Abstract
The natural pigment fucoxanthin has attracted global attention because of its superior antioxidant properties. The haptophyte marine microalgae Pavlova spp. are assumed to be promising industrial fucoxanthin producers as their lack of a cell wall could facilitate the commercialization of cultured cells as a whole food. This study screened promising Pavlova strains with high fucoxanthin content to develop an outdoor cultivation method for fucoxanthin production. Initial laboratory investigations of P. pinguis NBRC 102807, P. lutheri NBRC 102808, and Pavlova sp. OPMS 30543 identified OPMS 30543 as having the highest fucoxanthin content. The culture conditions were optimized for OPMS 30543. Compared with f/2 and Walne’s media, the use of Daigo’s IMK medium led to the highest biomass production and highest fucoxanthin accumulation. The presence of seawater elements in Daigo’s IMK medium was necessary for the growth of OPMS 30543. OPMS 30543 was then cultured outdoors using acrylic pipe photobioreactors, a plastic bag, an open tank, and a raceway pond. Acrylic pipe photobioreactors with small diameters enabled the highest biomass production. Using an acrylic pipe photobioreactor with 60-mm diameter, a fucoxanthin productivity of 4.88 mg/L/day was achieved in outdoor cultivation. Thus, this study demonstrated the usefulness of Pavlova sp. OPMS 30543 for fucoxanthin production in outdoor cultivation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fucoxanthin is synthesized by brown algae and diatoms as a major photosynthetic pigment; thus, it is the most abundant marine carotenoid and is widely distributed in nature (Dembitsky and Maoka 2007). Fucoxanthin has attracted considerable attention for use in the pharmaceutical, nutraceutical, and cosmetic industries because of its superior antioxidant properties (Peng et al. 2011). Fucoxanthin has also been studied for its anti-cancer activity in human cells (Hosokawa et al. 1999; Kotake-Nara et al. 2001), anti-type 2 diabetes and anti-obesity effects in mice and human cells (Gammone and d’Orazio 2015; Maeda et al. 2007), in vitro anti-cholesterol activity (Kawee-ai et al. 2013), anti-inflammatory effects in rats (Shiratori et al. 2005), anti-angiogenic effects in human cells (Sugawara et al. 2006), anti-malarial effects against Plasmodium falciparum (Afolayan et al. 2008), and anti-hypertensive effects in rats (Ikeda et al. 2003; Sivagnanam et al. 2015), as well as for the treatment of Alzheimer’s disease (Kawee-ai et al. 2013). Currently, fucoxanthin is produced commercially from brown algae such as Laminaria spp. and Undaria pinnatifida and diatoms such as Phaedactylum tricornutum (Gayen et al. 2019). Algatechnologies Inc. supplies FucovitalTM, which is manufactured from P. tricornitum, and this was the first fucoxanthin food ingredient product approved by the US Food and Drug Administration (NDI 1048, 2017). Fucoxanthin obtained from diatoms such as Chaetoceros gracilis and Odontella aurita also have potential industrial applications (Tokushima et al. 2016; Xia et al. 2018). Culture conditions such as light and nutrients have been reported to affect microalgal fucoxanthin production (Xia et al. 2013; Gómez-Loredo et al. 2016; Lu et al. 2018; Yang and Wei 2020). In O. aurita, cultivation in a high nitrate medium led to high fucoxanthin content and volumetric fucoxanthin production (Xia et al. 2013). In P. tricornutum, tryptone and urea were examined as supplemental nitrogen sources, and tryptone was found to improve cell growth and fucoxanthin production (Yang and Wei 2020).
In addition to brown algae and diatoms, haptophyte microalgae of Pavlova spp., such as P. lutheri and P. pinguis, can produce fucoxanthin (Hiller et al. 1988; Lananan et al. 2013). The marine microalga P. lutheri, which can produce considerable amounts of polyunsaturated fatty acids (PUFAs), is commonly employed as a larval feed in aquaculture (Brown et al. 1997; Guihéneuf and Stengel 2013), and its PUFA yield is increased via random mutagenesis (Meireles et al. 2003). P. pinguis contains abundant docosapentaenoic acid (Milke et al. 2008). As Pavlova spp. do not have a cell wall (Green 1980); they can be commoditized as whole foods without the need to extract intracellular fucoxanthin. Thus, Pavlova spp. are considered valuable fucoxanthin producers. However, there are no quantitative reports regarding fucoxanthin production by Pavlova spp.
