Abstract
Ventriculoperitoneal shunting is a widely accepted technique for the treatment of hydrocephalus. The standard procedure to insert the peritoneal catheter requires an abdominal incision, muscle dissection, and opening of the peritoneum. A number of complications related to the abdominal surgical phase have been reported. Laparoscopy-assisted ventriculoperitoneal shunting is a valid alternative procedure that reduces surgical trauma. We describe our experience and review the literature. A total of 30 laparoscopically guided ventriculoperitoneal shunting procedures were performed between January 2007 and June 2008, in collaboration with a general surgeon experienced in laparoscopy. Of these procedures, 25 were new shunt placements and 5 were revisions. Data about operative time, outcome, and complications were registered and compared with a group of 30 patients treated by means of standard laparotomy in the period 2005–2007. Laparoscopic shunt placement was successful in all patients. Operative duration, complications, and postoperative pain were all lower in patients treated by laparoscopy as compared to the laparotomy. In the laparoscopic group, an earlier peristalsis, quicker mobilization, and better cosmetic results were also noted. Laparoscopy in both ventriculoperitoneal shunt placement and revision is a safe, effective, and minimally invasive technique. It ensures proper abdominal placement of the distal catheter under direct vision allowing confirmation of its patency.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebrospinal fluid (CSF) shunting is the most used technique in neurosurgery for the treatment of hydrocephalus in both pediatric and adult patients [15, 18]. Shunts are used for treatment of hydrocephalus, drainage of arachnoid cysts, and postcraniotomy chronic subcutaneous fluid collections [33]. Peritoneum is the most common district recruited for draining CSF [22]. Usually, the distal catheter is inserted performing a small laparotomy in the right paraumbilical, right subcostal, or median supraumbilical region [2].
In literature many abdominal disadvantages associated with ventriculoperitoneal (VP) shunts are described. These include: obstruction of peritoneal catheter, peritonitis from shunt infection, hydrocele, CSF ascites, intestinal obstruction or perforation, volvolus, intestinal strangulation, postoperative pain, wound infection, and inguinal hernia (when shunts are inserted while processus vaginalis is patent) [11].
Moreover, about 50% of shunt-related admissions and costs are due to shunt revisions [29]. A high rate of surgical failure is related to the distal placement of the catheter, which is particularly difficult in obese patients, patients with previous abdominal surgeries, and patients with chronic inflammatory diseases [16, 33].
In order to reduce the number of complications and requirement for surgical revisions, many authors have suggested the use of the endoscope for the positioning of the distal catheter [9, 15, 16, 18, 30, 32, 33]. We describe our experience with the use of the endoscope in VP shunt placement and revision, and present a thorough review of the literature.
Historical perspective
VP shunts were first described in 1908, when Kausch used a rubber conduit to drain the lateral ventricle into the peritoneal cavity, but this concept did not receive much initial enthusiasm [22]. In 1967, because of the development of silastic catheters, which were well tolerated in the abdomen, Ames and Matsumoto resurrected the concept of VP procedures [22].
In 1978, Rodgers et al. described the use of laparoscopic techniques to determine the cause of shunt malfunction and to reposition the catheter in children with VP shunt distal obstruction [35]. In 1984, Lockhart et al. described blind percutaneous insertion of the peritoneal portion of shunt catheters using the Veress needle [23]. This procedure did not require any abdominal incision but was associated with a high risk of visceral and vascular injuries [10]. Recently, Ochalski et al. have described a modification of the original “blind” technique of Lockhart based on carbon dioxide insufflation to establish pneumoperitoneum, which can reduce these complications [28].
It was Armbruster in 1993 who first explored the feasibility of laparoscapically assisted implantation of VP shunts [4]. Thereafter, several reports have commented on this technique [7, 8, 15, 16, 18, 30, 32–34].
We started performing laparoscopically assisted shunt placement at our institution in 2007 with the aid of a general surgeon experienced in laparoscopy. The surgical technique is presented along with the obtained results and a review of the literature.
