Abstract
The present study investigates the seasonal changes in lipid components, fucoxanthin (Fx), and functional long-chain n-3 (omega-3) polyunsaturated fatty acids (PUFAs) and also evaluates for their antioxidant activity of two tropical brown algae, Nizamuddinia zanardinii and Cystoseira indica, collected from the southeast coast of Iran. Results show a significant amount of fucoxanthin that is possibly due to the specific conditions of sampling area in terms of oxidative stress and the waves of southwest monsoon and thermal stress. Total lipid in October and November was higher compare to rest of the year. The percent of PUFA in N. zanardinii increased coinciding with a period of relative cold weather, but PUFA in C. indica increased in May when the weather is warm. However, in both species, seasonal variation in DPPH radical scavenging activity, CUPRAC, and total phenolic content did not show a particular pattern. A correlation between fucoxanthin content and DPPH radical scavenging activity was evident. Both algal species had higher phenolic content over the peak of maturity and fertility when the plant was tallest (October to December for both species and April for N. zanardinii). In general, variation of these factors in a tropical area such as Oman Sea is different from temperate regions. These changes in many cases are not as tangible, and further investigations are required to assess the variations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Seaweed can be divided into the brown (Phaeophytes), green (Chlorophytes), and red seaweeds (Rhodophytes) (Dawczynski et al. 2007; Jaswir et al. 2013a). Red and brown algae have been used as human food sources and traditional medicine since ancient times (Dawczynski et al. 2007; Rohani-Ghadikolaei et al. 2012). Polyunsaturated fatty acids (PUFA), mostly omega 3 fatty acids and the carotenoid fucoxanthin, which are found in brown seaweeds, have a significant effect on human health (Haugan and Liaaen-Jensen 1994; Dembitsky and Maoka 2007; Miyashita et al. 2011; D’Orazio et al. 2012). Based on its unique molecular structure, fucoxanthin shows high biological activity, including antioxidant (Nomura et al. 1997; Heo and Jeon 2009; Sudhakar et al. 2013), anticancer (Kotake-Nara et al. 2001, 2005; Hosokawa et al. 2004; Jaswir et al. 2013a), and antidiabetic (Maeda et al. 2006; Hosokawa et al. 2010) activities. At present, the main source of n-3 PUFA is marine fish, however, considering reduction in marine fish stock, finding a new source of PUFA is necessary (Nomura et al. 2013). Several studies have reported that the amount of lipid in seaweed varies with respect to factors such as geographic location, season, temperature, salinity, and light intensity (Sánchez-Machado et al. 2004; Terasaki et al. 2009; Gerasimenko et al. 2010, 2011; Nomura et al. 2013).
Thirty-nine species of brown algae have been reported from Iranian coastal area of Persian Gulf and Oman Sea (Gharanjik 2005), but there are few studies on the nutritional value and bioactive compounds of brown algae from Iran (Tabarsa et al. 2012; Moghadam et al. 2013). In this study, we examined fluctuation of antioxidant properties, fatty acid, and fucoxanthin content with seasons. Nizamuddinia zanardinii and Cystoseira indica are tropical brown algae distributed in southwest Asia including India, Oman, Qatar, Pakistan, Yemen, and Iran (Silva et al. 1996; Wynne and Jupp 1998; Sahoo et al. 2001; Abid et al. 2005; Gharanjik 2005). The main reason for choosing these two species for the present study is their high density and biomass in Iran’s southeast coast (Abkenar et al. 2008) and also their great potential for mariculture. Cultivated C. indica in the tidal zone of Chabahar Bay, Iran, shows faster growth rate and higher biomass in comparison with some other algae in the area (Farahpour et al. 2010). Chabahar Bay is located in the southeast of Iran and northern part of the Oman sea (25° 17′ 28″ N, 60° 38′ 15″ E). The coastal area is an exposed area to strong ultraviolet radiation due to the location in a tropical area. This can cause the development of reactive radical species (ROS) and other strong oxidizing agents, and marine algae growing in such warm water change their metabolism to produce antioxidants as a defense (Zubia et al. 2007).
The aim of this study was to determine the seasonal variation of fucoxanthin, n-3, and n-6 PUFAs, total lipid, and the antioxidant potential in different seasons of the brown algae N. zanardinii and C. indica from Iran with regard to their potential for food and medicinal purposes.
Materials and methods
Two brown seaweed, Nizamuddinia zanardinii (Schiffner) P.C.Silva and Cystoseira indica (Thivy & Doshi) Mairh, samples were collected seasonally from Chabahar coast located in southeast of Iran and north part of Oman sea in 2013 and 2014. Immediately after collection, the seaweed samples were cleaned and washed with seawater to remove sand, debris, epiphytes, and other extraneous matter attached to the thalli and transported to the laboratory where they were frozen at −80 °C.