In the present study, screening of several Pavlova spp. to identify a strain with high fucoxanthin content revealed that Pavlova sp. OPMS 30543 is a promising producer. Culture conditions for OPMS 30543 were examined and optimized, and factors affecting biomass and fucoxanthin production were investigated in laboratory experiments. Large-scale and outdoor cultivation of OPMS 30543 was also conducted using various culture facilities.
Materials and Methods
Strains and Laboratory-Scale Cultivation
Pavlova pinguis NBRC 102807 and P. lutheri NBRC 102808 were obtained from the National Biological Resource Center (NBRC) of the National Institute of Technology and Evaluation. Pavlova sp. OPMS 30543 was isolated from brackish water from Okinawa Main Island, Japan. Microalgae were photoautotrophically cultivated in artificial seawater (Marine Art SF-1, Tomita Pharmaceutical, Tokushima, Japan) enriched with either Daigo’s IMK (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan), f/2 (Guillard and Ryther 1962), or Walne’s (Walne 1970) elements (Table 1). Culture conditions were as follows, unless otherwise noted in the figure legends: 800 mL of medium in 1-L sterilized bottles, illumination with white fluorescent lamps at an intensity of 150 µmol photons/m2/s with a 12-h:12-h light/dark cycle, and continuous aeration of 0.25 mL/mL/min. Cells were harvested using 0.7-μm pore size glass fiber filter paper GF/F (Cytiva, Tokyo, Japan), washed with distilled water, and dried at 120 °C for 2 h before measurement of dry cell weight (DCW). To examine alternative nitrogen sources for Daigo’s IMK, media were prepared as shown in Table 2.
Pigment Analysis
Approximately 10 mg of dried cells was suspended in 1 mL of acetonitrile, mixed by vortexing for 1 min, and disrupted by sonication for 10 min. After centrifugation at 10,000×g for 2 min, the supernatant was analyzed by high-performance liquid chromatography (Shimadzu, Kyoto, Japan) under the following conditions: reverse-phase column, COSMOSIL 5C18-AR-II, 4.6 mm I.D. × 150 mm (Nacalai Tesque, Kyoto, Japan); column oven temperature, 40 °C; mobile phase, 80% acetonitrile aqueous containing 0.1% formic acid; flow rate, 1 mL/min; and detection, 450 nm using a photodiode array detector. Fucoxanthin signals were identified and quantified using a standard curve generated using the fucoxanthin standard (FUJIFILM Wako Pure Chemical Corp.).
Large-Scale Cultivation
OPMS 30543 was cultivated outdoors under natural sunlight using the following common cultivation systems: (1) 60-mm outer diameter and 5-mm thickness acrylic pipe photobioreactor (PBR), (2) 114-mm outer diameter and 5-mm thickness acrylic pipe PBR, (3) 216-mm outer diameter and 5-mm thickness acrylic pipe PBR, (4) 267-mm outer diameter and 5-mm thick acrylic pipe PBR, (5) 450-mm outer diameter and 0.1-mm thickness plastic bag, (6) 200-L polycarbonate open tank, and (7) 500-L raceway pond, in 50% artificial seawater containing 2× Daigo’s IMK elements described above (Table 1). Agitation was performed by aeration at 0.25 mL/min for (1) and (2), and 0.1 mL/min for (3), (4), (5), and (6) except for the raceway pond, in which the flow rate was adjusted to 0.5 m/s by stirring with a paddle. During cultivation, the pH was adjusted to 8 by supplying 100% CO2.