Patients and methods
Thirty patients treated with complete laparoscopically guided VP shunt procedures were followed prospectively from January 2007 to December 2008. They included 17 men and 13 women; pediatric patients were not included in the study. The average age was 62.6 years (range 24–85). Twenty-five received the placement of new shunt system; five underwent shunt revision. Urgent cases and pure distal shunt revisions were not included. No other exclusion criteria were used in the enrollment of the patients.
Collected data were compared with those of a group of consecutive 30 patients, retrospectively reviewed, that were treated by standard minilaparotomy in the period 2005–2007 before the endoscopic study was initiated. In the minilaparotomic group the male–female ratio was 14:16. Mean age was 63.6 years (range 38–84). Twenty three received the placement of new shunt system, and seven underwent shunt revision.
The median value of the follow-up was 13 months for the laparoscopic group and 19 months for the control group. Demographic data for the two groups are summarized in Table 1.
Statistics
Differences of outcome and complications between treatment options were evaluated using the Student's t test and the chi-square (χ 2) test. Kaplan–Meier curves in combination with log rank test were used for the analysis of complication-free survival.
Surgical technique
The surgical and the neurosurgical teams were present in all surgical procedures.
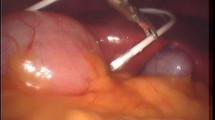
Under general anesthesia, the patient is placed supine on the operative table with the head resting on a horseshoe headrest turned about 30° toward the side. Head, neck, chest, and abdomen are prepared and draped in the usual fashion, leaving the sites of incision and the trajectory of the catheter exposed under a sterile transparent adhesive drape. A frontal burr hole is performed through a straight skin incision about 3 cm lateral to the midline and 2 cm anterior to the coronal suture. A curvilinear incision is placed in the retroauricular region to create the valve pocket. The ventricular catheter is placed in the lateral ventricle, passed subcutaneously to the retroauricular region, and connected to the valve. We used two types of valves based on each patient case: Medtronic medium pressure valve (Medtronic, Inc. Minneapolis, MN) or Codman®–Hakim programmable valve (Codman & Shurtleff, Inc., Raynham, MA). With a malleable tunneler, the distal catheter is then passed to the abdominal site of entrance, usually located in the right paraumbilical region, exteriorized through a small skin incision, and temporarily plunged in a sterile antibiotic soaked gauze covering. The general surgery team performs, usually contemporarily to the neurosurgical maneuvers, the introduction of the laparoscope with the open Hasson technique: a 15-mm curvilinear incision is created in the infraumbilical region, the peritoneal cavity is entered and a 12-mm blunt-tip trocar (Endo-Path Excel; Ethicon Endo-Surgery Inc., Cincinnati, OH) is inserted. Carbon dioxide pneumoperitoneum is created up to a pressure of 12 mmHg using the blunt-tip trocar and a 10-mm 30° videoscope (Karl Storz GmbH & Co., Tuttlingen, Germany), connected to a light source and a viewing device, is inserted through the trocar into the abdomen. The abdominal cavity is then inspected for possible undiagnosed pathologies (e.g., masses, hernias) or adhesions and for choosing a suitable site for the distal catheter insertion. If adhesions are encountered, another 5-mm trocar for the ultrasound scissors is introduced on the side of the abdomen opposite to the adhesions and adhesiolysis is performed. A split trocar (Codman & Shurtleff, Inc.) is inserted through the right paraumbilical incision and under videoscopic visualization, the trocar can be seen penetrating the ventral peritoneal wall. The distal catheter is then delivered into the peritoneal cavity through the split trocar, which serves as a guide, and directed to the desired site verifying spontaneous CSF flow. Once the position and the shunt function are confirmed, the split trocar is removed. The pneumoperitoneum is deflated, and the videoscope and the Endo-Path Excel trocar are removed. The subumbilical access is closed with three to four nonabsorbable stitches and the right paraumbilical access with one nonabsorbable stitch. At the same time, the neurosurgeon performs subcutaneous and skin closure of the frontal and retroauricular incisions with absorbable and nonabsorbable stitches, respectively.
