Abstract
Sponges have been experimentally farmed for over 100 years, with early attempts done in the sea to supply “bath sponges”. During the last 20 years, sponges have also been experimentally cultured both in the sea and in tanks on land for their biologically active metabolites, some of which have pharmaceutical potential. Sea-based farming studies have focused on developing good farming structures and identifying the optimal environmental conditions that promote production of bath sponges or bioactive metabolites. The ideal farming structure will vary between species and regions, but will generally involve threading sponges on rope or placing them inside mesh. For land-based sponge culture, most research has focused on determining the feeding requirements that promote growth. Many sea- and land-based studies have shown that sponges grow quickly, often doubling in size every few months. Other favorable results and interesting developments include partially harvesting farmed sponges to increase biomass yields, seeding sexually reproduced larvae on farming structures, using sponge farms as large biofilters to control microbial populations, and manipulating culture conditions to promote metabolite biosynthesis. Even though some results are promising, land-based culture needs further research and is not likely to be commercially feasible in the near future. Sea-based culture still holds great promise, with several small-scale farming operations producing bath sponges or metabolites. The greatest potential for commercial bath sponge culture is probably for underdeveloped coastal communities, where it can provide an alternative and environmentally friendly source of income.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sponges have lived in our oceans for at least 580 million years, and over 8,000 species exist today (Bergquist 1978; Van Soest et al. 2008). They are found in marine and freshwater habitats and can be dominant in the benthic community in terms of biomass and abundance. Sponges interact with the wider community in a variety of important relationships, from competing for space with sessile organisms to filtering small suspended particulate matter and transferring energy from the pelagic to the benthic zone. Lacking true tissues, a sponge individual is a collection of cell types covered by pinacoderm and supported by an endoskeleton consisting of spicules and/or spongin fibers (Bergquist 1978).
Sponge species that possess only spongin fibers may have commercial value as “bath sponges”. With cosmetic, bath, or industrial use, the value of a bath sponge depends on the quality of its spongin skeleton, with soft, durable, and elastic fibers demanding highest price. The general morphology of commercial bath sponge species ranges from spherical to vase like (Pronzato and Manconi 2008), with spherical-shaped sponges most desired and thus most commonly studied. Our use of bath sponges dates back thousands of years, with early Egyptians and Creeks free diving to collect sponges for bathing and cleaning (Storr 1957). During the last 200 years, sponge fishing spread from the Mediterranean Sea to tropical regions of the western Atlantic and Pacific Oceans. Historically, sponge fishing was an important and lucrative industry. From 1913 to 1938, yearly landings and value of bath sponges collected in Florida regularly exceeded 400,000 pounds and US$1 million (Storr 1964). In Libya, bath sponge production peaked in the 1920s, averaging 70 tons each year, but less than 10 tons a year is now harvested (Milanese et al. 2008). Overfishing and periodic disease outbreaks have decimated many natural populations of bath sponges, and global demand today far exceeds supply. In 2003, for example, global trade in bath sponges was 2,127 metric tons but global production from harvesting was 55 metric tons (FAO 2004).
Disease outbreaks on bath sponges are often severe, destroying both wild populations (Lauckner 1980) and experimental farms (Smith 1941). Disease outbreaks have been recorded for over 100 years (Pronzato 1999) and appear to be increasing in frequency possibly due to increased environmental stress (Webster 2007). The causative agent of sponge disease is often difficult to positively identify (Webster 2007), but includes viruses, fungi, cyanobacteria, and bacterial strains (Gaino and Pronzato 1989; Webster 2007). Sponge disease can destroy tissue and weaken fibers, resulting in a worthless and unmarketable bath sponge (Pronzato 1999).
Recently, a new commercialization of sponge products has begun. Sponges and their symbiotic microorganisms biosynthesize a wide range of biologically active metabolites that aid in growth and survival, such as preventing predation, deterring spatial competitors, and fighting microbial infection. Thousands of sponge-derived bioactive metabolites have been isolated and identified so far, and some have pharmaceutical potential as anticancer, antiviral, and antiinflammatory drugs or as biomedical tools (Blunt et al. 2009). Many of these bioactive metabolites are found at trace amounts within a sponge, often at concentrations of milligrams of the target metabolite per kilogram of sponge biomass (Schmitz et al. 1993). Harvesting wild populations to supply sufficient quantities is not therefore economically or environmentally feasible, except for the early phases of drug development. Sponge aquaculture is one possible method that could supply sufficient and sustainable quantities of sponge metabolites for drug development and manufacture.