Total lipid content
The methods of Nomura et al. (2013) with slight modification were used to determine total lipid. All extractions were carried out under dim light, and air in the extraction vessel was replaced with nitrogen to prevent possible degradation of carotenoids or lipids. Whole parts of the algae were sextracted with ten times (w/v) the amount of chloroform–methanol (1:2, v/v) at room temperature for 2 h. The solution was filtered, and this step was repeated two times. Ten times (w/v) the amount of chloroform–methanol–water (1:2:0.8, v/v/v) was added to the residue obtained by the second extraction. After extraction, all three filtrates were combined, and the solvent was removed under vacuum using a rotary evaporator. The dried extract was dissolved again with chloroform–methanol–water (8:4:3, v/v/v) in a separator funnel. After shaking the funnel vigorously, the lower layer was evaporated under vacuum in a rotary evaporator. The last traces of the remaining solvents and water were removed under high vacuum and a small amount of ethanol in order to obtain total lipids (TL). TL content was expressed as mg g−1 DW. This was calculated from fresh weight-based measurements of TL and the moisture content of the fresh samples. The moisture content was measured by oven drying the fresh sample at 105 °C (AOAC 1997) until a constant weight was obtained.
Analysis of Fucoxanthin content by HPLC
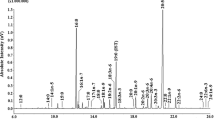
All HPLC analyses were carried out using a KNAUR Smartline system SYSS003 (Germany) equipped with a pump (Smartline Pump 1050), auto-sampler (Smartline Autosampler 3950 with sample cooling), and a photo diode-array spectrophotometric detector (Smartline UV Detector 2600 with fiber optics). Fucoxanthin content in the TL was determined by reversed phase HPLC (RP-HPLC) with methanol–acetonitrile (7:3 v/v) as the mobile phase at a flow rate of 1.0 mL min−1 (Terasaki et al. 2009). All RP-HPLC analyses were carried out at 28 °C using a C18 column (250 × 4.6 Nucl Eo DUR 100-5 C18). An aliquot of TL was dissolved in the mobile phase and filtered with a 0.22-μm membrane filter. Then, an aliquot of the filtered sample was submitted to HPLC analysis. The detection wavelength was 450 nm for detecting fucoxanthin (Jaswir et al. 2013b; Mori et al. 2004; Nomura et al. 2013; Terasaki et al. 2009). A calibration curve prepared using authentic standard was used for quantification. The standard fucoxanthin (>95 % purity) was isolated from C. indica lipids as outlined below.
The method described by Haugan et al. (1992) with slight modification was adopted for the extraction and purification of fucoxanthin. The ground dry algae were extracted several times with cold acetone–methanol (7:3 v/v) on ice bath and left standing at room temperature in darkness under N2. The extract was evaporated to dryness at 30 to 35 °C on a rotary evaporator, and the residue was dissolved in methanol. The residue was partitioned in a separator funnel between n-hexane and 90 % aqueous methanol (v/v). The n-hexane phase was discarded, and the aqueous hypophase was transferred to diethyl ether. The extract is evaporated to dryness on a Rotavapor, and the pigments were dissolved in benzene and applied to a silica gel (Kiesel gel 60, Merck, Germany) column. Elution was initially with n-hexane (100 %), and fucoxanthin was recovered in the n-hexane/acetone fraction (6:4 v/v). Column chromatography was repeated two or three times. The residue from the hexane/acetone (6:4 v/v) evaporation step was re-dissolved in methanol. The concentration of fucoxanthin was checked by HPLC (1 mL min−1 at 450 nm, methanol, and acetonitrile were the mobile phase). Purified fucoxanthin was determined by Fourier transform infrared spectroscopy (FT-IR). The characteristic wave number of specific functional group identified in purified sample was: OH group (3400 cm−1), C–H stretch (3005–2850 cm−1), allene (1924 cm−1), C═O acetate (1732 cm−1), conjugated C═O (1645 cm−1), CH2 stretch (1605–1455 cm−1), geminal methyl (1380–1360 cm−1), C–O acetate (1338, 1258, and 1249 cm−1), and trans-distributed –C═C– (1210–963 cm−1) which agreed with the previous reported data (Haugan et al. 1992; Rajauria and Abu-Ghannam 2013).
Fatty acid analysis of total lipids
Fatty acid methyl esters were prepared as per the method of Prevot and Mordret (1976). Briefly, 1 mL n-hexane and 0.2 mL 2 N sodium hydroxide in methanol were added to an aliquot of total lipid (20 mg), vortex for 10 s, and incubated at 50 °C for 30 min. After incubation, 0.2 mL of 2 N HCl in methanol was added to the solution and vortex for 60 s to obtain the upper n-hexane layer containing the fatty acid methyl esters (FAMEs). The fatty acid analysis was by gas chromatography (GC) (Shimadzu) using a flame ionization detector (FID) and a capillary column (BPX-70 25 M × 0.22 MM 0.25 μM). The carrier gas was nitrogen at a flow rate of 1.3 mL min−1. The detector, injector, and column temperatures were 280, 250, and 25 °C, respectively.