Results
The laparoscopic group (Lg) and the non-laparoscopic group (NLg) were compared for the following variables: preoperative abdominal surgical procedures, cause of hydrocephalus, technical problems during the operation, intra- and postoperative complications, surgery duration, postoperative pain using the visual analog scale (VAS), time to resume of oral intake, X-ray confirmation of catheter placement, and short and long-term clinical outcome.
Etiology
The patient diagnoses in the Lg were as follows: 11 patients had normal pressure hydrocephalus (NPH), 17 patients had obstructive hydrocephalus, and two patients had aresorptive hydrocephalus.
The patient diagnoses in the NLg were as follows: 8 patients had NPH, 20 patients had obstructive hydrocephalus, and 2 patients had aresorptive hydrocephalus (Table 1). Ten of patients, who received laparotomic VP shunt placement, had had previous abdominal surgeries; eight patients were obese.
We underline that until 2008 the shunt represents our gold standard for the treatment of hydrocephalus even if still now no randomized studies comparing endoscopic third ventriculostomy with shunt exist [20].
Surgical time and intraoperative complications
The total surgical time was less than 30 min with proximal (catheter placement and tunneling of the shunt down to the abdomen) and distal procedures (trocars insertion, abdominal inspection, distal catheter insertion, control of shunt function, and closure of abdominal incisions) performed simultaneously (Table 2). In the laparotomic group, the total surgical time ranged from 45 to 80 min instead (Table 2). The difference between the two groups was statistically significant (α < 0.05). It is noteworthy that the laparotomic procedure was performed by a single team of surgeons.
No intraoperative complications were recorded in the Lg.
In an obese elder patient, during the laparoscopic procedure, extensive adhesions were noted; therefore, a 5-mm trocar for the ultrasound scissors was introduced on the side of the abdomen opposite to the adhesions and adhesiolysis was successfully performed before distal catheter introduction.
In the NLg, one case of bowel perforation occurred during surgery, requiring the assistance of a general surgeon.
Postoperative course
Patients of the laparoscopic group referred minimal pain in the subumbilical access. On postoperative day 1, patients assigned a value on average of 1.6 to their pain (VAS scale, Table 2). On the contrary, patients who did not belong to the laparoscopic group felt much more pain, giving a value on average of 3.8 to the VAS scale (Table 2). The difference between the two groups was statistically significant (α < 0.05). In five patients of the laparoscopic group and in nine patients of the laparotomic one, VAS was not recorded because of their non-collaboration. Analgesic drug consumption was minimal in both groups.
Clinical signs and symptoms, including abdominal distension, bowel sounds, passage of feces and/or flatus, pain, nausea, and vomiting, were evaluated for the determination of the end of postoperative ileus. No significant differences were recorded between the two groups in terms of recovery of intestinal activity and feeding.
Head computed tomography and X-ray of catheter course were performed in all patients on postoperative day 1.
Length of stay was not considered in this study because affected by too many variables: etiology of hydrocephalus, age of patients, GCS on admittance, multiple operations during hospital stay, etc.
Outcome
Based on timing, we divided the surgical outcome into three categories: (1) immediate, when complications occurred within the first 10 days after surgery; (2) short term, when complications occurred within 6 months from the surgical procedure; and (3) long term, when complications occurred after more than 6 months (Table 3).
The immediate complications were as follows: a valve malfunction in the Lg required surgical revision; in the NLg, three cases of distal malposition of the catheter required a revision (in two cases the catheter was placed in the subcutaneous tissue and in one case in the submuscular layer), one patient died during the hospital stay of breathing complications, one patient had proximal malfunction of the catheter, and in one patient, an intraoperative bowel perforation occurred.