Results
Screening of Pavlova Strains for Fucoxanthin Production
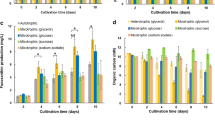
To develop a fucoxanthin production method using Pavlova spp., three strains (i.e., P. pinguis NBRC 102807, P. lutheri NBRC 102808, and P. sp. OPMS 30543) were examined in this study (Fig. 1a). The strains were cultured in 50% seawater containing 2× Daigo’s IMK at 25 °C to identify a promising strain with high fucoxanthin production. Strain NBRC 102808 exhibited the lowest biomass production, whereas NBRC 102807 exhibited the highest biomass production, 1.54 g DCW/L at day 12 (Fig. 1b). In contrast, among these Pavlova strains, strain NBRC 102807 exhibited the lowest fucoxanthin content (2.06 mg/g DCW, day 3) (Fig. 1c). OPMS 30543 exhibited measurable biomass production of 0.85 g DCW/L over 12 days and achieved the highest fucoxanthin content, 12.88 mg/g DCW at day 9. Fucoxanthin production (calculated by multiplying the biomass and fucoxanthin content) of 9.01 mg/L at day 9 was achieved by OPMS 30543, which was higher than that of strains NBRC 102807 (2.32 mg/L, day 12) and NBRC 102808 (0.61 mg/L, day 9) (Fig. 1d). Thus, OPMS 30543 was identified as a promising Pavlova strain for fucoxanthin production.
Examination of Culture Medium for OPMS 30543
To determine the optimal medium for fucoxanthin production, biomass and fucoxanthin content were investigated using OPMS 30543 grown in 50% seawater enriched with either 2× Daigo’s IMK, f/2 (Guillard and Ryther 1962) or Walne’s (Walne 1970) elements (Table 1). Among these conditions, cultivation in 2× Daigo’s IMK medium resulted in higher biomass (0.92 g DCW/L) relative to f/2 (0.55 g DCW/L) and Walne’s (0.56 g DCW/L) media after 14 days of cultivation (Fig. 2a). In addition, the fucoxanthin content of OPMS 30543 grown in 2× Daigo’s IMK medium was significantly higher (2.62 mg/g DCW, day 14) than that of cells grown in f/2 (1.48 mg/g DCW, day 7) or Walne’s (1.39 mg/g DCW, day 7) media (Fig. 2b). Fucoxanthin production of 1.51 mg/L on day 14 was achieved by culturing cells in 2× Daigo’s IMK medium, which was double the production of cells grown in medium containing f/2 (0.73 mg/L, day 7) or Walne’s (0.79 mg/L) elements (Fig. 2c). Thus, these data suggest that the use of 2× Daigo’s IMK was the most suitable for maximizing OPMS 30543 biomass and fucoxanthin production.
Examination of Culture Conditions for OPMS 30543
To improve the biomass production of OPMS 30543, various culture conditions (i.e., seawater concentration, pH, and temperature) were examined. When cultivated in 2× Daigo’s IMK with different concentrations of seawater, biomass production was observed only in the presence of seawater; OPMS 30543 did not grow in 0% seawater medium (Fig. 3a). The highest biomass of 6.16 g DCW/L on day 14 was achieved in the medium with 50% seawater. The effect of varying the culture pH by supplying CO2 gas to the medium was also examined (Fig. 3b). OPMS 30543 biomass production was reduced when the pH was adjusted to 6, whereas the highest biomass of 3.78 g DCW/L on day 6 was observed when pH was adjusted to 8. Culture temperature was investigated over the range of 15–35 °C (Fig. 3c). Within this temperature range, OPMS 30543 produced higher biomass at higher temperatures, and cultivation at 35 °C resulted in the highest biomass production of 3.32 g DCW/L on day 6. Thus, cultivation in 50% seawater medium at 35 °C and pH 8 was determined to be the optimal condition for OPMS 30543 biomass production.
Modification of IMK Medium by Replacing Nitrogen Sources and Adding Carbon Sources
To further improve OPMS 30543 biomass production and fucoxanthin content, the effect of varying the nitrogen source in the medium was examined. The modified IMK medium was prepared by replacing NaNO3 in 1× Daigo’s IMK with either NaNO3, KNO3, CO(NH2)2, or NH4Cl (Table 2). After 9 days of cultivation, cells cultured in the modified IMK medium containing KNO3 exhibited the highest biomass of 1.8 g DCW/L (Fig. 4a). Both urea CO(NH2)2 and NH4Cl were found to be available as nitrogen sources for OPMS 30543 cultivation, and biomass production of 1.58 and 0.82 g DCW/L at 10 days was observed, respectively. Use of NaNO3-containing medium resulted in higher fucoxanthin content (12.74 mg/g DCW) than in media with KNO3 (5.57 mg/g DCW), CO(NH2)2 (8.38 mg/g DCW), or NH4Cl (7.80 mg/g DCW) (Fig. 4b). Fucoxanthin production was the highest when NaNO3 was used as the nitrogen source (Fig. 4c). Fucoxanthin production of OPMS 30543 grown in modified IMK medium containing NaNO3, KNO3, CO(NH2)2, or NH4Cl was 17.84, 10.03, 13.24, and 6.40 mg/L, respectively. Thus, these data suggest that NaNO3 is the best nitrogen source for maximizing OPMS 30543 fucoxanthin production.
