Abstract
Cyclophilins are a set of ubiquitous proteins present in all subcellular compartments, involved in a wide variety of cellular processes. Comparative bioinformatics analysis of the rice and Arabidopsis genomes led us to identify novel putative cyclophilin gene family members in both the genomes not reported previously. We grouped cyclophilin members with similar molecular weight and subtypes together in the phylogenetic tree which indicated their co-evolution in rice and Arabidopsis. We also characterized a rice cyclophilin gene, OsCyp2-P (Os02g0121300), isolated from a salinity-tolerant landrace, Pokkali. Publicly available massively parallel signature sequencing (MPSS) and microarray data, besides our quantitative real time PCR (qRT-PCR) data suggest that transcript abundance of OsCyp2-P is regulated under different stress conditions in a developmental and organ specific manner. Ectopic expression of OsCyp2-P imparted multiple abiotic stress tolerance to transgenic tobacco plants as evidenced by higher root length, shoot length, chlorophyll content, and K+/Na+ ratio under stress conditions. Transgenic plants also showed reduced lipid peroxidase content, electrolyte leakage, and superoxide content under stress conditions suggesting better ion homeostasis than WT plants. Localization studies confirmed that OsCyp2-P is localized in both cytosol and nucleus, indicating its possible interaction with several other proteins. The overall results suggest the explicit role of OsCyp2-P in bestowing multiple abiotic stress tolerance at the whole plant level. OsCyp2-P operates via reactive oxygen species (ROS) scavenging and ion homeostasis and thus is a promising candidate gene for enhancing multiple abiotic stress tolerance in crop plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cyclophilins and FK506 binding proteins (FKBPs) are two subfamilies of immunosuppressant receptors or immunophilins (IMMs) superfamily (Kim et al. 2012; Kumari et al. 2013). Cyclophilins and FKBPs bind to immunosuppressive drugs cyclosporin A (CsA) (Handschumacher et al. 1984) and FK-506/rapamycin, respectively. Despite their lack of structural similarity, these two families share a common peptidyl-prolyl-cis-trans isomerase (PPIase) domain, catalyzing the rotation of X-Pro peptide bonds from a cis to trans conformation, a rate-limiting step in protein folding (Wang et al. 2010b). They are highly conserved in prokaryotes as well as eukaryotes (Wang et al. 2005). Cyclophilins are present in all subcellular compartments, involved in a wide variety of processes including protein trafficking and maturation (Shieh et al. 1989; Ferreira et al. 1996; Galat 1999), receptor complex stabilization (Leverson and Ness 1998), apoptosis (Lin and Lechleiter 2002), receptor signaling (Brazin et al. 2002; Yurchenko et al. 2002), RNA processing (Krzywicka et al. 2001), immune response (Wiederrecht et al. 1992), spliceosome assembly (Horowitz et al. 2002), miRNA activity (Smith et al. 2009), and RISC assembly (Iki et al. 2012). They are broadly classified into two groups: single-domain (SD) cyclophilins having a single PPIase or cyclophilin-like domain (CLD) and multidomain (MD) cyclophilins having a PPIase or CLD in conjunction with domains of other functions such as tetratricopeptide repeat (TPR), WD40, coiled-coil domain (CCD), and internal repeats domain (RPT), RRM, Zinc Finger etc. (Kumari et al. 2013).
The availability of high-quality finished genome sequences of rice and Arabidopsis and their expression data in the form of massively parallel signature sequencing (MPSS) and microarray makes it possible to analyze and study specific gene families. Bioinformatics-based tools have enabled the discovery of new members of several gene families in Arabidopsis and rice such as homeobox gene family (Jain et al. 2008), protein phosphatase 2C family (Xue et al. 2008), nucleocytoplasmic transport receptors family (Huang et al. 2010), etc. Our group has also recently carried out detailed genome-wide analysis and expression profiling of few important gene families, i.e., two-component system (Pareek et al. 2006), CBS domain containing proteins (Kushwaha et al. 2009), and glyoxalase gene family (Mustafiz et al. 2011). Since cyclophilins have multigene members in plants, we have carried out genome-wide analysis to comment on their number and domain organization in rice in comparison to Arabidopsis. In addition, comprehensive analysis of cyclophilin genes, including putative domain architecture, genomic distribution on respective chromosomes, subcellular localization, phylogenetic relationship between family members, and MPSS gene expression in Arabidopsis and rice, and tiling array gene expression analysis of cyclophilins under various environmental stresses in Arabidopsis has also been conducted.
Using subtractive hybridization screening, we previously cloned and identified a salinity-inducible SD cyclophilin member OsCyp2 from a salinity-tolerant landrace of rice, Pokkali. Although enhanced total PPIase activity has been correlated with stress-tolerant genotypes (Sharma and Singh 2003), effect of individual PPIase members on stress tolerance has not been addressed. With a view to investigate the role of OsCyp2 in enhancing multi-stress tolerance ability in plants, overexpression studies were extended to model plant tobacco. Transgenic approach was used wherein OsCyp2-P gene was ectopically expressed in tobacco plant using Agrobacterium-mediated transformation which resulted in a profound increase in tolerance against a variety of major abiotic stresses. Based on this study, it is proposed that this gene may serve as a “candidate” for enhancing multiple abiotic stress tolerance in crop plants. It is important to mention here that this is the first report where functional characterization of a cyclophilin ortholog from a salt-tolerant landrace, Pokkali, has been attempted, and good correlative evidence between the messenger RNA (mRNA) expression, protein expression, and tolerance towards multiple abiotic stresses in plants has been presented.
Materials and methods
Isolation of cDNA clone
The complementary DNA (cDNA) clone of OsCyp2 (EF576508) was obtained from Pokkali subtractive cDNA library of differentially expressed genes under salinity stress as described earlier (Kumari et al. 2009a).
Identification of CYPs gene families
Putative cyclophilin gene members in Arabidopsis thaliana and Oryza sativa were identified using protein profiles of cyclophilin (PF00016) from Pfam database (http://pfam.sanger.ac.uk/) using HMMER 3.0 software (http://hmmer.janelia.org/) against genome browser databases TAIR 9 for A. thaliana (http://www.Arabidopsis.org/) and TIGR Rice 6.1 (http://rice.plantbiology.msu.edu/), respectively.
Subcellular localization of cyclophilin genes
The subcellular localization of cyclophilins was predicted using programs such as PSORT (http://wolfpsort.org/), Predotar v.1.03 (https://urgi.versailles.inra.fr/predotar/predotar.html), and TargetP 1.1 server (http://www.cbs.dtu.dk/). Signal peptide cleavage sites and chloroplast transit peptides were predicted using SignalP 4.1 and ChloroP 1.1 servers (http://www.cbs.dtu.dk/), respectively, in a protein sequence.
Chromosomal location and gene structure of IMMs
Chromosomal locations of cyclophilin genes were determined using the TAIR Arabidopsis genome browser (http://www.Arabidopsis.org/) and O. sativa genome browser (http://rice.plantbiology.msu.edu/) along with the ensemble genome browser (http://plants.ensembl.org/index.html), and mapped on their respective chromosomes. The A. thaliana IMMs genomic distribution were crosschecked using chromosomal map tool (http://www.Arabidopsis.org/jsp/ChromosomeMap/tool.jsp). Gene duplication and their presence on duplicated chromosomal segments were also investigated in Arabidopsis and Oryza. The scaled position of cyclophilin genes on respective chromosomes and segmental duplication have been drawn using gplots package of open source R software. For nomenclature of new members of rice and Arabidopsis cyclophilin gene family, we have employed the previously established system for Arabidopsis. The cyclophilin genes were named “CYP” with a prefix “Os” for rice and “At” for Arabidopsis and a suffix number to indicate the predicted molecular weight. Members with same molecular weight were designated by Arabic numeral followed by a small case alphabet to indicate alternatively spliced form.
Domain annotations of CYPs
Cyclophilin members of A. thaliana and O. sativa from database search were analyzed for the presence of cyclophilin-like domain (CLD) using Pfam (http://www.kks/) and SMART (http://www.jjkjs/) databases. Only those sequences having CLD were used for further analysis. Based on domain architecture of these genes, they have been classified into separate groups, and a representative picture of each of these groups has been drawn using gplots package of open source R statistical software.
Phylogenetic analysis
A phylogenetic tree was constructed with the aligned A. thaliana and O. sativa cyclophilin sequences using Clustal X (version 2.1; http://www.clustal.org/) and using neighbor-joining (NJ) method with default options. For statistical reliability, we conducted bootstrap analysis with 1000 replicates. The constructed tree file was visualized and finally drawn by Iterative Tree of Life (iTOL) (Letunic and Bork 2011). Similarly, the classification of cyclophilin members of rice and Arabidopsis into various subtypes was performed using KOBAS 2.0 database. Cyclophilin genes are classified into single-domain (SD) and multidomain (MD) members depending upon the number of domains present. SD members possess only CLD catalytic domain while MD members contain various additional functional domains other than the catalytic CLD, such as tetratricopeptide repeat (TPR), WD40 domain, RNA binding domain (RNP), zinc finger domain, RNA recognition motif (RRM), ATPase (AAA) family, transposase family (tnp2), etc. The membrane-bound cyclophilin members (having a characteristic transmembrane domain) of rice and Arabidopsis and those localized in nucleus (possessing nuclear localized signals (NLS)) (Tables 1 and 2) were identified using WOLF PSORT (http://wolfpsort.org/) and Nucpred (http://www.sbc.su.se/).