Short-term complications: In the laparoscopic group, four patients died within 6 months of their primary disease; in the non-laparoscopic group, one short-term complication (malfunction) related to the presence of a coiling in the distal catheter occurred, one patient died of his primary disease, one patient required surgical revision of the proximal catheter, and one presented with a subacute subdural hematoma.
Long-term complications: In the laparoscopic group, two patients died of their primary disease, and in one case, a valve malfunction occurred requiring substitution; in the NLg, two proximal malfunctions, one obstruction of the distal catheter due to severe peritoneal adhesions, and one death (not related to the hydrocephalus) were noted.
No patients in both groups presented with shunt infections requiring surgical removal of the catheter; in two patients in the NLg, the postoperative time was characterized by fever and high values of indices of inflammation with a negative CSF culture, thus they were subjected to appropriate antibiotic treatment.
In Lg, no distal complications occurred and this has influenced the total number of complications. Indeed, there was a significant difference in overall complication rate between the two groups in the follow-up period (P = 0.0069, Fig. 1). Reviewing the complications linked with the abdominal surgical procedure, we observed a significant difference in the two groups: six abdominal complications occurred in the NLg and none in the Lg (α = 0.05).
In order to establish the impact of risk factors on the outcome, we reviewed the number of distal complications occurring in obese patients and in those with previous surgical procedures. In the laparoscopic group, no distal complications occurred neither in the immediate nor in the short or long-term periods. In the non-laparoscopic group, all complications occurred in those patients who had had previous surgical procedures; the only obese patient who presented complications had also had a previous operation.
Discussion
Ventriculoperitoneal shunt placement is a widespread technique for the treatment of hydrocephalus. Despite that its use has been described by many authors [2], the cost and the complications associated to this procedure prompted the research for alternative techniques. According to Bondurant and Patwardhan, VP shunts constitute a significant medical problem, in terms of urgency of treatment and economic costs, which was greater than $1 billion in the United States in the year 2000 [29].
Distal failure of VP shunt placement accounts for 25–30% of shunt revisions [16].
The highest number of distal complications in the VP placement has been described in obese patients as well as in patients with previous abdominal surgeries [15, 16, 32]. All these conditions reduce the shunt placement survivability [39].
Abdominal problems associated to obesity and adherences from previous surgeries are issues that were already faced by general surgeons and that in some cases were dealt with the aid of the endoscope. In this perspective, different authors started using the laparoscopic assisted technique for the placement of the distal catheter in the peritoneum [6, 9, 15, 16, 18, 30, 33–35, 39, 40]. It revealed to be a good technique that can lower the surgery duration and complications [3, 5, 16, 21, 39] although it requires the availability of two surgical teams, a dedicated instrumentation, and a variable number of surgical incisions, with an overall increased cost. However, it should be noted that there are some reports of lower complication rates of distal failures associated with laparotomic treatment. [17, 24, 25].
Because of these drawbacks, some authors suggest the use of the endoscope-assisted technique only in selected cases [15, 16, 18, 33, 40], while others consider it a helpful procedure in all patients [5, 39].
We presented our experience with the use of the laparoscopic assisted VP shunt placement comparing our results with a retrospectively collected cohort of patients treated with standard laparotomy.
In our experience, the requirement of both the surgical and the neurosurgical team led us to include in the present study only elective cases while emergencies were treated in the standard fashion only by the neurosurgeons.
The presence of a general surgeon only in the laparoscopic technique in our experience as well as in most of the literature raises the question whether the reduced incidence of complications is due to the expertise of the general surgeon or to the advantages of the laparoscopic technique. It is difficult to address this question based on the available information. Further studies are warranted. It is our opinion that a general surgical training is mandatory for neurosurgeons who want to perform the endoscopic approach; furthermore, the use of an “open” laparoscopic technique, which has been described as less risky for bowel and vascular perforation (compared to the blind introduction of the Veress needle) [27], requires a surgical expertise that is not part of the standard training of neurosurgeons.