Since about 1990, numerous studies have explored the potential of farming sponges to supply bioactive metabolites. This research is roughly equally divided between in situ or sea-based culture, where sponges are farmed in the sea, and in vitro or land-based culture, where sponges are farmed in tanks on land. Regardless of culture location, the research goals have been to develop farming protocols that promote sponge growth, survival, and biosynthesis (production) of the target metabolite. Many studies have also examined farming bath sponges, with all research being sea-based; the value of bath sponges is too low to justify the more costly land-based culture option. The main goal of this research has been to develop low-technology farming methods that produce large numbers of good quality bath sponges. Cell culture via cell suspension or aggregates (primmorphs) was reviewed recently by Müller et al. (2004) and Pomponi (2006) and will not be discussed here.
Sponge aquaculture for spongin or metabolite production capitalizes on the high regenerative abilities of many sponge species (Ayling 1983; Duckworth 2003), where one individual is cut into many smaller pieces or explants that heal and regrow. This is comparable to asexual reproduction where storms or predation may break a sponge into several pieces, which then re-attach and grow. Because sponges have indeterminate growth, where growth and final size is determined mostly by environmental conditions and not genetics (Sebens 1987), explants can be cut to a standardized size and randomized to experimental treatments, thus eliminating any potential bias of initial size or age. For initial farm set-up, sponge explants will be sourced from natural populations. Once the farm is established and operating, new sponge explants will be produced from existing farmed sponges. To increase farm production and profitability, these broodstock sponges should have high growth and survival rates and contain good quality spongin or high levels of the target metabolite. This review will examine the research and commercial potential of sea- and land-based aquaculture to supply sponge metabolites or spongin (bath sponges).
Bath Sponge Aquaculture
Due to the collapse of bath sponge populations in the Mediterranean Sea and tropical eastern Atlantic Ocean in the eighteenth and nineteenth centuries, research into bath sponge aquaculture has a relatively long history in both regions. These early studies focused on developing a good farming method or structure to culture sponges. Various methods were trialed, ranging from attaching explants to wooden crates or concrete disks to suspending them in mid-water secured with threaded bamboo poles or insulating wire (Moore 1910; Crawshay 1939). Although experimental, some studies showed great promise. Bath sponge explants farmed by Crawshay (1939), for example, tripled in volume in 1 year. Commercial sponge farming never started, however, mostly because of opposition and sabotage from sponge fisherman concerned about the competition (Moore 1910) and disease outbreaks killing explants (Smith 1941).
During the last 20 years, bath sponge farming has generated renewed interest and is now seen as the only viable technique to satisfy global demand for bath sponges. These new farming studies have tested synthetic materials (e.g., nylon ropes), with explants generally farmed off-bottom and suspended in the water column. Some studies, however, have grown sponges on horizontal lines laid on or close to the bottom (e.g., Duckworth et al. 1997; Pronzato et al. 1999; de Voogd 2007; Louden et al. 2007), which is advantageous in locations where water depth is shallow such as in lagoons. Most commercial sponge farms will be situated in deeper water (>5 m), with sponge explants cultured throughout the water column. This allows for more sponges to be grown per site, increasing productivity. In addition, sponges generally grow quicker when farmed in the water column (Duckworth et al. 1997), probably because of greater water flow and food availability.
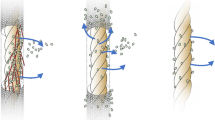
Two main farming methods have been trialed, with sponges grown either inside mesh or on threaded rope. Both methods have advantages and disadvantages. Survival is lower for some bath sponges species farmed on rope because of explant damage resulting from the threading process (Verdenal and Vacelet 1990; Duckworth et al. 2007; Duckworth and Wolff 2007). Other species in contrast survive well when cultured on rope (Pronzato et al. 1999; Fig. 1). Sponges farmed on threaded rope can also be ripped off during periods of strong water flow (Duckworth and Battershill 2003b) or grow away from the threaded rope forming a “donut” shape that is not marketable (Duckworth et al. 2007; Fig. 2). This donut growth pattern does not occur for all bath sponge species farmed on rope (Verdenal and Vacelet 1990; Pronzato et al. 1999). These differences in farming response are common even between sponge species grown in the same location and thus experiencing similar environmental conditions (Verdenal and Vacelet 1990; Pronzato et al. 1999; Duckworth and Battershill 2003b; Duckworth et al. 2007). Interspecific differences result from variation in regenerative ability, susceptibility to infection after cutting, growth potential, and general hardiness.