Antioxidant extraction and analysis
Because different components in the crude extracts of seaweeds, antioxidant activities depend on different reactions and mechanisms. Therefore, in this study, three methods were employed to evaluate the total antioxidant activity. All antioxidant extractions were carried out away from direct sunlight to reduce the possibility of oxidation. Methanol (15 mL) was added to the sample (300 mg) and homogenized for 1 h at room temperature. The methanol extract was evaporated to dryness at 30 °C using a rotary evaporator, and the residue was dissolved in methanol (20 mL).
Analysis of antioxidant activity
DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging assay
The scavenging effects of methanol extract were determined using the method of (Duan et al. 2006). Briefly, 2.0 mL of 0.16 mM DPPH solution (in methanol) was added to a test tube containing 2.0 mL of sample and incubated for 30 min in the dark at room temperature. The absorbance of samples was measured at 517 nm and using methanol as the blank. The scavenging effect (%) was calculated as:
where Asample is the absorbance of the sample with treatment, Asample blank is the absorbance of sample only without DPPH, and Acontrol is the absorbance of methanol with the treatment.
Copper-reducing antioxidant capacity (CUPRAC)
The CUPRAC reagent was prepared according to the method of Apak et al. (2004). Briefly, 1 mL sample and 0.1 mL distilled water were added to a test tube containing 1 mL of copper (II) chloride solution (10−2 M), neocuproine (Nc) solution (7.5 × 10−3 M), and ammonium acetate (NH4Ac) buffer at pH 7.0 to make up a final volume of 4.1 mL. After shaking, the test tube was left in the dark for 30 min and the absorbance measured at 450 nm using distilled water as the blank.
Total phenolic content
Distilled water (50 mL) was added to dried samples (2 g) and autoclaved at 121 °C for 15 min. The extract was centrifuged (2220×g for 10 min) and filtered. The total phenolic content of algal extracts, prepared as above, was determined according to the method of Chaovanalikit and Wrolstad (2004). To 0.5 mL aliquot of algal extract, 0.5 mL of Folin-Ciocalteu reagent and 7.5 mL of distilled water were added. After 10 min, 1.5 mL of 20 % Na2CO3 solution was added. The sample was thoroughly mixed and placed at room temperature for 1 h. Absorbance was measured at 755 nm, and gallic acid was used for the preparation of the standard curve.
Results
Figure 1 shows photographs of N. zanardinii and C. indica. Observations of the algae at different seasons showed that N. zanardinii has two peaks of productivity over the period late October to late November and late March to late April when the plants were tallest. On the other hand, C. indica only has one peak during late October to late November. In the late September, juvenile algae are dominant while in late October and November, adult spore-bearing plants are present and with sporulation begin to reproduce. With the warm weather in July, C. indica was not found and the density of N. zanardinii was low.
Table 1 shows the changes in total lipid (TL) and fucoxanthin (Fx) contents (mg g−1 DW) of C. indica and N. zanardinii. TL content of N. zanardinii in February is significantly (p < 0.05) higher than in June (p < 0.05), and in C. indica, the highest TL content was in December (86.1 ± 6.50 mg g−1 DW) and the lowest in February (69.12 ± 5.20 mg g−1 DW) respectively. The content of Fx in C. indica in all seasons was higher than in N. zanardinii (p < 0.05). The highest value of Fx in N. zanardinii was recorded in June (1.65 ± 0.17 mg g−1 DW), and in C. indica, it was recorded in December (3.56 ± 0.20 mg g−1 DW).
In the TL of C. indica and N. zanardinii, the major fatty acids were myristic (14:0), palmitic (16:0), oleic (18:1n-9), SDA (18:4n-3), AA (20:4n-6), and EPA (20:5n-3) (Tables 2 and 3). Among them, 16:0 was found in all the samples at highest concentration, followed by oleic acid, AA, SDA, myristic acid, and EPA. Variations of total saturated fatty acids (SFAs), total monounsaturated fatty acids (MUFA), total n-3 PUFAs, and total n-6 PUFAs in two studied species in 4 months are shown in Tables 2 and 3. The percentage of total PUFA in N. zanardinii (25.95 %) in February and in C. indica (37.91 %) in April was significantly higher than other seasons (p < 0.05). The higher level of PUFAs in April was more in the TL of C. indica (Tables 2 and 3).
The results of DPPH radical scavenging activities are shown in Table 4. The highest and lowest scavenging activity for N. zanardinii was in June (96.93 ± 1.41 %) and the lowest (85.7 ± 1.63 %) in October; for C. indica, the highest was in December and April (89.23 ± 1.81 %) and the lowest (73.96 ± 3.02 %) in April.
C. indica had a higher CUPRAC than N. zanardinii in the methanolic extracts (Table 4). The CUPRAC assay showed that N. zanardinii has high antioxidant activity in June (1.08 ± 0.11) and C. indica in December (1.26 ± 0.06).