The low incidence of complications in the Lg is probably due to the assistance of an endoscopic-experienced surgeon who can better deal with adherences and distorted anatomy in the most difficult cases. It seems to be reasonable that the assistance of a general surgeon could lower the rate of complications also in the standard technique, but it must be stressed that the advantages of the endoscope for dealing with adherences are well demonstrated in the surgical literature [9, 16, 33, 35, 36].
Further advantages of the endoscopic technique are suggested by our results; the endoscope indeed allows a direct visualization of the placement of the distal catheter and of its function. This is extremely useful to assess the correct functioning of VP shunt when their failure is suspected instead of proceeding with direct shunt substitution. All these considerations, supported by those of other authors [33, 35], strongly support the usefulness of the laparoscopic technique in standard patient care.
Whether the laparoscopic technique has to be used only in selected cases or in all patients is still a matter of debate; our results once again stress the advantages of this technique especially for those patients who have undergone previous surgical procedures.
The importance of laparoscopy in the placement of VP shunt is further underlined by the development of more refined techniques such as the one described by Turner who proposes the “….tunneling of the distal shunt catheter from the head into the peritoneum under laparoscopic guidance without skin incision overlying the distal catheter insertion site” [39]. This study on 111 patients further underlines the advantages of fast recovery time and reduction of misplaced catheters commonly associated to the standard laparotomic technique.
However, the laparoscopic approach is not without drawbacks; Roth and Yu together with other authors [33, 40] described some cardiovascular and respiratory complications deriving from the induced pneumoperitoneum (Table 4). Sekula reported how a conversion to an open procedure was required after failure to establish pneumoperitoneum in 3% of patients [37]. It is important to remember the theoretical risk of pressure transmission along the shunt system as suggested by Yu that can be lowered by a slowly induced pneumoperitoneum [40].
We did not observe any similar anesthetic problem although all these issues further suggest the need for a dedicated training.
Conclusion
Laparoscopic assisted VP shunt placement is gaining popularity in recent times with the progressive diffusion of endoscopic techniques. It is still confined to some excellent centers where there is the availability of both a surgical and a neurosurgical team.
Our results as well as those found in the recent surgical literature are encouraging although some points have to be better clarified such as the opportunity of its widespread use or limited to the most difficult cases.
It is our opinion that a good training is essential for mastering this technique properly but, once achieved, can be broadly used allowing good results also in those cases where complications are more frequent.
The problems related to the costs of the instrumentation as well as the need for another surgical team can also be offset by good training for neurosurgeons and by the significant reduction in the rate of complications.
References
Acharya R, Ramachandran CS, Singh S (2001) Laparoscopic management of abdominal complications in ventriculoperitoneal shunt surgery. J Laparoendosc Adv Surg Tech A 1(3):167–170
Apuzzo MJ (1993) Cerebrospinal fluid diversion procedures. In: Apuzzo MJ (ed) Brain surgery: complication avoidance and management. Churchill Livingstone, New York, pp 1471–1474
Argo JL, Durgamani KY, Ballem N, Harrigan MR, Fisher WS III, Wesley MM, Taylor TH, Clemnts RH (2009) Laparoscopic versus open approach for implantation of the peritoneal catheter during ventriculoperitoneal shunt placement. Surg Endosc 23:1449–1455. doi:10.1007/s00464-008-0245-x