Although survival is higher for some sponge species farmed in mesh because damage is less, low growth rates may result from the mesh strands reducing water flow and thus food availability (Duckworth and Battershill 2003b). Biofouling of ascidians, bryozoans, and algae on the mesh can also reduce water flow to the farmed sponges. These problems are reduced or eliminated by using mesh consisting of thin strands and large holes and good site selection. Mesh structures that have successfully grown bath sponges include lanterns (Kelly et al. 2004) and pearl panels (Duckworth et al. 2007; Fig. 3). Some studies have also examined the potential of combining both methods by using a “nursery period” (Duckworth et al. 2007; Duckworth and Wolff 2007), with sponges initially farmed in mesh until they have healed all cut surfaces and regenerated an intact aquiferous system and then threading them on rope to promote their growth before harvesting. This farming strategy, however, is labor intensive and costly, and sponge growth and survival rates are no better than if farmed solely in mesh (Duckworth et al. 2007; Duckworth and Wolff 2007). It is therefore not a viable or worthwhile strategy. Bath sponges farmed in mesh, however, should be transplanted as they grow into structures with larger mesh to promote larger final size. During this process, small non-growing explants can be weeded out and sold.
The best farming method to commercially grow bath sponges will vary between sponge species and regions. The bath sponge Coscinoderma matthewsi (Lendenfeld 1886), for example, is farmed on threaded line in Micronesia (MacMillan 1996), but is best cultured in modified pearl panels in tropical Australia (Duckworth et al. 2007; Duckworth and Wolff 2007).
Commercial farming also requires an understanding or knowledge of the environmental conditions that maximize sponge growth and survival. Although the optimal environmental conditions will vary among species, there are several general trends. Sponges are active suspension feeders but rely greatly on the passive flow of water to provide bacteria and microalgae; thus, site selection is critical for commercial success. Most sponges grow quickest and reach its largest size in sites with high water movement or flow (Wilkinson and Vacelet 1979; Leichter and Witman 1997; Duckworth et al. 2004). A high flow rate promotes sponge growth directly through increased food availability, indirectly by breaking down the food-depleted boundary layer around the individual (Leichter and Witman 1997), or by increasing the internal flow through the aquiferous system (Vogel 1974). However, there is not a linear relationship between growth rates and flow rates. In areas of very strong water flow, such as at shallow depths exposed to storm surge, sponges have reduced growth (Duckworth et al. 1997, 2004) resulting from decreased feeding efficiency and energy diverted to strengthen skeletal structures (Palumbi 1984).
Culture depth also influences light intensity, which can affect farming response. Fouling from algae can be severe at shallow depths, which can reduce water flow, decreasing growth rates and final size (Duckworth et al. 1997). UV radiation, highest at shallow depths, can reduce sponge growth (Wilkinson and Vacelet 1979) and survival (Jokiel 1980). Most bath sponge species do not contain photosynthetic symbionts; thus, exposure to light is not critical for farming success. Generally, bath sponges will be commercially cultured at depths >5 m to reduce exposure to high levels of light intensity and wave shock.
The transplant and farming season can also greatly influence sponge growth and survival. Many tropical and temperate sponges survive best if transplanted when water temperate is relatively low (Wilkinson and Vacelet 1979; Duckworth et al. 1997, 2004; van Treeck et al. 2003). Cooler water promotes survival by lowering respiration (Cheshire et al. 1995), encourages pinacoderm healing (Duckworth et al. 1997), and decreases microbial growth (Hummel et al. 1988), which collectively reduces the chance of infection and stress during transplanting. Sponge growth, in contrast, is generally greatest when water temperature is rising or relatively high (Verdenal and Vacelet 1990; Duckworth and Battershill 2003b; Handley et al. 2003; Page et al. 2005), although there are exceptions (Duckworth et al. 1997). In temperate waters, seasonal patterns of food abundance likely cause the seasonal growth of farmed sponges. In tropical regions, where environmental conditions are more stable, the effect of any seasonal variation in food abundance on sponge growth is unknown. Reproductive cycles that would divert energy away from somatic growth may also contribute to temporal variation in sponge growth.