Phenolic content in N. zanardinii was high in April (Table 4) (p < 0.05), with a value of 1.52 ± 0.06 mg g−1 DW), but in C. indica, it was lower than in other months (0.86 ± 0.03 mg g−1 DW). In both algae, phenolic content was low in February. In general, phenolic content of N. zanardinii was higher than in C. indica in all months (Table 4).
In the 5 months of data from N. zanardinii and C. indica in Tables 3 and 4, fucoxanthin showed a closer relation with DPPH scavenging activity than with the CUPRAC assay results and total phenolic content; correlation coefficients were 0.81 and 0.918 respectively for the two studies (Table 5).
Discussion
The lipid contents of seaweed ranged from 1 to 5 % of dry matter and varies strongly according to the species (Pise and Sabale 2010; Terasaki et al. 2009; van Ginneken et al. 2011), geographical location (Nomura et al. 2013), and season (Gerasimenko et al. 2010; Nomura et al. 2013; Terasaki et al. 2009). Tropical seaweed species have significantly lower total lipid than those from cold regions (Narayan et al. 2004). Lipid analysis of the Sargassaceae, from the subarctic zone (Terasaki et al. 2009), showed higher total lipid in contrast with tropical zone (Narayan et al. 2004; Rohani-Ghadikolaei et al. 2012). However, many researchers have reported high level of total lipids in brown algae from tropical areas (Gosch et al. 2012; McDermid and Stuercke 2003; Thinakaran et al. 2012). High levels of total lipid in the present study with seasonal change also were observed (3.1 to 7.9 % of dry weight for N. zanardinii and 7.2 to 8.6 % of dry weight in C .indica). This variation in C. indica showed less change because of the disappearance of the algae and the lack of sample for analysis.
It is documented that, in temperate regions, lipid formation directly changes with season and increases in the winter. Total lipid content of brown algae increases from winter to spring in temperate and subarctic zones of the North Pacific coinciding with seedling growth (Honya et al. 1994; Nelson et al. 2002; Gerasimenko et al. 2011). In the present study, the total lipid increased especially in October and December when seedlings and juvenile algae are predominant (Table 1).
Fucoxanthin is a major carotenoid present in brown seaweeds and, along with chlorophylls and β-carotene, plays an important role in light harvesting, photoprotection during excess light, and upregulation of photosynthesis (D’Orazio et al. 2012; McKew et al. 2013). The different fucoxanthin contents in different seasons are mainly due to light exposure and temperature fluctuations (Nomura et al. 2013). According to the results in Table 1, fucoxanthin content in N. zanardinii and C. indica is significantly higher than other species from tropical areas (Nomura et al. 2013; Lann et al. 2012; Jaswir et al. 2013a; Fung et al. 2013; Terasaki et al. 2009). It is likely that fucoxanthin in the studied species not only has a light harvesting role but also acts as a strong photoprotectant. In these algae, high light exposure with high oxygen concentration probably leads to formation of free radicals and other strong oxidative stress. Therefore, these conditions lead to the algae producing essential antioxidant compounds, especially in this tropical area, to protect them against oxidative stress such as UV radiation. There are many studies on the antioxidant properties of fucoxanthin against oxidative stress caused by UV-B radiation, scavenging of free radicals, and also quenching effect of singlet oxygen (Heo and Jeon 2009; Sudhakar et al. 2013; Nomura et al. 1997; Sachindra et al. 2007).
Sampling areas in the present study were rocky shores and tidal pools with high waves where organisms are exposed to many factors including sediment scouring, strong wave action, nutrient loading, herbivores, heat and desiccation, and high radiation exposure in some seasons. Organisms living in the mid- and high-shore areas of tropical rocky shores experience a greater degree of heat and desiccation in comparison with temperate shores (Macusi and Deepananda 2013). The higher content of fucoxanthin in June for N. zanardinii (1.65 ± 0.17 mg g−1 DW) can be due to the presence of strong wave action of the southwest monsoon of the Indian Ocean (SWM) and, consequently, creation of reactive oxygen species and thermal and light stress.
The major fatty acids of the total lipids of N. zanardinii and C. indica were myristic acid, palmitic acid, oleic acid, C18, and C20 PUFA as in other brown seaweeds (Dawczynski et al. 2007; Hofmann and Eichenberger 1997; Khotimchenko 1998; Kumari et al. 2010; Li et al. 2002; Sánchez-Machado et al. 2004) (Tables 3 and 4). The fatty acid composition of seaweeds is sensitive to temperature (Khotimchenko 1998; Nomura et al. 2013; Sewón et al. 1997). A decrease in temperature usually results in an increase of polyunsaturated fatty acid (PUFA) and a decrease of saturated fatty acid (SFA) (Nomura et al. 2013). A sma increase in PUFA in the cold season in N. zanardinii was observed (Table 3), but a remarkable point in this study is the higher amount of PUFA in C. indica than in N. zanardinii, especially in April (37.91 %). However, Kumari et al. (2010) reported that the amount of PUFA in C. indica is 47.5 ± 2.71 % which is comparable with temperate area species. This difference may be due to temperature differences at the different locations.