Armbruster C, Blauensteiner J, Ammerer HP, Kriwanek S (1993) Laparoscopically assisted implantation of ventriculoperitoneal shunts. J Laparoendosc Surg 3:191–192
Bani A, Hassler WE (2006) Laparoscopy-guided insertion of peritoneal catheters in ventriculoperitoneal shunt procedures: analysis of 39 children. Pediatr Neurosurg 42:156–158. doi:10.1159/000091858
Bani A, Telker D, Hassler W, Grundlach M (2006) Minimally invasive implantation of the peritoneal catheter in ventriculoperitoneal shunt placement for hydrocephalus: analysis of data in 151 consecutive adult patients. J Neurosurg 105:869–872
Cautico W, Vannix D (1995) Laparoscopically guided peritoneal insertion in ventriculoperitoneal shunts. J Laparoendosc Surg 5:309–311
Esposito C, Colella G, Settimi A, Centonze A, Signorelli F, Ascione G, Palmieri A, Gangemi M (2003) One-trocar insufflation: a valid procedure to treat abdominal complications in children with peritoneal shunt for hydrocephalus. Surg Endosc 17(5):828–830. doi:10.1007/s00464-002-9063-8
Esposito C, Porreca A, Gangemi M, Garipoli V, De Pasquale M (1998) The use of laparoscopy in the diagnosis and treatment of abdominal complications of ventriculo-peritoneal shunts in children. Pediatr Surg Int 13(5–6):352–354
Goiten D, Papasvas P, Gagné D, Ferraro D, Wilder B, Caushaj P (2006) Single trocar laparoscopically assisted placement of central nervous system-peritoneal shunts. J Laparoendosc Adv Surg Tech A 16(1):1–4
Greenberg MS (2006) Treatment of hydrocephalus. In: Greenberg MS (ed) Handbook of Neurosurgery, 6th edn. Thieme Medical Publishers, New York, pp 185–187
Handler MH, Callahan B (2008) Laparoscopic placement of distal ventriculoperitoneal shunt catheters. J Neurosurg Pediatr 2:282–285
Jea A, Al-Otibi M, Bonnard A, Drake JM (2007) Laparoscopy-assisted ventriculoperitoneal shunt surgery in children: a series of 11 cases. J Neurosurg 106(6):421–425
Kavic SM, Segan RD, Taylor MD, Roth JS (2007) Laparoscopic management of ventriculoperitoneal and lumboperitoneal shunt complications. JSLS 11:14–19
Khaitan L, Brennan EJ Jr (1999) A laparoscopic approach to ventriculoperitoneal shunt placement in adults. Surg Endosc 13(10):1007–1009
Khosrovi H, Kaufman HH, Hrabovsky E, Bloomfield SM, Prabhu V, el-Kadi HA (1998) Laparoscopic-assisted distal ventriculoperitoneal shunt placement. Surg Neurol 49(2):127–134
Kiefer M, Eymann R (2010) Gravitational shunt complications after a five-year follow-up. Acta Neurochir Suppl 106:107–112
Kirshtein B, Benifla M, Roy-Shapira A, Merkin V, Melamed I, Cohen Z, Cohen A (2004) Laparoscopically guided distal ventriculoperitoneal shunt placement. Surg Laparosc Endosc Percutan Tech 14(5):276–278
Kostantinidis H, Balogiannls I, Foroglu N, Spiliotopoulos A, Magras I, Keslsoglou I, Selviaridis P (2007) Laparoscopic placement of ventriculoperitoneal shunts: an innovative simplification of the existing techniques. Minim Invasive Neurosurg 50:62–64. doi:10.1055/s-2007-976513
Kulkarni A, Drake J, Kestle J, Mallucci CL, Sgouros S, Constantini S (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67(3):588–593
Li B, Zhang Q, Liu J, Hualong Yu, Sanyuan Hu (2007) Clinical application of a laparoscope in ventri-peritoneal shunting. Minim Invasive Ther Allied Technol 16(6):367–369. doi:10.1080/13645700701699547
Lifshutz Jason I, Walter J (2001) History of hydrocephalus and its treatments. Neurosurg Focus 11(2):1–5
Lockhart C, Selman W, Rodziewiez G, Speltzer RF (1984) Percutaneous insertion of peritoneal shunt catheter with use of the Veress needle. J Neurosurg 60:444–446
Meier U, Kiefer M, Sprung C (2004) Evaluation of the Miethke dual-switch valve in patients with normal pressure hydrocephalus. Surg Neurol 61:119–127