Expression analysis using MPSS database
Expression evidence from massively parallel signature sequencing (MPSS) tags was determined from the Arabidopsis MPSS project (http://mpss.udel.edu/at/) and Oryza MPSS project (http://mpss.udel.edu/rice) mapped to Arabidopsis and Oryza gene models. The signature was considered to be significant if it uniquely identifies an individual gene and shows perfect match (100 % identity over 100 % length of the tag). The normalized abundance (tags per million, tpm) of these signatures for a given gene in a given library represents a quantitative estimate of expression of that gene. The description of MPSS libraries in A. thaliana and O. sativa is provided in Supplementary Tables 1 and 2. Expression values obtained from MPSS database for respective cyclophilin genes were used for making the heatmap using gplots package of open source R software.
Expression analysis using microarrays
The microarray data showing expression of cyclophilin gene family members under various abiotic stress conditions such as cold, osmotic, salt, drought, genotoxic, oxidative, UV-B, wounding, and heat stress were retrieved from The Arabidopsis Information Resource (TAIR) and Rice Oligonucleotide Array Database (http://www.ricearray.org/expression/expression.php). The datasets obtained were corresponding to shoot tissues at different time sets of stress namely 0.5, 1, 3, 6, 12, and 24 h. For rice, the data was obtained for drought, salt, and cold stress. Fold increase in transcript abundance under stress conditions were calculated with respect to their controls. The hierarchical clustering analysis and the heatmaps were made using gplots package of R software as described earlier (Kushwaha et al. 2011).
Plant material and stress treatments for transcript analysis of OsCyp2-P
Rice seeds were rinsed with distilled water and germinated in a hydroponic system for 14 days (28 ± 1 °C; 12 h day/night) in half strength Hoagland medium. Seedlings were exposed to salinity stress by transferring them to Hoagland medium containing 200 mM NaCl. For heat stress, seedlings were transferred to a growth chamber maintained at 42 °C. For drought stress, medium was supplemented with 5 % PEG 6000. The untreated samples were taken as control. Control and treated shoots were harvested after 30 min and 24 h of stress treatment and kept in liquid nitrogen until further use. In the case of tobacco, pot-grown wild type (WT) and transgenic mature plants maintained under greenhouse conditions (28 ± 1 °C; 12 h day/night) were taken for leaf disc assay. RNA was isolated from control (half-strength Hoagland medium) and treated (half-strength Hoagland medium +150 mM NaCl) leaf discs after 5 days of stress.
Total RNA isolation and cDNA synthesis
Total RNA was isolated using TRI reagent (Sigma, USA) according to the manufacturer’s instructions. The concentration of RNA was determined using spectrophotometer and its integrity was checked on agarose gel. cDNA synthesis was carried out according to the manufacturer’s protocol (Thermo Scientific, UK) using 5 μg of total RNA.
Real-time PCR
Primers for real-time PCR analysis of the OsCyp2-P gene members were designed using Primer Express 3.0 software (PE Applied Biosystems, USA). The sequences for these primers OsCYPRT FP: TTCGCCAGTATCAGTCGTCTTG, OsCYPRT RP: CGACACGAACTCATAAAAAGACT were designed from within the gene and 3′UTR region of the gene which gave a 104 bp amplicon. Real-time PCR was performed employing ABI Prism 7500 Sequence Detection System and software (PE Applied Biosystems, USA) using standard RT-PCR protocol. The specificity of amplification was tested by dissociation curve analysis. The expression of each gene in different RNA samples was normalized with the expression of internal control gene, elongation factor 1α for rice samples, and actin-specific primers for tobacco. The mRNA levels for each candidate gene in different tissue samples were calculated relative to its expression in control seedlings using ΔΔCT method of SDS 1.4 software (Applied Biosystems). Three technical as well as biological replicates were analyzed for each sample (n = 3).
Protein extraction
One gram of sample tissue was taken and homogenized in liquid nitrogen to a fine powder form. The powder obtained was suspended in extraction buffer (Zivy et al. 1983). PVPP (50 mg/g fresh weight of the tissue) was added while homogenizing the samples to get rid of the phenolics. The crude homogenate was centrifuged twice at 12,000 rpm for 15 min at 4 °C and the total protein present in the supernatant were precipitated overnight at −20 °C using 8 volumes of prechilled acetone containing 10 mM 2-mercaptoethanol. The precipitated proteins were then solubilized in Laemmli buffer (Laemmli 1970), followed by heating at 100 °C for 5 min. The solubilized proteins were recovered by centrifugation at 15,000 rpm for 15 min and an aliquot of supernatant was used for protein quantification by Bradford assay (Bradford 1976).
Construction of binary vector and tobacco transformation
Full-length OsCyp2-P (519 bp) was cloned into plant expression vector pCAMBIA1304 under the control of 35SCaMV promoter by using the primer set (FP: 5′-GAAGATCTCATGTCGAACACGAGGGTGTTC-3′, RP: 5′-GGACTAGTGCTAGGAGAGCTGGCCGCAG-3′), at the BglII and SpeI site. Transformation of tobacco was done using Agrobacterium-mediated leaf disc method (Kumar et al. 2012). Leaf discs of 1-cm diameter were excised from 1-month-old tobacco leaves and incubated with Agrobacterium containing pCAMBIAOsCyp2-P (OD600 = 0.8 was further diluted with MS media upto OD = 0.3 to avoid overgrowth of Agrobacterium) for 15 min with intermittent shaking for co-infection. The co-infected leaf discs were co-cultivated on MS agar media in dark at 26 °C for 48 h. After 48 h, the co-cultivated leaf discs were further transferred to MS medium, containing 10 mM BAP, 1 mM NAA, 250 mg/l augmentin or cefotaxime (for combating Agrobacterium overgrowth sticking on surface of leaf tissue), and 25 mg/l hygromycin (for plant selection), for regeneration.
PCR for screening putative transgenic plants
Discs of about 0.5 to 0.7 cm from leaf tissue were punched into a 2-ml microcentrifuge tube using a standard one-hole paper punch. Then, 100 μl of extraction solution was added to the tube and vortexed briefly to mix properly, according to the manufacturer’s protocol (Red Extract-N-Amp, Sigma, USA). The mix was incubated at 95 °C for 10 min. Furthermore, 100 μl of dilution solution was added and vortexed. The leaf extract was then used as template for PCR reaction. The PCR reaction was prepared using 10 μl of the supplied Red Extract-N-Amp PCR ReadyMix, 4 μl of the leaf extract, and 0.4 μM of each primer in a 20 μl of total reaction volume. Transgenic plants were screened using gene-specific forward and vector-specific reverse primer set (FP: 5′-CATGTCGAACACGAGGGTGTTC-3′, RP: 5′-CAAGAATTGGGACAACTCCAG-3′). The plasmid pCAMBIA1304OsCyp2-P was taken as the positive control template for the PCR reaction transformation.
Southern blotting
For Southern hybridization, 10 μg of digested genomic DNA (BglII and SpeI) was electrophoresed on a 0.8 % agarose gel and transferred to nylon membrane using the capillary transfer method as described in Sambrook and Russel (2001). The membrane was prehybridized for 3 h at 65 °C in buffer containing 5X SSC, 5X Denhardt reagent (0.5 % Ficoll, 0.5 % PVP, 0.5 % BSA), 0.1 % SDS, 100 μg/ml denatured salmon sperm DNA, and 10 % dextran sulphate. Probes were prepared by labeling the PCR-amplified samples of cDNA clones of the desired gene with α32P-dATP (BRIT, Hyderabad, India) using HexaLabel DNA labeling kit (Thermo Scientific, UK) and purified using PCR purification kit (QIAGEN, USA). Thereafter, radiolabeled probe (pre-denatured by keeping on boiling water for 5 min) was added to the prehybridization solution. After incubation at 65 °C for 16–18 h in the hybridization solution, membrane was washed twice with 2X SSC for 5 min, 2X SSC +0.1 % SDS for 10 min, and 0.1 % SDS for 5 min. Washed membrane was exposed to phosphorimager plate (Amersham Pharmacia Biotech, England) for 15 h and scanned.
Raising of polyclonal antisera against OsCYP2-P
For expressing the recombinant protein OsCYP2, the coding region of 519 bp was cloned in the bacterial expression vector pET28a (Novagen, USA) at the multiple cloning site using the enzymes EcoRI and XhoI. The confirmed construct of pET28aOsCYP2 was transformed into BL21 (DE3) and induction of the protein OsCYP2 was carried out at 37 °C using 0.5 mM IPTG. Maximum induction of the OsCYP2 protein of the expected size of 18.6 kDa was observed at 37 °C at 4 h which was also confirmed using anti-HIS antibody Western blotting. The induced band was excised from the 12.5 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and confirmed again by peptide fingerprinting using MALDI-TOF-TOF. The spectrum gave 15 peaks with 36.6 % coverage, revealing high homology with O. sativa cyclophilin 2.
