neurol 47:371–379
George B, Lot G, Velut S (1993) Pathologie tumorale du foramen magnum. Neurochirurgie 39:8–9, 11–30; 31–42; 65–73
Gilsbach JM, Eggert HR, Seeger W (1987) The dorsolateral approach in ventrolateral craniospinal lesions. In: Voth D, Gees P (eds) Disease in the cranio-cervical junction. Walter de Gruyter, Berlin, pp 359–364
Goel A, Desai K, Muzumdar D (2001) Surgery on anterior foramen magnum meningiomas using a conventional posterior suboccipital approach: a report on an experience with 17 cases. Neurosurgery 49:102–106
Guidetti B, Spallone A (1980) Benign extramedullary tumors of the foramen magnum. Surg Neurol 13:9–17
Guidetti B, Spallone A (1988) Benign extramedullary tumors of the foramen magnum. Adv Tech Stand Neurosurg 16:83–120
Heroes RC (1986) Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg 64:559–562
Howe JR, Taren JA (1973) Foramen magnum tumors: pitfalls in diagnosis. JAMA 225:1061–1066
Kandenwein JA, Richter HP, Antoniadis G (2009) Foramen magnum meningiomas–experience with the posterior suboccipital approach. Br J Neurosurg 23:33–39
Kano T, Kawase T, Horiguchi T, Yoshida K (2010) Meningiomas of the ventral foramen magnum and lower clivus: factors influencing surgical morbidity, the extent of tumour resection, and tumour recurrence. Acta Neurochir 152:79–86
Kim KS, Weinberg PE (1981) Foramen magnum meningioma. Surg Neurol 17:287–289
Koos WTH, Spetzler RF, Pendl G, Perneczky A, Lang J (1985) Color atlas of microneurosurgery, vol 118. Thieme, Stuttgart, pp 125–134
Kratimenos GP, Crockard HA (1993) The far lateral approach for ventrally placed foramen magnum and upper cervical spine tumours. Br J Neurosurg 7(2):129–140
Lang J (1986) Craniocervical region, blood vessels. Neuro-orthopedics 2:55–69
Lang J (1987) Craniocervical region, surgical anatomy. Neuro-orthopedics 3:1–26
Lecuire J, Dechaume JP (1971) Les meningiomes de la fosse cerebrale posterieure. Neurochirugie 17:1–146
Margalit NS, Lesser JB, Singer M, Sen C (2005) Lateral approach to anterolateral tumors at the foramen magnum: factors determining surgical procedure. Neurosurgery 56(2 Suppl):324–336
Meyer FB, Ebersold MJ, Reese DF (1984) Benign tumors of the foramen magnum. J Neurosurg 61:136–142
Nicolato A, Foroni R, Pellegrino M, Ferraresi P, Alessandrini F, Gerosa M, Bricolo A (2001) Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg 44:211–217
Pamir MN, Kiliç T, Ozduman K, Türe U (2004) Experience of a single institution treating foramen magnum meningiomas. J Clin Neurosci 11:863–867
Perneczky A (1986) The posterolateral approach to the foramen magnum. In: Samii M (ed) Surgery in and around the brain stem and the third ventricle. Springer, Berlin, pp 460–466
Pirotte BJ, Brotchi J, DeWitte O (2010) Management of anterolateral foramen magnum meningiomas: surgical vs conservative decision making. Neurosurgery 49:102–106
Pritz MB (1991) Evaluation and treatment of intradural tumors located anterior to the cervicomedullary junction by a lateral suboccipital approach. Acta Neurochir 113:74–81
Resnikoff S, Cardenasy J (1964) Meningioma at the foramen magnum. J Neurosurg 21:301–303
Salas E, Sekhar LN, Ziyal IM, Caputy AJ, Wright D (1999) Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg: Spine 90(2):206–219
Samii M, Klekamp J, Carvalho G (1996) Surgical results for meningiomas of the craniocervical junction. Neurosurgery 39:1086–1095
Scott EW, Rhoton AL (1991) Foramen magnum meningiomas. In: Al-Mefty O (ed) Meningiomas. Raven, New York, pp 543–568
Seeger W (1978) Atlas of topographical anatomy of the brain and surrounding structures. Springer, Wien, pp 485–489
Sekhar LN, Jannetta PJ, Burkhart LE, Janosky JE (1990) Meningiomas involving the clivus: a six-year experience with 41 patients. Neurosurg 27:764–781
Sekhar LN, Javed T (1973) Meningiomas with vertebrobasilar artery encasement: review of 17 cases. Skull Base Surgery 3:91–106
Sekhar LN, Swamy NK, Jaiswal V, Rubinstein E, Hirsch WE Jr, Wright DC (1994) Surgical excision of meningiomas involving the clivus: preoperative and intraoperative features as predictors of postoperative functional deterioration. J Neurosurg 81:860–868