Meier U, Lemcke J, Al-Zain F (2008) Course of disease in patients with idiopathic normal pressure hydrocephalus (iNPH): a follow-up study 3, 4 and 5 years following shunt implantation. Acta Neurochir Suppl 102:125–127
Nfonsam V, Chand B, Rosenblatt S (2008) Laparoscopic management of distal ventriculoperitoneal shunt complications. Surg Endosc 22:1860–1870. doi:10.1007/s00464-007-9728-4
Nuzzo G, Giuliante F, Tebala GD, Vellone M, Cavicchioni C (1997) Routine use of laparoscopic operations. J Am Coll Surg 184(1):58–62
Ochalski PG, Horowitz MB, Mintz AH, Hughes SJ, Okonkwo DO, Kassam AB, Watson AR (2009) Minimal-access technique for distal catheter insertion during ventricular peritoneal shunt procedures: a review of 100 cases. J Neurosurg 13:1–5. doi:10.3171/2009.2.JNS08454
Patwardhan RV, Nanda A (2005) Implanted ventricular shunts in the United States: the billion-dollar-a-year cost of hydrocephalus treatment. Neurosurgery 56:139–144. doi:10.1227/01.NEU.0000146206.40375.41
Reimer R, Wharen RE, Jr PPDM (1998) Ventriculoperitoneal shunt placement with video-laparoscopic guidance. J Am Coll Surg 187:637–639
Rolle U, Gräfe G, brock D, Grosser K (1998) Laparoscopy-assisted abdominal shunt revisions in children with hydrocephalus. Eur J Pediatr Surg 8(1):60
Roth JS, Park E, Gewirtz R (2000) Minilaparoscopically assisted placement of ventriculoperitoneal shunts. Surg Endosc 14:461–463. doi:10.1007/s004640020017
Roth J, Sagie B, Szold A, Elran H (2007) Laparoscopic versus non-laparoscopic-assisted ventriculoperitoneal shunt placement in adults. A retrospective analysis. Surg Neurol 68(2):177–184. doi:10.1016/j.surneu.2006.10.069
Schievink WI, Wharen RE, Jr RR, Pettit PDM, Seiler JC, Shine TSJ (1993) Laparoscopic placement of ventriculoperitoneal shunts: preliminary report. Mayo Clin Proc 68:1064–1066
Schubert F, Fijen BP, Krauss JK (2005) Laparoscopically assisted peritoneal shunt insertion in hydrocephalus: a prospective controlled study. Surg Endosc 19(12):1588–1591. doi:10.1007/s00464-005-0164-z
Schukfeh N, Tschan CA, Kuebler JF, Hermann EJ, Nustede R, Krauss JK, Ure B, Gluer S (2009) Laparoscopically assisted ventriculoperitoneal shunt placement in infants with previous multiple abdominal operations. Eur J Pediatr Surg 19:168–170
Sekula RF, Marchan EM, Oh MY, kim DK, Frederickson AM, Pelz G, Uchal M (2009) Laparoscopically assisted peritoneal shunt insertion for hydrocephalus. Br J Neurosurg 23(4):439–442. doi:10.1080/02688690902755605
Tepetes K, Tzovaras G, Paterakis K, Spyridakis M, Xautouras N, Hatzitheofilou C (2006) Inoe trocar laparoscopic placement of peritoneal shunt for hydrocephalus: a simplified technique. Clin Neurol Neurosurg 108(6):580–582
Turner RD, Rosenblatt SM, Chand B, Luciano MG (2007) Laparoscopic peritoneal catheter placement: results of a new method in 111 patients. Neurosurgery 61:167–172, discussion 172–174
Yu S, Bensard DD, Partrick DA et al (2006) Laparoscopic guidance or revision of ventriculoperitoneal shunts in children. JSLS 10:122–125
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Hartmut Collmann, Würzburg, Germany
In this paper dealing with shunt surgery in adults, the authors focus on the risks associated with the insertion of the peritoneal catheter, namely malpositioning and bowel perforation. In a mainly retrospective study on two cohorts of 30 patients, respectively, the authors compare the conventional technique of distal catheter insertion via a small laparatomy with a laparascopically guided trocar technique. With the conventional technique, they report one case of bowel injury and five additional cases of malpositioned catheters, while these complications did not occur in the laparascopically treated group. The authors conclude that laparascopic control significantly improves the safety of distal catheter placement. For now, they do not advocate the laparascopic technique as a routine, mainly for logistic reasons, i.e., the need of a separate surgical team.