Examination of alternative nitrogen sources and additional carbon sources. a Biomass, b fucoxanthin content, and c fucoxanthin production of cells grown in 50% seawater enriched with modified IMK and different nitrogen sources. d Biomass, e fucoxanthin content, and f fucoxanthin production of cells grown in 50% seawater enriched with 2× Daigo’s IMK with additional carbon sources, illuminated with red, blue, and white LEDs at a total intensity of 300 µmol photons/m2/s with a 12-h:12-h light/dark cycle
The effect of adding various carbon sources to the medium was also examined to enhance biomass and fucoxanthin production. Modified IMK medium was prepared by adding either glucose, methanol, sodium acetate, or sodium bicarbonate to 50% seawater enriched with 1× Daigo’s IMK. Each of the additional carbon sources increased biomass production compared with that with the normal 1× Daigo’s IMK (Fig. 4d). After 4 days of cultivation, OPMS 30543 grown in medium with sodium acetate exhibited the highest biomass of 1.79 g DCW/L, whereas OPMS 30543 biomass in medium containing glucose, methanol, and sodium bicarbonate was 1.19, 0.71, and 1.28 g DCW/L, respectively. Use of medium containing methanol resulted in the highest fucoxanthin content (7.26 mg/g DCW) relative to medium containing glucose (4.25 mg/g DCW), sodium acetate (4.11 mg/g DCW), or sodium bicarbonate (2.99 mg/g DCW) (Fig. 4e). Fucoxanthin production was the highest when sodium acetate was added to the medium (Fig. 4f). Fucoxanthin production by OPMS 30543 grown with glucose, methanol, sodium acetate, and sodium bicarbonate was 5.06, 5.15, 7.36, and 3.83 mg/L, respectively. Thus, sodium acetate was suggested as the optimal carbon source for enhancing fucoxanthin production.
Large-Scale Outdoor Cultivation of OPMS 30543
A large-scale outdoor OPMS 30543 cultivation test was performed to evaluate the potential of fucoxanthin production outdoors. Acrylic pipe PBRs (5-mm thickness with different outer diameters of 114, 216, and 267 mm), a plastic bag (0.1-mm thickness with 450-mm outer diameter), a 200-L polycarbonate open tank, and a 500-L raceway pond were used for cultivation (Fig. 5). Six days of cultivation outdoors in acrylic pipe PBRs with 114-, 216-, and 267-mm outer diameter produced biomass of 0.73, 0.39, and 0.31 g DCW/L, respectively (Fig. 6a). Cultivation using a plastic bag, a 200-L polycarbonate open tank, and a 500-L raceway pond produced 0.24, 0.26, and 0.10 g DCW/L, respectively, on day 6. Thus, the acrylic pipe PBRs with smaller outer diameters achieved higher biomass production than the plastic bag, open tank, or raceway pond. To further examine these results, OPMS 30543 was cultivated using an acrylic pipe PBR with a 60-mm outer diameter. Biomass of 1.82 g DCW/L and 2.20 g DCW/L were observed on days 6 and 8, respectively (Fig. 6b), both of which were higher than the biomass production achieved using the acrylic pipe PBR with a 114-mm outer diameter. The fucoxanthin content on day 8 was 20.86 mg/g DCW, which was higher than that achieved with any of the laboratory-scale cultivations in this study. Using a PBR with a 60-mm outer diameter, biomass productivity of 0.23 g DCW/L/day and fucoxanthin productivity of 4.88 mg/L/day were demonstrated in large-scale outdoor cultivation.