In addition, nutritionists and nutrition standards suggest a ratio of omega-6 to omega-3 essential fatty acids of 1:1.15 to 1:2 (Hamazaki and Okuyama 2003), whereas in the western diet this ratio is between 15:1 and 16.7:1 (Simopoulos 2002). In this study, the n-6:n-3 ratio for N. zanardinii is between 2.01:1 and 1.14:1, which indicates an acceptable level for use as an additive in the human diet for balancing the ratio of omega 3 and omega 6 fatty acids.
In both species, we observed a slight monthly variation of total phenolic content but with a different pattern. Seasonal phenolic contents measured in this study are in good agreement with previous studies, with maximum levels accruing in different seasons (Kamiya et al. 2010; Mannino et al. 2013; Plouguerné et al. 2006; Steinberg 1995). Total phenolic contents are generally affected by different abiotic factors such as temperature, irradiance levels, nutrient availability and salinity, or biotic factors such as grazing pressure and the reproductive state of the algae (Airanthi et al. 2011; Kamiya et al. 2010; Mannino et al. 2013; Steinberg 1995; Stiger et al. 2004). Algae past their peak of maturity and fertility show higher phenolic content (Steinberg 1989; Stiger et al. 2004). According to our results, both algal species displayed higher phenolic content over these peaks when the plant was tallest (October to December for both species and April for N. zanardinii). In this study, N. zanardinii had two peaks of maturity and fertility which is agreement with a previous study for N. zanardinii at the Socotra Archipelago off Yemen (Kemp 1998). Pavia et al (1997) reported that increased UV-B radiation led to an increase in phlorotannins in Ascophyllum nodosum. Furthermore, in plant tissue, phenolic compound synthesis increases with increase of water temperature (Aquino-Bolaños and Mercado-Silva 2004). Ragan and Jensen (1978) reported that in Fucus vesiculosus, there is a high correlation between surface water temperature and phenolic content. Moreover, phenolic content in nutrient-rich waters is increased (Arnold et al. 1995; Stiger et al. 2004). In the present study, the higher amount of phenolic content is in June, possibly due to increased water temperature and increased nutrients caused by the summer monsoon and the existence of high waves in this area.
The major active compounds in brown algae extract detected with DPPH radical scavenging assay is polyphenol and fucoxanthin (Airanthi et al. 2011; Ganesan et al. 2008). There is a synergic relationship between antioxidant activity of brown seaweeds and the combination of sucoxanthin and phenolic content (Airanthi et al. 2011; Fung et al. 2013). In this study, a relationship between fucoxanthin content and DPPH radical scavenging activity was evident with correlation coefficient of (r 2 = 0.81) for N. zanardinii and (r 2 = 0.91) for C. indica. In CUPRAC assay, the correlation with fucoxanthin was reasonable in C. indica (r 2 = 0.8). Similarly, the correlation of fucoxanthin content of Undaria pinnatifida with CUPRAC assay gave a correlation coefficient of 0.752 (Fung et al. 2013), indicating that our results are comparable with previous reports for brown algae.
Phlorotannins, the largest group of phloroglucinol-based polyphenols that are accumulated only in marine brown algae, suggested that they were the most likely active component responsible for the main antioxidant activity in brown seaweeds (Li et al. 2011). They exist in various types of different molecular size and degree of polymerization, and this may influence the antioxidant activity (Wang et al. 2012). In this study, one of the reasons for different antioxidant levels in different months between DPPH radical scavenging, CUPRAC, and Folin assays could be due to the structural variations in the phenolic compounds and the selectivity of each assay. The Folin assay measures only hydrophilic antioxidants, and DPPH radical scavenging is a solvent dependent assay, whereas the CUPRAC assay is more selective and able to simultaneously assay lipophilic and hydrophilic antioxidants (Apak et al. 2008).
CUPRAC assay values are comparable to ABTS/TEAC and Folin assay values for polyphenols, but simple sugars and citric acid are not oxidized in CUPRAC (Badarinath et al. 2010; Prior et al. 2005). Çelik et al (2008) reported that the correlation between total phenolic content and CUPRAC assay in herbal tea gave a correlation coefficient of 0.966, showing that total phenolic content and CUPRAC correlated well in herbal plant extracts. The negative correlation between TPC and CUPRAC requires further investigation to better understand this variation apart from the fucoxanthin and polyphenol contents in brown algae.
Our present study is the first report on the isolation, purification, identification, and seasonal change of bioactive compounds, fucoxanthin, fatty acids, and also the antioxidant potential of the tropical marine brown algae, N. zanardinii and C. indica, with significant pharmaceutical, medicinal, cosmetic, nutraceutical, food, and agricultural importance from the Chabahar coast of Iran.
In a general, brown algae have important bioactive compounds such as fucoxanthin, SDA, omega 3 EPA, and omega 6 ARA. Among these compounds, fucoxanthin is the key for better functional understanding of brown algal lipid features. This study has also demonstrated that fluctuation in the fatty acid and fucoxanthin contents cannot be attributed to specific environmental changes.