Polyclonal antisera against gel-purified OsCyp2-P was raised in rabbits as per the standard procedure (Harlow and Lane 1988) Three New Zealand White rabbits (3 months old) were selected for raising antibodies at the Animal House, JNU. Pre-immune serum was obtained before immunization. Primary immunization was carried out with 100 μg protein antigen dissolved in normal saline (0.9 % NaCl). Before immunization, the protein antigen was emulsified with the complete Freund’s adjuvant (CFA, Sigma, USA). Immunization with immunogens was carried out subcutaneously as well as intramuscularly. When immunized subcutaneously, the antigen was injected at multiple sites. For intramuscular immunization, single injection was given. Fifteen days after priming the animal, first booster shot was given. Purified antigen (50 μg protein) was emulsified with incomplete Freund’s adjuvant (IFA) before injection. Subsequent booster shots were given weekly with 50 μg protein. After five booster shots, serum was collected separately from each animal and tested individually for cross reaction by performing Western analysis. High titer anti-OsCyp2 antisera were collected after five booster shots.
Western blotting using polyclonal antisera against OsCyp2-P
Thirty micrograms of protein separated on SDS-PAGE were transferred to Hybond-C+, membrane (GE Healthcare, India) using Mini Transblot Electrophoretic cell (Bio-Rad, USA). Electroblotting buffer consisted of 150 mM glycine, 20 mM Tris, and 20 % methanol (pH 8.0). Prestained molecular weight markers (Sigma, USA) were employed to check the efficiency of protein transfer. The blot was rinsed briefly in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4.7H2O, 1.4 mM KH2PO4 (pH 7.3)) containing 0.1 % Tween-20 and then incubated in blocking solution (5 % non-fat dried milk dissolved in PBS) for 45 min. Blot was washed thrice with PBS containing 0.1 % Tween-20 at 5-min interval and thereafter, incubated with primary antibodies at 1:1000 dilution with blocking buffer for 1 h. Blot was again washed thrice with PBS containing 0.1 % Tween-20 at 10-min interval followed by incubation in alkaline-phosphatase conjugated secondary antibodies for 1 h. The blot was washed thrice with PBS containing 0.3 % Tween-20 for 5 min. Thereafter, it was washed thrice with PBS containing 0.1 % Tween-20 for 5 min. The protein-antibody complex was developed in 5-bromo-4-chloro-3-indolyl phosphate (BCIP)/nitroblue tetrazolium (NBT) containing solution (0.1 M Tris–HCl (pH 9.5), 0.1 M NaCl, 5 mM MgCl2 containing 150 μg/ml of NBT and 75 μg/ml of BCIP). The reaction was terminated by rinsing the blot in water.
Subcellular localization of OsCYP2-P in rice
For the extraction of subcellular protein fractions, 10 g of sample tissue was used according to the manufacturer’s instruction (Celllytic PN isolation kit, Sigma USA). Aliquots of 20 μg of protein of total, cytosolic, and nuclear protein was separated on 12.5 % SDS-PAGE and electroblotted onto Hybond-C membrane (Amersham, UK). Purity of the extracted cytosolic and nuclei proteins was checked by Western blots using primary antibodies raised against cytosolic marker enzyme—aldolase (CST, USA) and nuclear marker protein—histone 3 (CST, USA). Western analysis of OsCYP2-P was carried out using anti-CYP antibodies raised in our laboratory. All the three antibodies were treated with anti-rabbit secondary antibody conjugated with horseradish peroxidase (Sigma, USA) and developed via standard enhanced chemiluminescence method.
Leaf disc senescence assay
Leaf discs of 1-cm diameter excised from healthy and fully expanded leaves of tobacco plants maintained under greenhouse conditions (28 ± 1 °C; 12 h day/night) were kept in half-strength Hoagland media containing 150 mM NaCl. Leaf discs kept in half-strength Hoagland were taken as control.
Chlorophyll estimation
Estimation of chlorophyll from leaf discs or seedlings were done following Arnon’s method (Arnon 1949): 100 mg of tissue was homogenized thoroughly in 1 ml of 80 % acetone and centrifuged at 3000 rpm for 2–3 min. The supernatant was retained and absorbance was recorded using spectrophotometer at 663 and 645 nm. The experiment was repeated thrice with transgenic line L5 (n = 3).
Seed germination and measurement of growth under stress conditions
T2 generation seeds of OsCyp2-P ectopically expressing transgenic tobacco lines along with WT were subjected to various abiotic stresses. Half MS agar plates containing 150 mM NaCl (for high salt stress), 5 mM H2O2 (sublethal dosage of oxidative stress), 200 mM mannitol (high osmotic stress), or 5 % PEG (drought stress) were prepared. Seeds of transgenic lines and WT were sterilized by washing with 70 % ethanol for 1 min and subsequently washed with double-distilled autoclaved water to remove any trace of residual ethanol. The sterilized seeds were sown on the freshly prepared half MS agar plates (containing various stress inducing agents). After 21 days, images were taken and various physiological parameters, e.g., % seed germination, root length, shoot length, and chlorophyll content, were measured. Data from one representative line L5 is shown here (n = 3).
K+/Na+ estimation
For determination of endogenous K+ and Na+ contents, 100 mg of tissue (control or salinity stressed) was taken and digested in 0.1 % HNO3 (Singla-Pareek et al. 2003). Ions were extracted in distilled H2O by boiling for 30 min twice. The filtered extract thus obtained was used to measure specific ions with flame photometer. Experiment was repeated thrice and standard error was calculated (n = 3).
The assessment of membrane damage through lipid hydroperoxide assay
Lipid hydroperoxide accumulation was detected as per manufacturer’s protocol (LPO assay kit, Cayman Chemical, USA). Tissue homogenate from unstressed and stressed seedlings were added into 500 μl of Extract R saturated methanol and vortexed followed by addition of 1 ml of chloroform. This mixture was centrifuged at 15,000 rpm for 5 min at 0 °C followed by collection of bottom layer. Then, 500 μl of bottom chloroform layer was mixed with 450 μl of chloroform methanol solvent mixture and thereafter 50 μl of freshly prepared chromagen was added. Tubes were kept at room temperature for 5 min and absorbance was measured at 500 nm. Final amount of lipid hydroperoxide in the sample was calculated as per the manufacturer’s protocol. Experiment was repeated thrice and standard error was calculated (n = 3).
Electrolyte leakage
For measuring electrolyte leakage, 100-mg tissue was taken from salt-stressed seedlings (along with the unstressed controls). They were washed immediately with distilled water and electrolyte leakage was measured as described earlier (Kumar et al. 2009). The tissue was dipped in 20 ml deionized water and incubated at 32 °C. The electrical conductivity (E1) of the solution was measured after 2 h using a conductivity meter (ELEINS Inc., India). The total conductivity (E2) was determined further by autoclaving the same immersion solution for 15 min at 121 °C, and the conductivity was measured after cooling it down to room temperature. Relative electrical conductivity was calculated as (EL = E1/E2 × 100). Experiment was repeated thrice and standard error was calculated (n = 3).
In planta histochemical estimation of H2O2
Accumulation of H2O2 was examined based on histochemical staining by 3, 3′-diaminobenzidine (DAB) as described earlier (Kumar et al. 2009). Leaves from 21-day-old seedlings WT and transgenic plants subjected to various stresses (as described above) were vacuum infiltrated into 1 mg/ml fresh DAB solution (pH 3.8) prepared in 10 mM phosphate buffer (pH 7.8) and placed in a plastic box under high humidity and light until brown spots were observed (5 to 6 h). The stained leaves were then fixed with a solution of 3:1:1 ethanol: lactic acid: glycerol and photographed. Experiment was repeated thrice and standard error was calculated (n = 3).
Peptidyl-prolyl-cis-trans activity assay
The fine chemicals and reagents used in this study were purchased from Sigma Chemical Co, St. Louis, USA. Peptidyl-prolyl-cis-trans isomerase activity was assayed in a coupled reaction with chymotrypsin, as described earlier (Kumari et al. 2009a). The assays were performed at 15 °C for 360 s. The 1 ml assay mixture contained 80 micro M N-succinyl-ala-ala-prophe- p-nitroanilidine as test peptide, assay buffer (50 mM HEPES (pH 8.0), 150 mM NaCl, 0.05 % Triton X-100) along with varying concentration of the plant protein from either WT or transgenic line. The reaction was initiated by the addition of chymotrypsin (300 mg/ml), and the change in absorbance at 390 nm was monitored using a spectrophotometer (Perkin-Elmer Mol Biotechnol Lambda Bio20) equipped with Peltier temperature control system. For calculating the PPIase activity, the difference between the catalyzed and uncatalyzed first-order rate constants, derived from the kinetics of the absorbance change at 390 nm, was multiplied by the amount of the substrate in each reaction.