Facilities used for outdoor cultivation. a Acrylic pipe photobioreactors (5-mm thickness with outer diameters of 114, 216, and 267 mm) and a plastic bag (0.1-mm thickness with a 450-mm outer diameter). b 200-L polycarbonate open tank. c 500-L raceway pond. d Acrylic pipe photobioreactor (60-mm outer diameter)
Large-scale outdoor cultivation of OPMS 30543. a Biomass of OPMS 30543 cultivated using natural light in acrylic pipe PBRs (5-mm thickness with different outer diameters of 114, 216, and 267 mm), a plastic bag (0.1-mm thickness with a 450-mm outer diameter), 200-L polycarbonate open tank, and 500-L raceway pond. b Biomass of OPMS 30543 cultivated outdoors under natural light in an acrylic pipe PBR with a 60-mm outer diameter. In these experiments, 50% seawater enriched with 2× Daigo’s IMK was used as the medium. Aeration was provided except for the raceway pond. In the raceway pond, cells were stirred using a paddle. During cultivation, the pH was adjusted to 8 by blowing CO2.
Discussion
In previous studies, P. lutheri and P. pinguis were examined as aquatic feed producers that accumulate high levels of ω-3 fatty acids, including docosahexaenoic acid and eicosapentaenoic acid (Guihéneuf and Stengel 2013; Guihéneuf et al. 2015; Fernandes et al. 2020). However, these organisms have not been studied extensively for their use as fucoxanthin producers, despite several reports describing fucoxanthin production by P. lutheri (Hiller et al. 1988; Lananan et al. 2013) and the advantages of the lack of a cell wall in Pavlova spp. (Green 1980). To develop a useful fucoxanthin production method, this study first compared fucoxanthin production in three Pavlova strains and identified Pavlova sp. OPMS 30543 as a promising strain owing to its significantly higher fucoxanthin production than that of P. pinguis NBRC 102807 and P. lutheri NBRC 102808 (Fig. 1d).
To determine the optimal conditions for OPMS 30543 cultivation, three types of media were examined. The use of 2× Daigo’s IMK medium resulted in higher fucoxanthin production than with either f/2 or Walne’s medium (Fig. 2c). A likely reason is that 2× Daigo’s IMK contains a much higher level of nitrate (400 mg/L NaNO3) than f/2 (75 mg/L NaNO3) or Walne’s (100 mg/L NaNO3) (Table 1). Nitrate supplementation has been reported to increase fucoxanthin production in the diatoms Phaeodactylum tricornutum and O. aurita (Xia et al. 2013; McClure et al. 2018). Nitrogen supplementation with tryptone improved fucoxanthin production in P. tricornutum (Yang and Wei 2020). This study also investigated different nitrogen sources with which to modify 2× Daigo’s IMK and found that the use of NaNO3 resulted in the highest fucoxanthin accumulation (Fig. 4c). Microalgae growth and fucoxanthin generally show a positive relationship, except under some conditions such as nitrogen depletion, under which fucoxanthin content decreases (Xia et al. 2018). In this study, the modified IMK medium containing KNO3 led to the highest biomass (Fig. 4a), although the fucoxanthin content was the lowest (Fig. 4b). This might be because the nitrogen source was depleted in the KNO3 medium owing to the highest cell growth. The effect of the nitrogen source on fucoxanthin production has not been examined in detail in previous studies. Absorption and assimilation of different nitrogen sources were investigated in Pelagophycea Aureococcus anophagefferens, which also accumulates fucoxanthin (Ou et al. 2018). Different from the results of this study, cultivation using urea resulted in the highest fucoxanthin content in this microalga compared with cultivation with NaNO3, NH4Cl, or glutamic acid. Although the effects differ among algae species, these results suggest that supplementation and type of nitrogen source are important factors affecting fucoxanthin accumulation.