Sen CN, Sekhar LN (1990) An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery 27:197–204
Sen CN, Sekhar LN (1991) Surgical management of anteriorly placed lesions at the craniocervical junction-an alternative approach. Acta Neurochir 108:70–77
Smolik EA, Sachs E (1954) Tumors of the foramen magnum of spinal origin. J Neurosurg 11:161–172
Stein BM, Leeds NE, Taveras JM, Pool JL (1963) Meningiomas of the foramen magnum. J Neurosurg 20:740–751
Welling B, Al-Mefty O (1996) Foramen magnum tumors. In: Cohen AR (ed) Surgical disorders of the fourth ventricle. Blackwell Science, Oxford, pp 251–261
Wu Z, Hao S, Zhang J, Zhang L, Jia G, Tang J, Xiao X, Wang L, Wang Z (2009) Foramen magnum meningiomas: experiences in 114 patients at a single institute over 15 years. Surg Neurol 72:376–382
Yasargil MG, Mortara RW, Curcic M (1980) Meningiomas of basal posterior fossa. Adv Tech Standards Neurosurg 7:3–115
Yasuoka S, Okazaki H, Okazaki H, Daube JR, MacCarthy CS (1978) Foramen magnum tumors. J Neurosurg 49:828–838
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Michaël Bruneau, Jacques Brotchi, Brussels, Belgium
This article reflects the wide experience of the authors about the surgical treatment of anterior and lateral foramen magnum meningiomas. In this large series based on 64 cases, total removal was achieved in 81 %, while in the remaining cases, a remnant was judiciously left in place due to adherences to perforators, to the vertebral artery, or to the brainstem. The authors noted, as experienced by others, difficulties generated by encasement of important neurovascular structures, excessive bleeding, hard tumor consistency, and aggressive tumor behavior inducing dural invasion and the absence of the arachnoidal plane. Their results were excellent with, respectively, 74, 77, and 27 % of improvement of preoperative long tracts, cerebellar and cranial nerve deficits. The authors noted 21 new cranial deficits postoperatively and pointed out the importance of swallowing disturbances. We agree that these must be systematically checked postoperatively as soon as possible in order to prevent aspiration. In all cases, their approach consisted in a far-lateral approach. This approach is associated with the lowest morbidity rate and allows an adequate exposure of these tumors. In our experience, the drilling of the medial aspect of the foramen magnum lateral wall must only be performed in selected cases and, when required, can always be very limited. It is extremely important to be able to anticipate the position of the lower cranial nerves. In lateral tumors, their position depends on the relation of the meningioma with the vertebral artery. Tumors growing below the vertebral artery (which is the most common situation) displace the lower cranial nerves upwards and posteriorly. Unfortunately, while growing above the vertebral artery, the lower cranial nerves can be displaced in any direction.
William T. Couldwell, Salt Lake City, USA
The authors have presented their surgical results of a series of 64 patients treated at their institution over a 20-year period. They had many older patients (>65 years, n = 29). They provide an honest appraisal of the complications associated with removal of these tumors, especially the lower cranial nerve palsies.
This is a great contribution to the literature and will represent the best example of contemporary microsurgical results for the treatment of meningiomas in this location. It provides a thorough review of other series in the recent literature and also sets the standard for which to compare outcomes of evolving anterior transfacial and endoscopic techniques.
Helmut Bertalanffy, Hannover, Germany
I wish to congratulate the authors and particularly the senior author (AB) for the nice presentation of their patient series and their good results in this special group of skull base meningiomas. The authors' expertise is also reflected in the remarkable number of patients treated at a single institution.