Results
Multi-stress responsive CYP gene family has expanded in rice as compared to Arabidopsis
In the present study, we have employed genome-wide analysis of Arabidopsis and rice in order to identify their respective cyclophilin gene family members. Detailed features of these cyclophilin gene family members in Arabidopsis and rice have been shown in Tables 1 and 2, respectively. We identified 31 distinct cyclophilin genes in Arabidopsis including two additional genes to that of 29 identified by Romano et al. (2004) encoding 41 putative proteins. In silico analysis of the O. sativa genome retrieved 29 CYPs genes encoding 46 putative proteins instead of 27 (Ahn et al. 2010) and 28 (Trivedi et al. 2012) gene members reported previously. Rice cyclophilin gene members show comparatively higher incidence of alternative splicing as compared to Arabidopsis. Chromosomal localization analysis showed that cyclophilin gene family members of Arabidopsis and rice were dispersed throughout their respective genomes. In Arabidopsis, the family of 31 cyclophilin genes was distributed randomly on all the 5 chromosomes while in Oryza, a family of 29 cyclophilin genes was distributed on 10 of the 12 chromosomes (Supplementary Fig. Ia, b).
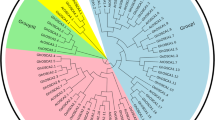
In order to classify and to investigate their evolutionary relationships, derived protein sequences of Arabidopsis and rice cyclophilins were subjected to phylogenetic analyses. The detailed phylogenetic tree depicting evolutionary relationship among cyclophilin members of rice and Arabidopsis is shown in Supplementary Fig. II, inner circle. The classification of cyclophilin members of rice and Arabidopsis into various subtypes was performed using KOBAS 2.0 database as shown by middle circle (middle circle). Cyclophilin members falling in same molecular weight category and subtype were grouped together in the phylogenetic tree indicating their co-evolution in rice and Arabidopsis. The outermost circle depicts the domain architecture of cyclophilin members of rice and Arabidopsis. The membrane-bound cyclophilin members (having a characteristic transmembrane domain) of rice and Arabidopsis and those localized in nucleus (possessing nuclear localized signals (NLS)) (Tables 1 and 2) identified using WOLF PSORT (http://wolfpsort.org/) and Nucpred (http://www.sbc.su.se/) have also been shown in the outer circle. The TPR containing cyclophilin members include AtCYP40, OsCYP40b, and OsCYP40a (Supplementary Fig. II, outer circle).
We have used the publicly available 17 and 20 bp MPSS libraries of Arabidopsis and rice for carrying out expression analysis of cyclophilin genes identified in this study. Expression analyses of Arabidopsis cyclophilin genes revealed that AtCyp19-1 transcript is most abundant in various tissues and stress treatments (Supplementary Fig. IIIa), thereby indicating its functional significance at all the developmental stages and stress treatments. Expression analysis of rice cyclophilin genes using MPSS database revealed that OsCyp19-3 and OsCyp40-2 were the only cyclophilin genes that were highly expressed in mature pollen and developing seeds, respectively (Supplementary Fig. IIIb). One of the cytosolic cyclophilin gene member from rice, OsCyp19-1, showed highly abundant transcript accumulation at various developmental stages and tissues under control and both biotic and abiotic stresses, as evident in the MPSS data.
Microarray data analysis of cyclophilin genes of rice and Arabidopsis depicts differential yet specific stress-inducible nature of these genes. Heat maps based on expression pattern of these genes under various stresses show distinct classes of these proteins, as shown by the hierarchical clustering (Supplementary Fig. IVa, b). AtCyp19-2, ortholog of rice gene OsCyp2 (OsCyp19-1), was found to be highly induced by both high- and low-temperature stress, besides showing moderate to high expression under salt and osmotic stress. Microarray analysis of rice cyclophilin genes in response to cold, salinity, and drought revealed only few members to be perturbed by low-temperature stress whereas a large number of genes were altered under salinity and drought stress (Supplementary Fig. IVb). This analysis clearly indicated towards uniquely orchestrated action of various members of the cyclophilin family under various abiotic stresses and developmental stages. Microarray data analysis of cyclophilin genes in Arabidopsis and rice supports the MPSS data for their stress- and tissue-specific expression patterns (Supplementary data I).
Expression of OsCyp2-P gene is induced by multiple stresses in rice, both at transcript and protein level
As per MPSS and microarray data, cytosolic single-domain cyclophilin, OsCyp19-1, shows stress-dependent and developmental stage-specific transcript induction. In our previous study, we had obtained a single-domain cyclophilin in salinity-specific subtractive library (referred as OsCyp2 in Kumari et al. (2009a, b) and now being referred as OsCyp2-P). On closer inspection, based on sequence similarity, it was found that OsCyp19-1 and OsCyp2-P show 100 % sequence similarity. To revalidate if OsCyp2-P is regulated by multiple abiotic stresses, qRT-PCR was carried out with cDNA prepared from shoots of IR-64 (salt sensitive) and pokkali (salt tolerant) rice seedlings (14 days old) subjected to heat, osmotic, and salinity stress. Analysis of transcript abundance of OsCyp2 in shoot tissue of these two contrasting cultivars of rice at seedling stage revealed its differential stress-inducible expression (Fig. 1a). This analysis indicated that OsCyp2 is upregulated rapidly (after 30 min) under salinity stress in both IR-64 and Pokkali. However, the induction in Pokkali is much higher (7–8-fold) than IR-64. Similar observations were made when the transcript abundance for OsCyp2-P was analyzed in seedlings in response to high temperature or osmotic stress.
qRT-PCR and Western blot analysis of OsCyp2-P gene in contrasting cultivars of rice. a Real-time PCR analysis of OsCyp2-P expression in shoots of 14-day-old rice seedlings under various abiotic stresses such as heat (42 °C), salinity (200 mM NaCl), and drought (5 % PEG) for various durations, i.e., 30 min and 24 h. The expression of each gene in different RNA samples was normalized with the expression of internal control gene elongation factor 1α. Data are means ± SE. Each data set represents an average of minimum three separate experiments. Asterisk mark shows values significantly different from respective cultivar under control conditions at P < 0.05, by Student’s t test. b Western blot using anti-OsCYP2-P antisera showing OsCyp2-P protein accumulation in response to various stresses in contrasting rice genotypes i.e. IR64 and Pokkali
To corroborate these findings further at protein level, Western analysis was carried out with the crude protein extracts isolated from the above experimental setup. Polyclonal antibodies were raised against full-length OsCyp2-P protein and used for studying the protein accumulation in crude plant protein extract isolated from 4-day-old seedlings. A single specific band corresponding to 18.6 kDa of OsCyp2-P protein was obtained in the blots. Differential accumulation of OsCyp2-P protein was observed for the two genotypes under study. The tolerant cultivar showed higher level under both control and stress conditions (Fig. 1b), thus implying the role of OsCyp2-P in multi-stress response in rice.
OsCyp2-P is localized both in cytosol and nucleus in rice
OsCyp2-P protein lacks any nuclear signal (NLS) and is predicted to be cytosolic. To confirm it, we carried out Western analysis of the subcellular protein fractions in rice (Fig. 2). The cytosolic marker enzyme aldolase and nuclear marker protein histone H3 showed predominance in cytosolic and nuclear fractions, respectively, establishing the purity of these fractions. However, both the cytosolic and nuclear fractions showed the presence of OsCYP2-P protein suggesting the predominance of this protein in both cytosol and nucleus. The presence of this protein in the cytosolic fraction is much higher as compared to the nuclear, suggesting its major involvement in the cytosolic events.
Localization of OsCYP2-P protein by Western blots of subcellular protein fractions in rice leaves. Presence of OsCYP2-P could be seen clearly in the fractions corresponding to the cytosol and nucleus of the cell. Purity of the cytosolic and nuclear fractions was checked by the marker proteins—aldolase and histone H3, respectively
Transgenic tobacco plants ectopically expressing OsCyp2-P showed constitutive higher Cyp transcript and protein accumulation as well as higher PPIase activity
Since OsCyp2 was highly represented in a subtractive library and its overexpression conferred tolerance to Escherichia coli and Saccharomyces cerevisiae (Kumari et al. 2009b), further characterization of this gene OsCyp2-P (isolated from a salinity-tolerant landrace, i.e., Pokkali) and its ectopic expression in tobacco was carried out to assess its effect as a candidate gene conferring multi-stress tolerance to plants. The putative transgenic plants which formed proper roots on hygromycin containing MS media were screened using tissue PCR (see “Materials and methods” section) with a combination of gene-specific and vector-specific primer. Genomic DNA from WT (non-transgenic) tobacco cv. Petit Havana and pCAMBIA1304OsCyp2-P plasmid (Supplementary Fig. Va) were taken as negative and positive controls, respectively. Among ten lines screened, five lines were found to be positive (L1, L2, L4, L5, L6) as they showed the desired band amplified from the genomic DNA of the putative transgenic plants (Supplementary Fig. Vb).
Genomic Southern blot analysis of PCR positive plants (four lines L1, L2, L4, and L5) was carried out to check the stable integration of the transgene in the tobacco genome. The genomic DNA from each of the transgenic lines and WT tobacco plants was digested with BglII and SpeI which released the OsCyp2-P fragment of 519 bp. The digested DNA was fractionated on agarose gel (Supplementary Fig. Vc) and transferred to the nitrocellulose membrane and probed with full-length OsCyp2-P specific α-P32-radiolabeled probe (see “Materials and methods” section). Only a single specific cross hybridizing band, which exactly matched with the size of the positive control, was visible for all four samples and none in the WT (Supplementary Fig. Vd). These results confirmed the transgene (OsCyp2-P) insertion in the tobacco genome. However, based on visual analysis of thickness of band, it appears that lines L1 and L2 may be multicopy transgenic lines.