Among the Pavlova strains tested in this study, P. pinguis NBRC 102807 exhibited the highest biomass production (Fig. 1b). In contrast, Pavlova sp. OPMS 30543 could grow under a wide range of seawater concentrations, ranging from 25 to 100%, with similar biomass productivity (Fig. 3a). This robustness toward salinity is a valuable characteristic for seawater cultivation. OPMS 30543 did not produce biomass when cultured in medium with 0% seawater, possibly because Daigo’s IMK medium depends upon supplementation of Mg2+ and Ca2+ in seawater (Table 1). Of the three media examined, 2× Daigo’s IMK provided the highest OPMS 30543 biomass production (Fig. 2a), probably because it contained more nitrate than either f/2 or Walne’s media (Table 1). The effects of an additional carbon source were also examined. This analysis revealed that the addition of glucose, sodium acetate, or sodium bicarbonate to 2× Daigo’s IMK medium enhanced OPMS 30543 biomass production (Fig. 4d). In haptophyte Isochrysis galbana, glycerol was found to be the best additional carbon source to enhance biomass production, whereas acetate had no effect and glucose only slightly enhanced the growth rate (Alkhamis and Qin 2013). Overall, these data suggest that the addition of a suitable carbon is a promising approach for enhancing the biomass production of microalgae, including OPMS 30543.
In the large-scale outdoor cultivation experiment, the acrylic pipe PBRs demonstrated higher biomass production than the open tank or raceway pond (Fig. 6a). A possible reason for this result is that the open tank and raceway pond were highly contaminated with bacteria, fungi, and protozoa (data not shown). Among the acrylic pipe PBRs examined, those with a smaller diameter produced higher biomass, most likely because the higher surface area-to-volume ratio contributes to more efficient illumination. Using the 60-mm diameter acrylic pipe PBR, a fucoxanthin content of 20.86 mg/g DCW and fucoxanthin productivity of 4.88 mg/L/day was obtained after 8 days of cultivation (Fig. 6b). Fucoxanthin content in various microalgae and macroalgae has been reported in previous studies (Table 3). Microalgae such as haptophytes, diatoms, and chrysophytes generally show higher fucoxanthin content than macroalgae. In diatoms, P. tricornutum and Cylindrotheca closterium were reported to achieve 59.2 mg/g DCW and 25.5 mg/g DCW fucoxanthin content, respectively (McClure et al. 2018; Wang et al. 2018). Chrysophytes Mallomonas sp. also showed a high fucoxanthin content of 26.6 g/g DCW (Petrushkina et al. 2017). For commercialization of cultured cells as a whole food, however, these microalgae would not be favorable because they have a cell wall. In this study, as a cell wall-lacking microalga, Pavlova sp. OPMS 30543 achieved a fucoxanthin content of 20.86 mg/g DCW, which is higher than that achieved with Isochrysis aff. galbana (Kim et al. 2012). Thus, Pavlova sp. OPMS 30543 is a promising feedstock for fucoxanthin, characterized by both a high fucoxanthin content and the absence of cell wall. With the development of a large-scale outdoor cultivation method for OPMS 30543 fucoxanthin production as demonstrated in this study, the utilization of Pavlova cells as whole foods has taken a step toward successful commercialization.
Data Availability
The data supporting the findings of this study are available within this article or from the corresponding author upon reasonable request. Pavlova pinguis NBRC 102807 and Pavlova lutheri NBRC 102808 can be obtained from the National Biological Resource Center (NBRC).