References
Abid M, Zaki MJ, Khan MQ, Sattar A (2005) Use of marine algae for the management of root knot nematode (Meloidogyne javanica) in okra and tomato plants. Int J Phycol Phycochem 1:187–192
Abkenar AM, Payghambari SY, Sadjadi MM, Abidi MR (2008) Comparison of extracted alginic acid in brown algaes; Sargassum illicifolium, Cystoseira indica and Nizimuddinia zanardini in the Oman sea; Chabahar. Iranian J Paj Saz 3(21):49–54 (In Persian)
Airanthi M, Hosokawa M, Miyashita K (2011) Comparative antioxidant activity of edible Japanese brown seaweeds. J Food Sci 76:104–111
AOAC (1997) Official methods of analysis, 16th edn. Association of Official Analytical Chemists, AOAC International, Arlington, VA, USA
Apak R, Güçlü K, Özyürek M, Karademir SE (2004) Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J Agr Food Chem 52:7970–7981
Apak R, Güçlü K, Özyürek M, Bektas B, Bener M (2008) Cupric ion reducing antioxidant capacity assay for food antioxidants: vitamins, polyphenolics, and flavonoids in food extracts. Methods Mol Biol 477:163–93
Aquino-Bolaños EN, Mercado-Silva E (2004) Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol Technol 33:275–283
Arnold TM, Tanner CE, Hatch WI (1995) Phenotypic variation in polyphenolic content of the tropical brown alga Lobophora variegata as a function of nitrogen availability. Mar Ecol Prog Ser 123:177–183
Badarinath A, Rao KM, Chetty CMS, Ramkanth S, Rajan T, Gnanaprakash K (2010) A review on in-vitro antioxidant methods: comparisons, correlations and considerations. Int J PharmTech Res 2:1276–1285
Çelik SE, Özyürek M, Altun M, Bektaşoğlu B, Güçlü K, Berker KI, Özgökçe F, Apak’ R (2008) Antioxidant capacities of herbal plants used in the manufacture of Van Herby cheese:‘Otlu peynir’. Int J Food Prop 11:747–761
Chaovanalikit A, Wrolstad R (2004) Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J Food Sci 69:67–72
D’Orazio N, Gemello E, Gammone MA, de Girolamo M, Ficoneri C, Riccioni G (2012) Fucoxanthin: a treasure from the sea. Mar Drugs 10:604–616
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103:891–899
Dembitsky VM, Maoka T (2007) Allenic and cumulenic lipids. Prog Lipid Res 46:328–375
Duan XJ, Zhang WW, Li XM, Wang BG (2006) Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem 95:37–43
Farahpour M, Abkenar AM, Notash GR (2010) Alga cultural survey on three species: Sargassum ilicifolium, Hypnea musciformis and Cystoseira indica in Chabahar seashore, Iran. Iran J Med Aromat Plants 3:296–304 (in Persian)
Fung A, Hamid N, Lu J (2013) Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem 136:1055–1062
Ganesan P, Kumar CS, Bhaskar N (2008) Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol 99:2717–2723
Gerasimenko N, Busarova N, Moiseenko O (2010) Seasonal changes in the content of lipids, fatty acids, and pigments in brown alga Costaria costata. Rus Plant Physiol 57:205–211
Gerasimenko N, Skriptsova A, Busarova N, Moiseenko O (2011) Effects of the season and growth stage on the contents of lipids and photosynthetic pigments in brown alga Undaria pinnatifida. Russ J Plant Physiol 58:885–891
Gharanjik BM (2005) Persian Gulf and Oman Sea Algae Atlas. Iranian Fisheries Research Institute, Tehran
Gosch BJ, Magnusson M, Paul NA, Nys R (2012) Total lipid and fatty acid composition of seaweeds for the selection of species for oil‐based biofuel and bioproducts. GCB Bioenergy 4:919–930
Hamazaki T, Okuyama H (2003) The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev Nutr Diet 92:109–132
Haugan JA, Liaaen-Jensen S (1994) Algal carotenoids 54. Carotenoids of brown algae (Phaeophyceae). Biochem Syst Ecol 22:31–41
Haugan A, Aakermann T, Liaaen-Jensen S (1992) Isolation of fucoxanthin and peridinin. Meth Enzymol 213:231–245
Heo SJ, Jeon YJ (2009) Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J Photochem Photobiol B 95:101–107
Hofmann M, Eichenberger W (1997) Lipid and fatty acid composition of the marine brown alga Dictyopteris membranacea. Plant Cell Physiol 38:1046–1052
Honya M, Kinoshita T, Ishikawa M, Mori H, Nisizawa K (1994) Seasonal variation in the lipid content of cultured Laminaria japonica: fatty acids, sterols, β-carotene and tocopherol. J Appl Phycol 6:25–29
Hosokawa M, Kudo M, Maeda H, Kohno H, Tanaka T, Miyashita K (2004) Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta 1675:113–119
Hosokawa M, Miyashita T, Nishikawa S, Emi S, Tsukui T, Beppu F, Okada T, Miyashita K (2010) Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK- Ay mice. Arch Biochem Biophys 504:17–25