To confirm the expression of OsCyp2-P at the transcript level, semiquantitative PCR and qRT-PCR analysis were performed in transgenic tobacco ectopically expressing OsCyp2-P lines L1, L2, L4, and L5. Accumulation of OsCyp2-P transcripts in three lines L1, L4, and L5 could be clearly detected on gel (Fig. 3a). Though line L2 was found to be positive for the presence of transgene by PCR and Southern hybridization, it did not show any expression of the transgene. The results of qRT-PCR again confirmed these observations (Fig. 3b). It is possible that the transgene was silenced in the line L2, hence no expression of the transcript could be found. To examine whether increase in transcript expression in transgenic lines was also reflected in the protein accumulation, Western blot analysis was carried out for transgenic lines using anti-OsCyp2-P antibodies. High level of OsCyp2-P protein was detected in lines L1, L4, and L5, whereas wild-type tobacco plants and line L2 did not show accumulation of OsCyp2 protein (Fig. 3c, d). Though cyclophilin proteins are highly conserved in nature, the absence of an immunological homolog of OsCyp2-P in tobacco (non-transgenic) indicates toward the high quality of the antisera raised against OsCyp2-P. Among the four lines tested here, tobacco line L5 showed highest transcript as well as its corresponding protein accumulation as revealed by qRT-PCR and Western blot analysis, respectively. To test if the increased OsCyp2-P protein is also enzymatically active, we performed PPIase assay in WT and L5 transgenic lines using standard protocol as done earlier (Kumari et al. 2009b). The transgenic line L5 was found to have ~sixfold PPIase activity as compared to WT (Supplementary Fig. VI) which confirmed that the orthologous OsCyp2-P protein is also enzymatically active.
Confirmation of OsCyp2-P ectopically expressing transgenic tobacco plants and assessment of their tolerance towards salinity stress. a Ethidium bromide stained agarose gels showing semiquantitative RT-PCR amplicons of actin gene (lower panel) and OsCyp2-P gene (upper panel) in wild-type and transgenic lines. Note that lines L1, L4, and L5 are positive and line L2 is negative for the expression of OsCyp2-P. b Bar diagram showing relative expression of OsCyp2-P in wild type and OsCyp2-P ectopically expressing transgenic lines analyzed through qRT-PCR, again confirming the expression pattern for OsCyp2-P as observed in RT-PCR shown above. c Commassie stained SDS-PAGE showing total protein isolated from wild-type tobacco plants and OsCyp2-P ectopically expressing lines and d corresponding Western blot analysis for the presence of recombinant protein using anti-OsCYP2-P antisera. Note that lines L1, L4, and L5 are positive and line L2 is negative for the expression of OsCyp2-P protein as revealed by the immunoblot. e Leaf disc assay of wild-type tobacco plants and OsCyp2-P lines (L1, L4, and L5) to obtain a comparative estimate of chlorophyll retention capability under salinity stress (150 mM NaCl). Leaf discs floated in Hoagland medium served as the experimental controls. f Bar diagram depicting chlorophyll content retained in leaf discs after 48 h in OsCyp2-P lines along with the WT. The data represent mean ± SE of three biological replicates (n = 3). Asterisk shows values significantly different from respective control under same conditions at P < 0.05, by Student’s t test
All the transgenic plants overexpressing OsCyp2-P matured normally under control conditions. The seed setting of these transgenic plants was similar to that of the untransformed WT plants thereby suggesting that overexpression of the transgene did not hamper the yield potential. Furthermore, the basis of stress tolerance in OsCyp2-P ectopically expressing plants was studied by examining various physiological and biochemical parameters.
OsCyp2-P plants show better capability to retain chlorophyll under salinity stress
To study whether ectopic expression of OsCyp2-P in tobacco plants can alter their sensitivity towards salinity, leaf disc chlorophyll retention assay was performed. Fully expanded healthy leaves of WT tobacco plants and transgenic T2 lines were used for this assay. Leaf discs of 1-cm diameter were cut from each transgenic line and were kept in stress (i.e., half-strength Hoagland media supplemented with 150 mM NaCl) or control (i.e., half-strength Hoagland media). Significant differences in the “green-ness” of the leaf discs were observed among WT and transgenic plants (line no. L1, L4, L5) treated with 150 mM NaCl within 48 h (Fig. 3e). However, line no. L5 showed significant tolerance towards salinity as observed by its chlorophyll retention under stress condition (Fig. 3f). Under salinity stress, L5 had almost same levels of chlorophyll as under control (non-stressed conditions) as revealed by t test.
OsCyp2-P transgenic plants show enhanced tolerance to multiple abiotic stresses
In our study, OsCyp2-P (both mRNA and protein) has been found to be induced in rice seedlings in response to various abiotic stresses such as salinity, high temperature and drought. Previous studies have also documented similar induction of OsCyp2 under various stresses (Ruan et al. 2011). Based on these findings, we hypothesize that OsCyp2-P may be playing crucial role in providing tolerance to plants for multiple abiotic stresses. To validate this hypothesis, we have performed various physiological experiments with the tobacco plants ectopically expressing OsCyp2-P which is presented below.
Comparative analysis of germination and growth of T2 seeds of tobacco plants ectopically expressing OsCyp2-P and wild-type tobacco was carried out under multiple stress conditions. Results for one of the representative line, i.e., L5 has been presented here, since in the leaf disc assay (Fig. 3f), this line exhibited highest chlorophyll retention when exposed to salt stress. Equal number of seeds of line L5 and WT tobacco plants were inoculated on MS media supplemented with various stressors (see “Materials and methods” section). Differential growth response of WT and transgenic line towards various stresses was observed (Fig. 4a–f for salinity and oxidative stress response and Supplementary Fig VIIa–e for drought and osmotic stress response). Under these saline conditions, only 76 % of WT seeds could germinate. In contrast, 96 % seed germination was observed for OsCyp2-P transgenic plants under similar conditions. Similarly, germination percentage was found to be 86 and 98 % in L5 tobacco plants under drought and osmotic stress, respectively, while it was 80 and 94 % in WT plants under same conditions (Fig. 4e and Supplementary Fig VIIc). However, under oxidative stress, almost all of the WT as well as transgenic seeds could germinate.
Germination assay of OsCyp2-P ectopically expressing transgenic tobacco seeds on MS media supplemented with various stressors. For this purpose, medium was supplemented with either a NaCl (150 mM) for salinity stress or b H2O2 (5 mM) for oxidative stress. Bar diagram depicting c shoot length and d root length of wild-type and transgenic seedlings under control and stress conditions as indicated below the bars. e Bar diagram showing germination percentage of wild-type and transgenic seedlings (L5) under various stress conditions. f Bar diagram depicting K+/Na+ ratio in WT and transgenic lines grown under control and saline (NaCl, 150 mM) conditions. The data represent mean ± SE of three biological replicates (n = 3). Asterisk shows values significantly different from respective WT under same conditions at P < 0.05, by Student’s t test
Interestingly, in terms of growth under stress conditions, the root and shoot growth of the L5 transgenic plants showed significant difference in comparison to the respective WT under similar conditions. Imposition of different stress conditions resulted in significant decrease in shoot growth of both WT and OsCyp2-P L5, but the reduction in shoot length of L5 was significantly lower than WT (Fig. 4c and Supplementary Fig VIId). L5 transgenic line also showed decrease in root length under mannitol and salt stress, though as compared to control, it was significantly lower (Fig. 4d and Supplementary Fig VIIe); however, it showed significantly high root growth under drought stress (PEG), whereas the wild-type roots were severely retarded. It thus appears that the presence of OsCyp2-P prevented the decrease in root as well as shoot growth under drought stress. Using mutant rice lines, it has been demonstrated that OsCyp2 plays a critical role in degradation of auxin-responsive proteins and thus plays a crucial role in its lateral root initiation (Kang et al. 2013). Oxidative stress (5 mM H2O2) severely affected the root and shoot growth in both WT and transgenic plants. However, the decrease in shoot growth of L5 was significantly less than the WT under oxidative stress. On the contrary, root growth was adversely affected to the same extent in both wild-type and transgenic lines (Fig. 4d).
OsCyp2-P ectopically expressing plants show increased tolerance towards multiple abiotic stresses in terms of their K+/Na+ ratio, electrolyte leakage, and chlorophyll contents
We measured the Na+ and K+ concentration in the whole seedlings of WT plant and OsCyp2-P transgenic lines exposed to salinity stress (150 mM NaCl). It was observed that the OsCyp2-P ectopically expressing tobacco seedlings were able to maintain K+/Na+ homeostasis better than the WT plants under salinity stress (Fig. 4f). Another report by Ruan et al. (2011) showed that the transgenic rice plants overexpressing OsCyp2 maintain reduced K+/Na+ ratio than WT even under non-stress conditions. However, in our study, OsCyp2-P tobacco seedlings showed significant advantage over WT in terms of maintaining K+/Na+ ratio even under non-stress (control) conditions (Fig. 4f). The reasons for these observed differences needs to be explored further.