References
Afolayan AF, Bolton JJ, Lategan CA, Smith PJ, Beukes DR (2008) Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z Naturforsch C J Biosci 63:848–852
Alkhamis Y, Qin JG (2013) Cultivation of Isochrysis galbana in phototrophic, heterotrophic, and mixotrophic conditions. Biomed Res Int 2013:983465
Brown MR, Jeffrey SW, Volkman JK, Dunstan GA (1997) Nutritional properties of microalgae for mariculture. Aquaculture 151:315–331
Dang TT, Bowyer MC, Van Altena IA, Scarlett CJ (2017) Comparison of chemical profile and antioxidant properties of the brown algae. Int J Food Sci Technol 53:174–181
Dembitsky VM, Maoka T (2007) Allenic and cumulenic lipids. Prog Lipid Res 46:328–375
Fariman GA, Shastan SJ, Zahedi MM (2016) Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J Appl Phycol 28:1323–1331
Fernandes T, Martel A, Cordeiro N (2020) Exploring Pavlova pinguis chemical diversity: a potentially novel source of high value compounds. Sci Rep 10:339
Gammone MA, d’Orazio N (2015) Anti-obesity activity of the marine carotenoid fucoxanthin. Mar Drugs 13:2196–2214
Gao F, Teles Cabanelas Itd I, Wijffels RH, Barbosa MJ (2020) Process optimization of fucoxanthin production with Tisochrysis lutea. Bioresour Technol 315:123894
Gayen K, Bhowmick TK, Maity SK (2019) Sustainable Downstream Processing of Microalgae for Industrial Application. CRC Press, Boca Raton
Gómez-Loredo A, Benavides J, Rito-Palomares M (2016) Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J Appl Phycol 28:849–860
Green JC (1980) The fine structure of Pavlova pinguis Green and a preliminary survey of the order Pavlovales (Prymnesiophyceae). Br Phycol J 15:151–191
Guihéneuf F, Stengel DB (2013) LC-PUFA-enriched oil production by microalgae: accumulation of lipid and triacylglycerols containing n-3 LC-PUFA is triggered by nitrogen limitation and inorganic carbon availability in the marine haptophyte Pavlova lutheri. Mar Drugs 11:4246–4266
Guihéneuf F, Mimouni V, Tremblin G, Ulmann L (2015) Light intensity regulates LC-PUFA incorporation into lipids of Pavlova lutheri and the final desaturase and elongase activities involved in their biosynthesis. J Agric Food Chem 63:1261–1267
Guillard RRL, Ryther JH (1962) Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Hiller RG, Larkum AWD, Wrench PM (1988) Chlorophyll proteins of the prymnesiophyte Pavlova lutherii (Droop) comb. nov.: identification of the major light-harvesting complex. Biochimica et Biophysica Acta - Bioenergetics 932:223–231
Hosokawa M, Wanezaki S, Miyauchi K, Kurihara H, Kohno H, Kawabata J, Odashima S, Takahashi K (1999) Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci Technol Res 5:243–246
Ikeda K, Kitamura A, Machida H, Watanabe M, Negishi H, Hiraoka J, Nakano T (2003) Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 30:44–48
Kawee-ai A, Kuntiya A, Kim SM (2013) Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat Prod Commun 8:1381–1386
Kim SM, Kang SW, Kwon ON, Chung D, Pan CH (2012) Fucoxanthin as a major carotenoid in Isochrysis aff. galbana: Characterization of extraction for commercial application. J Korean Soc Appl Biol Chem 55:477–483
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
Lananan F, Jusoh A, Ali N, Lam SS, Endut A (2013) Effect of Conway medium and f/2 medium on the growth of six genera of South China Sea marine microalgae. Bioresour Technol 141:75–82
Lu X, Sun H, Zhao W, Cheng KW, Chen F, Liu B (2018) A hetero-photoautotrophic two-stage cultivation process for production of fucoxanthin by the marine diatom Nitzschia laevis. Mar Drugs 16:219
Maeda H, Fukuda S, Izumi H, Saga N (2018) Anti-oxidant and fucoxanthin contents of brown alga Ishimozuku (Sphaerotrichia divaricata) from the West Coast of Aomori Japan. Mar Drugs 16:255
Maeda H, Hosokawa M, Sashima T, Miyashita K (2007) Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem 55:7701–7706
Marella TK, Tiwari A (2020) Marine diatom Thalassiosira weissflogii based biorefinery for co-production of eicosapentaenoic acid and fucoxanthin. Bioresour Technol 307:123245
McClure DD, Luiz A, Gerber B, Barton GW, Kavanagh JM (2018) An investigation into the effect of culture conditions on fucoxanthin production using the marine microalgae Phaeodactylum tricornutum. Algal Res 29:41–48