The authors are certainly right, when they consider distal catheter placement a less than trivial procedure in some cases, particularly in elderly patients with slack, obese abdominal walls or with a history of complicated abdominal surgery. Nevertheless, their complication rate is unusually high and contrasts with several reports based on larger cohorts, some of which are cited in the text, while others are not (2, 3, 4). In fact, malposition of the distal catheter and bowel perforation are generally not considered a major problem of shunt surgery in elderly people (1).
Looking at the results in more detail, one is wondering why:
–In three out of 30 cases, the surgeons were not able to verify proper access to the peritoneal cavity during conventional laparatomy,
–Mere coiling of a distal catheter should result in shunt malfunction, and
–Peritoneal adhesions per se should lead to shunt malfunction after weeks or months, unless they cause a pseudocyst or localized ascites.
It is beyond question that the laparascopically guided trocar technique causes less postoperative pain. But, the same goal can be achieved by using the blind trocar technique as customary in many centers.
Finally, although in this study the laparascopic technique did not increase the rate of shunt infection, this result still needs validation since minimal handling of the shunt hardware is a classical imperative not yet disproved. Nevertheless, laparascopy should be considered a useful surgical aid in selected patients, e.g., with a history of major or complicated abdominal surgery. The authors provide valuable advice for practicing this technique in shunt surgery.
References
1. Bergsneider M, Black PM, Klinge P et al. (2005) Surgical management of idiopathic normal-pressure hydrocephalus. Neurosurgery 57 (Suppl 3):S29-S39
2. Boon AJ, Tans JD, Delwel EJ et al (1998) Dutch normal-pressure hydrocephalus study: randomized comparison of low- and medium-pressure shunts. J Neurosurg 88:490-495
3. Vanneste J, Augustijn P, Dirven C, Tan WF, Goedhart ZD (1992) Shunting normal pressure-hydrocephalus: do the benefits outweigh the risks? Neurology 42:54-59
4. Zemack G, Romner B (2002) Adjustable valves in normal-pressure hydrocephalus: a retrospective study of 218 patients. Neurosurgery 51:1392-1402
Sherif Elwatidy, Riyadh, Saudi Arabia
The authors presented their experience with laparoscopy-assisted VP shunt, which has the advantage of being a minimally invasive technique for placement of the peritoneal catheter and ensures proper placement of the catheter into the peritoneal cavity. There has been always difficulty in placing the distal catheter in patients with abdominal adhesions due to previous infections, laparoscopy-assisted technique solves this problem and facilitates the operation. However, the procedure requires experience with laparoscopic surgery or the presence of a general surgeon with experience in laparoscopic surgery, which will necessitate special arrangements particularly in emergency situation. Therefore, laparoscopy-assisted VP shunt would be highly recommended for complicated and redo cases.
Rights and permissions
About this article
Cite this article
Raysi Dehcordi, S., De Tommasi, C., Ricci, A. et al. Laparoscopy-assisted ventriculoperitoneal shunt surgery: personal experience and review of the literature. Neurosurg Rev 34, 363–371 (2011). https://doi.org/10.1007/s10143-011-0309-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-011-0309-6