Chlorophyll content measured in WT and different OsCyp2-P transgenic seedlings grown under greenhouse conditions revealed a clear advantage of OsCyp2-P ectopic expression under various stress conditions (Fig. 5a). This is in conformity with the observation related to chlorophyll retention leaf disc assay (Fig. 3e). Electrolyte leakage is correlated with cell membrane stability and integrity and is of primary concern in plants grown under abiotic stress conditions (Wang et al. 2010a). Compared to WT, the OsCyp2-P ectopically expressing transgenic lines showed significantly lower electrolyte leakage under all the stress conditions tested (Fig. 5b). For all these studies, the silenced line L2 exhibited the response similar to that of WT.
Assessment of various physiological parameters of WT and OsCyp2-P transgenic tobacco lines grown under control or stress conditions (i.e., mannitol, 200 mM; heat, 42 °C; NaCl, 150 mM and H2O2, 5 mM). Bar diagram depicting a chlorophyll retention; b electrolyte leakage (%). c Lipid hydroperoxide accumulation in WT and OsCyp2-P ectopically expressing lines grown under control and stress conditions. d Assessment of relative H2O2 levels in tobacco plants under stress conditions. Stress-induced levels of H2O2 in leaves of WT as well as OsCyp2-P ectopically expressing lines (L2, L1, L4, L5) were visualized by DAB staining. Bar diagram depicting relative quantity of H2O2 in these leaves in terms of their color intensity. Transgenic line L2 represents a silenced line taken as a control. The data represents the means of three biological replicates (n = 3). Asterisk shows values significantly different from respective WT under same conditions at P < 0.05, by Student’s t test
OsCyp2-P transgenics show reduced lipid peroxidation and reduced H2O2 accumulation under various abiotic stress conditions
To validate the protective effects of OsCyp2-P on the photosynthesis machinery (hub for ROS generation) in tobacco leaves, we compared salt stress-induced changes in the lipid peroxidation and reactive oxygen species (ROS) scavenging in OsCyp2-P transgenic and wild-type seedlings. The level of lipid hydroperoxide in plant tissues was used as an indicator of lipid peroxidation. Under control conditions, the lipid hydroperoxide levels were comparable in OsCyp2-P transgenic seedlings and wild-type seedlings (Fig. 5c). However, under stress conditions (osmotic, heat, salinity, H2O2), the lipid hydroperoxide levels were significantly lower in all OsCyp2-P transgenic lines (except the line L2 where the gene appears to be silenced), as compared to WT (Fig. 5c), indicating that OsCyp2-P ectopic expression decreased the lipid peroxidation levels. Diaminobenzidine tetrahydrochloride (DAB) staining was used to visualize the accumulation of H2O2 in stressed WT and OsCyp2-P ectopically expressing lines. Under stress conditions, OsCyp2-P transgenic tobacco seedlings contain substantially lower H2O2 levels than wild-type seedlings (Fig. 5d).
Discussion
Genome-wide analysis of cyclophilin members in Arabidopsis and rice revealed this family to have expanded in the latter via alternative splicing events. Similar observations were made when we compared the cases of alternative splicing of two-component system family (TCS) between Arabidopsis and rice (Pareek et al. 2006). Alternative splicing is a molecular mechanism utilized by broad range of eukaryotes to extend the repertoire of normal functions associated with a single gene (Haas 2008). It has been suggested that alternate splicing of many genes in rice is affected by stress conditions, suggesting that manipulation of alternate splicing may be an effective strategy of rice to adapt to abiotic stresses (Zhiguo et al. 2013). Expansion of Cyp family in rice may indicate towards involvement and varied functions of the members in handling diverse stresses. Random distribution of salinity-related ESTs on different chromosomes in rice has also been reported by our group recently (Kumari et al. 2009a). In contrast, we have also reported clustering of few metallothionein genes in rice which may be representing a kind of “regulon” (Kumar et al. 2012). This kind of genome-wide analysis allows studying the gene-specific molecular evolution of species possibly correlating with their adaptive responses towards abiotic stresses. Analysis of cyclophilin family in rice and Arabidopsis showed their random distribution on chromosomes and also showed that some of the members were duplicated on certain regions of the chromosome (for details, see the supplementary data).
Stress-dependent accumulation of cyclophilins has been documented in several plant species including bean, maize, sorghum, pigeon pea, Arabidopsis, and tomato (Marivet et al. 1994, 1995; Scholze et al. 1999; Godoy et al. 2000; Sharma and Singh 2003; Sekhar et al. 2010). These reports suggest a pivotal role of cyclophilins in plants under adverse conditions either through their direct role in chaperoning or transcriptional activation of other stress-regulated genes. Recently, OsCyp2 has also been shown to participate in auxin signal transduction by acting as molecular chaperone and interacting with the co-chaperone OsSGT1 (Kang et al. 2013), which further strengthens the possibility of its role as molecular chaperone. Mammalian CypA was predicted earlier as a cytosolic Cyp but it was found to be distributed in both the nucleus and cytosol of mouse macrophages and interact with a zinc-finger transcription factor (Krummrei et al. 1995). It has also been hypothesized that yeast cyclophilin A (which also lacks NLS) passes through nuclear pores passively due to its small size. Similarly, OsCyp2-P is also a small protein of 18.6 kDa and thus it can physically pass into the nucleus by diffusion. This leads us to hypothesize that OsCyp2-P is delivered into the nucleus through diffusion, similar to the yeast cyclophilin A, and interact with specific nuclear proteins thereby regulating the transcriptional activation of downstream genes. Furthermore, in contrast to mammalian multiprotein NADPH oxidase complex (Cross and Segal 2004), plant NADPH oxidases are single cytosolic proteins (Davis et al. 1998). AtCyp19 has been reported to be localized in cytoplasm and hypothesized to interact with cytosolic ROS-producing proteins, particularly with NADPH oxidase or act its upstream regulator to produce ROS (Pogorelko et al. 2014). These reports confirm our study and serve as an indication towards the key regulatory role played by OsCyp2-P as a modulator of the gene function and protein expression during stress responses.
Differential accumulation pattern of OsCyp2-P transcripts under various stresses indicate its possible role in multiple stress responses. This is in agreement with our previous findings where we found differential accumulation pattern of OsCyp2 transcripts at various developmental stages indicating its developmental and stress responsive regulation (Kumari et al. 2009b). The high salinity tolerance in L5 line was in strong correlation with the high abundance of transcript, protein, and activity of OsCyp2-P. In order to survive under higher concentrations of NaCl, plants have to either sequester or extrude excess Na+ ions and thereby maintain higher K+ ions required for various cellular processes (Zhu 2001). Maintenance of K+/Na+ ratio under stress condition is considered as a vital determinant of stress tolerance (Glenn et al. 1999). The OsCyp2-P transgenic plants could maintain higher K+/Na+ ratio and better membrane integrity than WT plants under salinity stress, thereby implying that the ectopically expressing lines showed unequivocal adaptive advantage in terms of ion homeostasis and membrane integrity. Thus, OsCyp2-P may be playing a role in protecting cellular membranes via its chaperoning activity as proposed earlier (Lippuner et al. 1994; Ruan et al. 2011).
Diverse stresses have been shown to ultimately lead to oxidative stress due to the disturbance in reactive oxygen species (ROS) production and scavenging, leading to generation of ROS such as H2O2 (Mittler et al. 2004; Huang et al. 2010; Ouyang et al. 2010). ROS are highly detrimental to biological systems. They partially reduce or activate derivatives of oxygen and thus damage DNA, proteins, and carbohydrates, resulting in cell death (Jambunathan et al. 2010). They have also been implicated in lipid peroxidation and cell membrane damage, and their levels can reflect the degree of damage to cellular components (Huang et al. 2010). Stress-induced H2O2 accumulation can result in increase in lipid peroxidation (Jambunathan et al. 2010). It has been proposed earlier that ROS act as primary signaling molecules during abiotic stresses, activating mitogen-activated protein kinase (MAPK) pathways (Karali et al. 2012). ROS are reported to be produced by cell wall, mitochondria, plastids, endoplasmic reticulum, peroxisomes, NADPH oxidase metabolisms, and due to the consequence of cell membrane electron transporting (Sharma et al. 2012). The study by Ruan et al. (2011) has also demonstrated the role of OsCyp2-P towards providing tolerance to salinity stress in rice seedlings via ROS scavenging. They have observed that OsCyp2-P transgenic rice seedlings had lower MDA content and H2O2, which is well correlated with higher antioxidant enzyme activities (such as SOD, CAT, and APX) than WT seedlings. It has been suggested that OsCyp2 up-regulates the activities of several important enzymes such as SOD, CAT, and APX at post-transcriptional level (Kong et al. 2001). This in turn protects photosynthetic components of leaves against oxidative stress. Taken together, our findings also indicate the role of OsCyp2-P as a key regulator controlling ROS levels.
The proliferated growth of OsCYP2-P transgenic plants under stress conditions (this study) are in confirmation with the earlier studies that reported improved germination and growth in the transgenic plants that overexpressed single-domain cyclophilin (Gjcyp-1) in red algae (Cho and Kim 2008) and ThCyp1 in tobacco (Chen et al. 2007). Overexpression of a pigeonpea cyclophilin gene, CcCyp in Arabidopsis, also resulted in improved germination and biomass accumulation in transgenic plants (Sekhar et al. 2010). Our results clearly show that OsCyp2-P plays a crucial role in preventing oxidative damage in cells thus protecting the photosynthetic machinery. Since generation of ROS is central to various abiotic stresses studied here and OsCyp2-P expressing plants exhibit considerable tolerance to these varied abiotic stresses, we confirm the role of OsCyp2-P as a key regulator controlling the levels of ROS in plants.