Meireles LA, Guedes C, Malcata FX (2003) Increase of the yields of eicosapentaenoic and docosahexaenoic acids by the microalga Pavlova lutheri following random mutagenesis. Biotechnol Bioeng 81:50–55
Milke LM, Bricelj VM, Parrish CC (2008) Biochemical characterization and nutritional value of three Pavlova spp. in unialgal and mixed diets with Chaetoceros muelleri for postlarval sea scallops Placopecten magellanicus. Aquaculture 276:130–142
Ou L, Cai Y, Jin W, Wang Z, Lu S (2018) Understanding the nitrogen uptake and assimilation of the Chinese strain of Aureococcus anophagefferens (Pelagophyceae). Algal Res 34:182–190
Peng J, Yuan JP, Wu CF, Wang JH (2011) Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs 9:1806–1828
Petrushkina M, Gusev E, Sorokin B, Zotko N, Mamaeva A, Filimonova A, Kulikovskiy M, Maltsev Y, Yampolsky I, Guglya E, Vinokurov V, Namsaraev Z, Kuzmin D (2017) Fucoxanthin production by heterokont microalgae. Algal Res 24:387–393
Shiratori K, Ohgami K, Ilieva I, Jin XH, Koyama Y, Miyashita K, Yoshida K, Kase S, Ohno S (2005) Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res 81:422–428
Sivagnanam SP, Yin S, Choi JH, Park YB, Woo HC, Chun BS (2015) Biological properties of fucoxanthin in oil recovered from two brown seaweeds using supercritical CO2 extraction. Mar Drugs 13:3422–3442
Sugawara T, Matsubara K, Akagi R, Mori M, Hirata T (2006) Antiangiogenic activity of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. J Agric Food Chem 54:9805–9810
Sun Z, Wang X, LiuJ, (2019) Screening of Isochrysis strains for simultaneous production of docosahexaenoic acid and fucoxanthin. Algal Res 41:101545
Susanto E, Fahmi AS, Abe M, Hosokawa M, Miyashita K (2016) Lipids, fatty acids, and fucoxanthin content from temperate and tropical brown seaweeds. Aquat Procedia 7:66–75
Tokushima H, Inoue-Kashino N, Nakazato Y, Masuda A, Ifuku K, Kashino Y (2016) Advantageous characteristics of the diatom Chaetoceros gracilis as a sustainable biofuel producer. Biotechnol Biofuels 9:235
Walne PR (1970) Studies on the food value of nineteen genera of algae to juvenile bivalves of the genera Ostrea, Crassostrea, Mercenaria, and Mytilis. Fish Invest 26:1–62
Wang S, Verma SK, Said IH, Thomsen L, Ullrich MS, Kuhnert N (2018) Changes in the fucoxanthin production and protein profiles in Cylindrotheca closterium in response to blue light-emitting diode light. Microb Cell Fact 17:110
Xia S, Gao B, Fu J, Xiong J, Zhang C (2018) Production of fucoxanthin, chrysolaminarin, and eicosapentaenoic acid by Odontella aurita under different nitrogen supply regimes. J Biosci Bioeng 126:723–729
Xia S, Wang K, Wan L, Li A, Hu Q, Zhang C (2013) Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar Drugs 11:2667–2681
Xiao X, Si X, Yuan Z, Xu X, Li G (2012) Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J Sep Sci 35:2313–2317
Yang R, Wei D (2020) Improving fucoxanthin production in mixotrophic culture of marine diatom Phaeodactylum tricornutum by LED light shift and nitrogen supplementation. Front Bioeng Biotechnol 8:820
Acknowledgements
The authors thank Dr. Takeshi Fujiwara, Dr. Takafumi Watanabe, and Ms. Yuko Koizumi for their technical assistance. We would like to thank NBRC for supplying Pavlova pinguis NBRC 102807 and Pavlova lutheri NBRC 102808.
Author information
Authors and Affiliations
Contributions
A. Kanamoto designed the study, conducted the experiments, and drafted the manuscript. Y. K., E. Y., T. H., and A. Kondo commented on the study, helped interpret results, and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A. Kanamoto was a CEO of OP Bio Factory at the time this study was conducted. A. Kanamoto participated in the experiments as a representative of OP Bio Factory. The corresponding author has full access to all the data in the study and is completely responsible for the data and its accuracy.
Rights and permissions
About this article
Cite this article
Kanamoto, A., Kato, Y., Yoshida, E. et al. Development of a Method for Fucoxanthin Production Using the Haptophyte Marine Microalga Pavlova sp. OPMS 30543. Mar Biotechnol 23, 331–341 (2021). https://doi.org/10.1007/s10126-021-10028-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-021-10028-5