Some neurosurgeons consider the surgical removal of foramen magnum meningiomas an easy task, as has occasionally been mentioned during oral presentations. The authors of this study have nicely shown that this may be an inadequate generalization and underestimation of the problems that can occur in treating a foramen magnum meningioma. Surgery can be quite challenging, for instance when they firmly adhere to the brainstem or in cases in which the tumor extends into the extradural space. Indeed, each type of tumor may require a tailored surgical technique. However, I am not in favor of distinguishing so many variations of exposure such as transfacetal, retrocondylar, partial transcondylar, complete transcondylar, extreme-lateral transjugular, and transtubercular that evolved in the recent literature. In analogy to different ways of exposing a medial sphenoid wing meningioma by various degrees of resecting the sphenoid wing, the amount of bony resection at the level of the lateral foramen magnum depends upon the local anatomy and the exact location and extent of the tumor. For an adequate exposure of a foramen magnum meningioma, I recommend exposing the vertebral artery up to the dural entrance that is hidden by the surrounding venous plexus. Initially, this venous plexus has to be dealt with properly either by injecting fibrin glue or by resecting the plexus and achieving good hemostasis with gentle packing of hemostatic sponges or Surgicel. Thus, the exact course of the horizontal portion of the vertebral artery becomes clearly visible: it is lateral to medial within the sulcus of the atlantal arch, but medial to lateral prior to piercing the dura mater. In a meningioma that completely encases the proximal intradural vertebral artery; I always open the dural ring around the artery to completely mobilize the vessel. This nicely exposes the tumor portions located ventral to the artery that may otherwise not be easily accessible. Mobilizing the artery by opening the dural ring is also required in the cases of intra- and extradural extension of a foramen magnum meningioma. In such case, the medial portions of the occipital condyle and of the lateral atlantal mass have to be drilled away lateral to the dural entrance of the vertebral artery [1].
We are grateful not only for the detailed description of the authors' technique but also for their nice overview of the pertinent literature on this subject.
References
1. H Bertalanffy, O Bozinov, O Sürücü, U Sure, L Benes, C Kappus, N Krayenbühl (2010) Dorsolateral approach to the craniocervical junction. In: P. Cappabianca et al. (eds.), Cranial, craniofacial and skull base surgery. Springer, Italy pp. 175–196
Engelbert Knosp, Vienna, Austria
Foramen magnum meningiomas are a rare and challenging pathology in neurosurgery. Although we achieved significant technological improvements during the time, the requirements in this specific pathology remained unchanged over the decades : namely detailed anatomical knowledge, an accurate approach and an excellent surgical technique.
In this publication, Dr Bricolo et al. presented a very large series of ventrally located foramen magnum meningiomas, which were collected over the period of 20 years and were treated in the same fashion over the years: semisitting position and using the dorsolateral approach. The authors focused on anatomical and surgical details of this confined area of the foramen magnum, lower clivus and upmost spinal canal. They addressed many specific problems arising in surgical treatment of these lesions and they provide the readers sound suggestions to avoid complications. Although it was rare in the senior authors hand, the suggestion to stop resection in case of hard and calcified tumors or encasement of perforators or loss of arachnoid plane at the brainstem is very helpful.
A topic still remained for discussion is, in which extent one has to resect the posterior part of the condyle and the necessity to remove arch of C1 or whether it is always necessary to dissect (and displace) the vertebral artery. As seen in the manuscript, the authors prefer an extensive extradural resection to reach the anterior rim of the foramen magnum.
In this publication the reader can appreciate a life long dedication to neurosurgery, furthermore his surgical philosophy in treating difficult pathologies like eg foramen magnum meningiomas. The results showed here are excellent and rich in details, well analysed and a must to read for every surgeon dealing with meningiomas of the foramen magnum.
Rights and permissions
About this article
Cite this article
Talacchi, A., Biroli, A., Soda, C. et al. Surgical management of ventral and ventrolateral foramen magnum meningiomas: report on a 64-case series and review of the literature. Neurosurg Rev 35, 359–368 (2012). https://doi.org/10.1007/s10143-012-0381-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-012-0381-6