Abbreviations
- At:
-
Arabidopsis thaliana
- Cyp:
-
Cyclophilin
- CLD:
-
Cyclophilin-like domain
- PPIase:
-
Peptidyl-prolyl-cis-trans isomerase
- Os:
-
Oryza sativa
- WT:
-
Wild type
References
Ahn JC, Kim DW, You YN, Seok MS, Park JM, Hwang H, Kim BG, Luan S, Park HS, Cho HS (2010) Classification of rice (Oryza sativa L. Japonica nipponbare) immunophilins (FKBPs, CYPs) and expression patterns under water stress. BMC Plant Biol 10:253
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brazin KN, Mallis RJ, Fulton DB, Andreotti AH (2002) Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci U S A 99:1899–1904
Chen AP, Wang GL, Qu ZL, Lu CX, Liu N, Wang F, Xia G (2007) Ectopic expression of ThCYP1, a stress-responsive cyclophilin gene from Thellungiella halophila, confers salt tolerance in fission yeast and tobacco cells. Plant Cell Rep 26:237–245
Cho EK, Kim M (2008) A red algal cyclophilin has an effect on development and growth in Nicotiana tabacum. Plant Physiol Biochem 46:868–874
Chou IT, Gasser CS (1997) Characterization of the cyclophilin gene family of Arabidopsis thaliana and phylogenetic analysis of known cyclophilin proteins. Plant Mol Biol 35:873–892
Cross AR, Segal AW (2004) The NADPH oxidase of professional phagocytes—prototype of the NOX electron transport chain systems. Biochim Biophys Acta 1657:1–22
Davis AR, Mascolo PL, Bunger PL, Sipes KM, Quinn MT (1998) Cloning and sequencing of the bovine flavocytochrome b subunit proteins, gp91-phox and p22-phox: comparison with other known flavocytochrome b sequences. J Leukoc Biol 64:114–123
Ferreira PA, Nakayama TA, Pak WL, Travis GH (1996) Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature 383:637–640
Galat A (1999) Variations of sequences and amino acid compositions of proteins that sustain their biological functions: an analysis of the cyclophilin family of proteins. Arch Biochem Biophys 371:149–162
Glenn EP, Brown JJ, Blumwald E (1999) Salt tolerance and crop potential of halophytes. Crit Rev Plant Sci 18:227–255
Godoy AV, Lazzaro AS, Casalongue CA, San Segundo B (2000) Expression of a Solanum tuberosum cyclophilin gene is regulated by fungal infection and abiotic stress conditions. Plant Sci 152:123–134
Haas BJ (2008) Analysis of alternative splicing in plants with bioinformatics tools. Curr Top Microbiol Immunol 326:17–37
Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW (1984) Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science 226:544–547
Harlow E, Lane D (1988) Antibodies; A laboratory manual. Cold Spring Harbor Laboratory Press, New York, New York
Horowitz DS, Lee EJ, Mabon SA, Misteli T (2002) A cyclophilin functions in pre-mRNA splicing. EMBO J 21:470–480
Huang JG, Yang M, Liu P, Yang GD, Wu CA, Zheng CC (2010) Genome-wide profiling of developmental, hormonal or environmental responsiveness of the nucleocytoplasmic transport receptors in Arabidopsis. Gene 451:38–44
Iki T, Yoshikawa M, Meshi T, Ishikawa M (2012) Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J 31:267–278
Jain M, Tyagi AK, Khurana JP (2008) Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J 275:2845–2861
Jambunathan N, Penaganti A, Tang Y, Mahalingam R (2010) Modulation of redox homeostasis under suboptimal conditions by Arabidopsis nudix hydrolase 7. BMC Plant Biol 10:173
Kang B, Zhang Z, Wang L, Zheng L, Mao W, Li M, Wu Y, Wu P, Mo X (2013) OsCYP2, a chaperone involved in degradation of auxin-responsive proteins, plays crucial roles in rice lateral root initiation. Plant J 74:86–97
Karali D, Oxley D, Runions J, Ktistakis N, Farmaki T (2012) The Arabidopsis thaliana immunophilin ROF1 directly interacts with PI(3)P and PI(3,5)P2 and affects germination under osmotic stress. PLoS One 7:e48241
Kim SK, You YN, Park JC, Joung Y, Kim BG, Ahn JC, Cho HS (2012) The rice thylakoid lumenal cyclophilin OsCYP20-2 confers enhanced environmental stress tolerance in tobacco and Arabidopsis. Plant Cell Rep 31:417–426
Kong HY, Lee SC, Hwang BK (2001) Expression of pepper cyclophilin gene is differentially regulated during the pathogen infection and abiotic stress conditions. Physiol Mol Plant Pathol 59:189–199
Krummrei U, Bang R, Schmidtchen R, Brune K, Bang H (1995) Cyclophilin-A is a zinc-dependent DNA binding protein in macrophages. FEBS Lett 371:47–51
Krzywicka A, Beisson J, Keller AM, Cohen J, Jerka-Dziadosz M, Klotz C (2001) KIN241: a gene involved in cell morphogenesis in Paramecium tetraurelia reveals a novel protein family of cyclophilin-RNA interacting proteins (CRIPs) conserved from fission yeast to man. Mol Microbiol 42:257–267
Kumar G, Purty RS, Sharma MP, Singla-Pareek SL, Pareek A (2009) Physiological responses among Brassica species under salinity stress show strong correlation with transcript abundance for SOS pathway-related genes. J Plant Physiol 166:507–520
Kumar G, Kushwaha HR, Sabharwal VP, Kumari S, Joshi R, Karan R, Mittal S, Singla-Pareek SL, Pareek A (2012) Clustered metallothionein genes are co-regulated in rice and ectopic expression of OsMT1e-P confers multiple abiotic stress tolerance in tobacco via ROS scavenging. BMC Plant Biol 12:107
Kumari S, Panjabi Nee Sabharwal V, Kushwaha HR, Sopory SK, Singla-Pareek SL, Pareek A (2009a) Transcriptome map for seedling stage specific salinity stress response indicates a specific set of genes as candidate for saline tolerance in Oryza sativa L. Funct Integr Genom 9:109–123
Kumari S, Singh P, Singla-Pareek SL, Pareek A (2009b) Heterologous expression of a salinity and developmentally regulated rice cyclophilin gene (OsCyp2) in E. coli and S. cerevisiae confers tolerance towards multiple abiotic stresses. Mol Biotechnol 42:195–204
Kumari S, Roy S, Singh P, Singla-Pareek SL, Pareek A (2013) Cyclophilins: proteins in search of function. Plant Sign Behav 8:8
Kushwaha HR, Singh AK, Sopory SK, Singla-Pareek SL, Pareek A (2009) Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics 10:200
Kushwaha HR, Kumar G, Verma PK, Singla-Pareek SL, Pareek A (2011) Analysis of a salinity induced BjSOS3 protein from Brassica indicate it to be structurally and functionally related to its ortholog from Arabidopsis. Plant Physiol Biochem 49:996–1004
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Letunic I, Bork P (2011) Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Res 39:W475–W478
Leverson JD, Ness SA (1998) Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol Cell 1:203–211
Lin DT, Lechleiter JD (2002) Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem 277:31134–31141
Lippuner V, Chou IT, Scott SV, Ettinger WF, Theg SM, Gasser CS (1994) Cloning and characterization of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J Biol Chem 269:7863–7868
Marivet J, Margis-Pinheiro M, Frendo P, Burkard G (1994) Bean cyclophilin gene expression during plant development and stress conditions. Plant Mol Biol 26:1181–1189
Marivet J, Frendo P, Burkard G (1995) DNA sequence analysis of a cyclophilin gene from maize: developmental expression and regulation by salicylic acid. Mol Gen Genet 247:222–228
Mittler R, Vanderauwera S, Gollery M, Van BF (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mustafiz A, Singh AK, Pareek A, Sopory SK, Singla-Pareek SL (2011) Genome-wide analysis of rice and Arabidopsis identifies two glyoxalase genes that are highly expressed in abiotic stresses. Funct Integr Gen 11:293–305
Ouyang SQ, Liu YF, Liu P, Lei G, He SJ, Ma B, Zhang WK, Zhang ZS, Chen SY (2010) Receptor-like kinase OsSIK1 improves drought and salt stress tolerance in rice (Oryza sativa) plants. Plant J 62:316–329
Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL (2006) Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol 142:380–397
Pogorelko GV, Mokryakova M, Fursova OV, Abdeeva I, Piruzian ES, Bruskin SA (2014) Characterization of three Arabidopsis thaliana immunophilin genes involved in the plant defense response against Pseudomonas syringae. Gene 538:12–22
Romano PG, Horton P, Gray JE (2004) The Arabidopsis cyclophilin gene family. Plant Physiol 134:1268–1282
Ruan SL, Ma HS, Wang SH, Fu YP, Xin Y, Liu WZ, Wang F, Tong JX, Wang SZ, Chen HZ (2011) Proteomic identification of OsCYP2, a rice cyclophilin that confers salt tolerance in rice (Oryza sativa L.) seedlings when overexpressed. BMC Plant Biol 11:34
Sambrook J, Russel DW (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, New York
Scholze C, Peterson A, Diettrich B, Luckner M (1999) Cyclophilin isoforms from Digitalis lanata. Sequences and expression during embryogenesis and stress. J Plant Physiol 155:212–219
Sekhar K, Priyanka B, Reddy RD, Rao KV (2010) Isolation and characterization of a pigeonpea cyclophilin (CcCYP) gene, and its over-expression in Arabidopsis confers multiple abiotic stress tolerance. Plant Cell Environ 33:1324–1338