Jaswir I, Noviendri D, Salleh HM, Taher M, Miyashita K (2013a) Isolation of fucoxanthin and fatty acids analysis of Padina australis and cytotoxic effect of fucoxanthin on human lung cancer (H1299) cell lines. Afr J Biotechnol 10:18855–18862
Jaswir I, Noviendri D, Salleh HM, Taher M, Miyashita K, Ramli N (2013b) Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J Liq Chrom Rel Technol 36:1340–1354
Kamiya M, Nishio T, Yokoyama A, Yatsuya K, Nishigaki T, Yoshikawa S, Ohki K (2010) Seasonal variation of phlorotannin in sargassacean species from the coast of the Sea of Japan. Phycol Res 58:53–61
Kemp JM (1998) The occurrence of Nizamuddinia zanardinii (Schiffner) RC.Silva (Phaeophyta :Fucales) at the Socotra archipelago. Bot Mar 41:345–348
Khotimchenko SV (1998) Fatty acids of brown algae from the Russian Far East. Phytochem 49:2363–2369
Kotake-Nara E, Kushiro M, Zhang H, Sugawara T, Miyashita K, Nagao A (2001) Carotenoids affect proliferation of human prostate cancer cells. J Nutr 131:3303–3306
Kotake-Nara E, Asai A, Nagao A (2005) Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett 220:75–84
Kumari P, Kumar M, Gupta V, Reddy C, Jha B (2010) Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem 120:749–757
Lann KL, Ferret C, VanMee E, Spagnol C, Lhuillery M, Payri C, Stiger‐Pouvreau V (2012) Total phenolic, size‐fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacific Ocean: spatial and specific variability. Phycol Res 60:37–50
Li X, Fan X, Han L, Lou Q (2002) Fatty acids of some algae from the Bohai Sea. Phytochemistry 59:157–161
Li YX, Wijesekara I, Li Y, Kim SK (2011) Phlorotannins as bioactive agents from brown algae. Process Biochem 46:2219–2224
Macusi ED, Deepananda KA (2013) Factors that structure algal communities in tropical rocky shores: what have we learned? Int J Sci Res Publ 3:490, ISSN 2250-3153
Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K (2006) Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int J Mol Med 18:147–152
Mannino A, Vaglica V, Oddo E (2013) Seasonal variation in total phenolic content of Dictyopteris polypodioides (Dictyotaceae) and Cystoseira amentacea (Sargassaceae) from the Sicilian coast. Fl Med 24:39–50
McDermid KJ, Stuercke B (2003) Nutritional composition of edible Hawaiian seaweeds. J Appl Phycol 15:513–524
McKew BA, Davey P, Finch SJ, Hopkins J, Lefebvre SC, Metodiev MV, Oxborough K, Raines CA, Lawson T, Geider RJ (2013) The trade‐off between the light‐harvesting and photoprotective functions of fucoxanthin‐chlorophyll proteins dominates light acclimation in Emiliania huxleyi (clone CCMP 1516). New Phytol 200:74–85
Miyashita K, Narayan B, Tsukui T, Kamogawa H, Abe M, Hosokawa M (2011) Brown seaweed lipids as potential source of omega‐3 PUFA in biological systems. In: Kim S-K (ed) Handbook of marine macroalgae: biotechnology and applied phycology. Wiley, Chichester, pp 329–339
Moghadam MH, Firouzi J, Saeidnia S, Hajimehdipoor H, Jamili S, Rustaiyan A, Gohari AR (2013) A cytotoxic hydroperoxy sterol from the brown alga, Nizamuddinia zanardinii. Daru 21(1):24
Mori K, Ooi T, Hiraoka M, Oka N, Hamada H, Tamura M, Kusumi T (2004) Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar Drugs 2:63–72
Narayan B, Miyashita K, Hosakawa M (2004) Comparative evaluation of fatty acid composition of different Sargassum (Fucales, Phaeophyta) species harvested from temperate and tropical waters. J Aquat Food Prod 13:53–70
Nelson M, Phleger C, Nichols P (2002) Seasonal lipid composition in macroalgae of the northeastern Pacific Ocean. Bot Mar 45:58–65
Nomura T, Kikuchi M, Kubodera A, Kawakami Y (1997) Proton‐donative antioxidant activity of fucoxanthin with 1, 1‐diphenyl‐2‐picrylhydrazyl (DPPH). Biochem Mol Biol Int 42:361–370
Nomura M, Kamogawa H, Susanto E, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K (2013) Seasonal variations of total lipids, fatty acid composition, and fucoxanthin contents of Sargassum horneri (Turner) and Cystoseira hakodatensis (Yendo) from the northern seashore of Japan. J Appl Phycol 25:1159–1169
Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B radiation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Ser 157:139–146
Pise N, Sabale A (2010) Biochemical composition of seaweeds along central west coast of India. Pharmacog J 2:148–150