Sharma AD, Singh P (2003) Effect of water stress on expression of a 20 kD cyclophilin-like protein in drought susceptible and tolerant cultivars of Sorghum. J Plant Biochem Biotechnol 12:77–80
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions, J Bot: 217037
Shieh BH, Stamnes MA, Seavello S, Harris GL, Zuker CS (1989) The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature 338:67–70
Singla-Pareek SL, Reddy MK, Sopory SK (2003) Genetic engineering of the glyoxalase pathway in tobacco leads to enhanced salinity tolerance. Proc Natl Acad Sci U S A 100:14672–14677
Smith MR, Willmann MR, Wu G, Berardini TZ, Moller B, Weijers D, Poethig RS (2009) Cyclophilin 40 is required for microRNA activity in Arabidopsis. Proc Natl Acad Sci U S A 106:5424–5429
Trivedi DK, Yadav S, Vaid N, Tuteja N (2012) Genome wide analysis of Cyclophilin gene family from rice and Arabidopsis and its comparison with yeast. Plant Signal Behav 17:1653–1666
Wang C, Jing R, Mao X, Chang X, Li A (2010a) TaABC1, a member of the activity of bc1 complex protein kinase family from common wheat, confers enhanced tolerance to abiotic stresses in Arabidopsis. J Expt Bot 63:1299–1311
Wang X, Shi X, Hao B, Ge S, Luo J (2005) Duplication and DNA segmental loss in the rice genome: implications for diploidization. New Phytol 165:937–946
Wang Z, Song J, Milne TA, Wang GG, Li H, Allis CD, Patel DJ (2010b) Pro isomerization in MLL1 PHD3-bromo cassette connects H3K4me readout to CyP33 and HDAC-mediated repression. Cell 141:1183–1194
Wiederrecht G, Hung S, Chan HK, Marcy A, Martin M, Calaycay J, Boulton D, Sigal N, Kincaid RL, Siekierka JJ (1992) Characterization of high molecular weight FK-506 binding activities reveals a novel FK-506-binding protein as well as a protein complex. J Biol Chem 267:21753–21760
Xue T, Wang D, Zhang S, Ehlting J, Ni F, Jakab S, Zheng C, Zhong Y (2008) Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genomics 9:550
Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, Guo H, Hung HC, Toole B, Gallay P, Sherry B, Bukrinsky M (2002) Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem 227:22959–22965
Zhiguo EZ, Wang L, Zhou J (2013) Splicing and alternative splicing in rice and humans. BMB Rep 46:439–447
Zhu JK (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Zivy M, Thiellement H, De VD, Hofmann JP (1983) Study on nuclear and cytoplasmic genome expression in wheat by two-dimensional gel electrophoresis: 1. First results on 18 alloplasmic lines. Theor Appl Genet 66:1–7
Acknowledgments
Work carried out in this paper has been supported by funds available from the Department of Biotechnology, Ministry of Science and Technology, Government of India and IAEA, Vienna. The authors acknowledge AIRF, JNU for confocal microscopy. Award of research fellowship from CSIR to SR and SK is also gratefully acknowledged.
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Sumita Kumari, Rohit Joshi, Khushwant Singh, and Suchismita Roy have equally contributed to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 25 kb)
Supplementary Table 1
(DOCX 11 kb)
Supplementary Table 2
(DOCX 15 kb)
Supplementary Fig. I
Chromosomal distribution and segmental duplication events for cyclophilin genes of a Arabidopsis; with prefix “At” and b rice; with prefix “Os.” Only the chromosomes having cyclophilin genes are shown and their number is indicated at their bottom in Romans. The scale is in megabase (Mb), and the centromeric regions are indicated by circles. The chromosomal positions (Mb) and orientation of each of the cyclophilin genes for Arabidopsis and rice are shown as horizontal bars and arrows, respectively. The cyclophilin genes present on duplicated chromosomal segments are connected by broken lines. (GIF 31 kb)
Supplementary Fig. II
Phylogenetic tree depicting evolutionary relationship, similarity to cyclophilin subtypes identified in human and the presence of conserved domains among the cyclophilin members from Arabidopsis and rice. The detailed phylogenetic tree depicting evolutionary relationship among cyclophilin members of Arabidopsis and rice is shown in inner circle, while the classification of cyclophilin members of Arabidopsis and rice into various subtypes according to their similarity to subtypes identified for cyclophilin gene members in human is shown in middle circle. The outermost circle depicts the domain architecture and presence of signal peptides, if any, of cyclophilin members of Arabidopsis and rice. NLS nuclear localization signal, RRM RNA recognition motifs, ATPase adenosine tri-phosphatase, UBOX E4 ubiquitination factor, Trans trans domain, TPR tetratrico peptide repeats, CLD cyclophilin-like domain, WD40 beta-transducin repeats, TM transmembrane domain (GIF 56 kb)
Supplementary Fig. III
Expression profile of cyclophilin genes from MPSS data (name of the MPSS libraries are shown below each heat map) in different tissues/organs of a Arabidopsis and b rice. The description of MPSS libraries is given at the website mentioned in the “Materials and methods” section. The heatmaps have been generated using TIGR MeV software package and represent hierarchical clustering of average log signal values of all cyclophilin genes in various tissues/organs (indicated at the top of each lane). The color bar below represents relative expression values; thereby, green color represents lowest expression levels, black represents medium expression levels, and red signifies highest expression level. (GIF 33 kb)
Supplementary Fig. IV
Heatmap analyses of Cyp genes from Arabidopsis and rice under stress conditions. a The microarray data for expression of Arabidopsis cyclophilin genes under various abiotic stress conditions such as cold, osmotic, salt, drought, genotoxic, oxidative, UV-B, wounding, and heat stress has been retrieved from TAIR. b The microarray data obtained for expression of rice cyclophilin genes under various abiotic stress conditions such as drought, salt, and cold stress (Rice Oligonucleotide Array Database). Color bar at the right represents expression values in terms of fold change, thereby green color representing lowest expression levels, black medium expression levels, and red signifies highest expression level. (GIF 56 kb)
Supplementary Fig. V
Confirmation of OsCyp2-P ectopically expressing transgenic tobacco plants. a Schematic diagram of OsCyp2-P gene construct in plant expression vector pCAMBIA1304. b Ethidium bromide gel showing PCR amplicons corresponding to OsCyp2-P and c genomic DNA from WT and OsCyp2-P ectopically expressing transgenic lines (L1, L2, L4, L5) digested with BglII and SpeI run on 0.8 % agarose gel stained with ethidium bromide. d Southern blot of respective lines probed with OsCyp2-P specific probe. Lines L1, L2, L4, L5, and L6 were found to be positive for transgene (GIF 41 kb)
Supplementary Fig. VI
Bar diagram depicting PPIase activity of WT and transgenic line L5 (showing highest OsCyp2-P protein accumulation) plants. Peptidyl-prolyl-cis-trans isomerases activity was assayed in a coupled reaction with chymotrypsin. The assays were performed at 15 °C for 360 s. The 1-ml assay mixture contained 80 IM N-succinyl-ala-ala-prophe-p-nitroanilidine as test peptide, assay buffer [50 mM HEPES (pH 8.0), 150 mM NaCl, 0.05 % Triton X-100] along with varying concentration of the plant protein from either WT or transgenic line. The reaction was initiated by the addition of chymotrypsin (300 mg/ml) and the change in absorbance at 390 nm was monitored using a spectrophotometer. For calculating the PPIase activity, the difference between the catalyzed and uncatalyzed first-order rate constant, derived from the kinetics of the absorbance change at 390 nm, was multiplied by the amount of substrate in each reaction. (GIF 43 kb)
Supplementary Fig. VII
Germination assay of OsCyp2-P ectopically expressing transgenic tobacco seeds on MS media supplemented with various stressors. For this purpose, medium was supplemented with either a PEG (5 %) for drought stress or b mannitol (200 mM) for osmotic stress. c Bar diagram showing germination percentage of wild-type and transgenic seedlings (L5) under various stress conditions. Bar diagram depicting d shoot length and e root length of wild-type and transgenic seedlings under control and stress conditions as indicated below the bars. The data represent mean ± SE of three biological replicates (n = 3). Asterisk shows values significantly different from respective WT under same conditions at P < 0.05, by Student’s t test. (GIF 212 kb)
Rights and permissions
About this article
Cite this article
Kumari, S., Joshi, R., Singh, K. et al. Expression of a cyclophilin OsCyp2-P isolated from a salt-tolerant landrace of rice in tobacco alleviates stress via ion homeostasis and limiting ROS accumulation. Funct Integr Genomics 15, 395–412 (2015). https://doi.org/10.1007/s10142-014-0429-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-014-0429-5