Plouguerné E, Le Lann K, Connan S, Jechoux G, Deslandes E, Stiger-Pouvreau V (2006) Spatial and seasonal variation in density, reproductive status, length and phenolic content of the invasive brown macroalga Sargassum muticum (Yendo) Fensholt along the coast of Western Brittany (France). Aquat Bot 85:337–344
Prevot AF, Mordret FX (1976) Utilisation des colonnescapillairesde verre pour l’analyse des corps gras par chromotographie en phase gazeuse. Revue Francaise des Corps Gras 23:409–423
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Ragan MA, Jensen A (1978) Quantitative studies on brown algal phenols. II. Seasonal variation in polyphenol content of Ascophyllum nodosum (L.) Le Jol. and Fucus vesiculosus (L.). J Exp Mar Biol Ecol 34:245–258
Rajauria G, Abu-Ghannam N (2013) Isolation and partial characterization of bioactive fucoxanthin from Himanthalia elongata brown seaweed: a TLC-based approach. Int J Anal Chem. doi:10.1155/2013/802573
Rohani-Ghadikolaei K, Abdulalian E, Ng WK (2012) Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian Gulf of Iran as potential food and feed resources. J Food Sci Tech 49:774–780
Sachindra NM, Sato E, Maeda H, Hosokawa M, Niwano Y, Kohno M, Miyashita K (2007) Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J Agric Food Chem 55:8516–8522
Sahoo D, Nivedita, Debasish (2001) Seaweeds of Indian coast. A.P.H. Publishing, New Delhi, p 283, xxi +3
Sánchez-Machado D, López-Cervantes J, López-Hernández J, Paseiro-Losada P (2004) Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem 85:439–444
Sewón P, Mikola H, Lehtinen T, Kallio P (1997) Polar lipids and net photosynthesis potential of subarctic Diapensia lapponica. Phytochemistry 46:1339–1347
Silva PC, Basson PW, Moe RL (1996) Catalogue of the benthic marine algae of the Indian Ocean. University of California Press
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Steinberg PD (1989) Biogeographical variation in brown algal polyphenolics and other secondary metabolites: comparison between temperate Australasia and North America. Oecologia 78:373–382
Steinberg PD (1995) Seasonal variation in the relationship between growth rate and phlorotannin production in the kelp Ecklonia radiata. Oecologia 102:169–173
Stiger V, Deslandes E, Payri CE (2004) Phenolic contents of two brown algae, Turbinaria ornata and Sargassum mangarevense on Tahiti (French Polynesia): interspecific, ontogenic and spatio-temporal variations. Bot Mar 47:402–409
Sudhakar MP, Ananthalakshmi JS, Nair BB (2013) Extraction, purification and study on antioxidant properties of fucoxanthin from brown seaweeds. J Chem Pharm Res 5:169–175
Tabarsa M, Rezaei M, Ramezanpour Z, Waaland JR, Rabiei R (2012) Fatty acids, amino acids, mineral contents, and proximate composition of some brown seaweeds. J Phycol 48:285–292
Terasaki M, Hirose A, Narayan B, Baba Y, Kawagoe C, Yasui H, Sag N, Hosokawa M, Miyashita K (2009) Evaluation of recoverable functional lipid components with special reference to fucoxanthin and fucosterol contents of several brown seaweeds of Japan. J Phycol 45:974–980
Thinakaran T, Balamurugan M, Sivakumar K (2012) Screening of phycochemical constituents qualitatively and quantitatively certain seaweeds from Gulf of Mannar biosphere reserve. http://agris.fao.org/aos/records/AV2012094453
van Ginneken VJ, Helsper J, de Visser W, van Keulen H, Brandenburg WA (2011) Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis 10:104
Wang T, Jónsdóttir R, Liu H, Gu L, Kristinsson HG, Raghavan S, Ólafsdóttir GN (2012) Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J Agric Food Chem 60:5874–5883
Wynne MJ, Jupp BP (1998) The benthic marine algal flora of the Sultanate of Oman: new records. Bot Mar 41:7–14
Zubia M, Robledo D, Freile-Pelegrin Y (2007) Antioxidant activities in tropical marine macroalgae from the Yucatan Peninsula, Mexico. J Appl Phycol 19:449–458
Acknowledgments
This research is from the MSc thesis of Salim Jangizehi Shastan at the Chabahar Maritime University (CMU). We thank CMU for the financial support. The authors also thank the Chabahar free zone (CFZ) Quality control laboratory and also the Fishery Research Center of Chabahar for their technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fariman, G.A., Shastan, S.J. & Zahedi, M.M. Seasonal variation of total lipid, fatty acids, fucoxanthin content, and antioxidant properties of two tropical brown algae (Nizamuddinia zanardinii and Cystoseira indica) from Iran. J Appl Phycol 28, 1323–1331 (2016). https://doi.org/10.1007/s10811-015-0645-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-015-0645-y