Farmed bath sponges can grow quickly, with several studies recording explants doubling or tripling in size in one year (Moore 1910; Crawshay 1939; Verdenal and Vacelet 1990; Corriero et al. 2004; Duckworth et al. 2007). Although these studies include both tropical and temperate sponges, tropical species generally grow faster. Crawshay (1939) and Verdenal and Vacelet (1990) have suggested that for bath sponge culture to be commercially viable, explants should double in size in 1 year.
One of the interesting results from many of these studies is that growth and survival can vary greatly between sponges cultured together and experiencing similar environmental conditions. Even explants from the same donor sponge can vary greatly in growth (Verdenal and Vacelet 1990). These differences could result from many factors, including initial variation in energy reserves, health, number of choanocyte (feeding) chambers, and explant size. For some sponges, percent growth and final survival is lowest for small explants (Duckworth et al. 1997; Duckworth and Wolff 2007). After cutting, small explants have a greater ratio of exposed surface area to volume and would need to invest comparatively more energy into regeneration, diverting energy away from somatic growth and increasing stress and mortality. For other species, small explants grow as quickly as larger explants (van Treeck et al. 2003; de Voogd 2007). Another consideration is that larger explants will likely grow to market size sooner. For each commercial sponge species, it is a trade-off between producing sufficient explants to transplant and farm, obtaining high growth and survival and producing large bath sponges to sell. Considering the importance of seasonal environmental variation on farming response, this optimum explant size may vary between seasons (van Treeck et al. 2003).
One exciting development in sponge culture that would eliminate some of these concerns involves culturing sponges from sexually produced larvae (Dubios 1914; de Caralt et al. 2007). First suggested 100 years ago (Moore 1910), it is most feasible for brooding sponge species. “Seeding” of larvae on farming structures would occur in tanks on land to maximize settlement. After a nursery period of several weeks, where juvenile sponges are fed, the farming structures plus sponges would be transplanted to the farm. Because this process would occur initially in tanks on land, it is essential to know the optimal environmental factors such as light intensity and feeding requirements, which promote larval settlement and subsequent juvenile survival and growth. These factors will be species specific.
Another exciting potential of sea-based culture is using sponge farms as “biofilters” (Müller et al. 1999; Pronzato et al. 1999; Milanese et al. 2003), either in integrated multi-trophic aquaculture to reduce environmental pollution from fish farms (Fig. 4) or as a final step in waste-water treatment plants. This capitalizes on the ability of sponges to filter large volumes of water with high retention rates for bacteria and other small microbes (Reiswig 1971; Pile et al. 1996; Duckworth et al. 2006). However, many sponges are susceptible to sediment smothering so farm location and distance from the pollution source is paramount (Verdenal and Vacelet 1990).
Although several temperate and tropical bath sponge species from various countries have been experimentally farmed with promising results, commercial farming is occurring only in Pohnpei with the sponge C. matthewsi (OEA 2004). Annual production is about 12,000 sponges, with most sold locally to residents or tourists (OEA 2004). These sponges are farmed on threaded line, with farming equipment and maintenance costing a few thousand dollars (MacMillan 1996) International demand for C. matthewsi is substantially greater, however. One British retail store requested 90,000 farmed bath sponges per year, but farming production could not supply sufficient numbers and the contract was terminated. New commercial bath sponge farms will soon start in two tropical Australian locations: Torres Strait, culturing C. matthewsi, and Palm Islands, farming C. matthewsi and Rhopaloeides odorabile (Thompson et al. 1987). Both Australian farms will be producing 100,000+ bath sponges each year. The commercial enterprises in Pohnpei, Torres Strait and the Palm Islands will be all community driven and managed, providing employment to local indigenous people.
Sea-Based Sponge Aquaculture for Bioactive Metabolites
The majority of research into farming sponges in the sea for their bioactive metabolites has occurred in the Mediterranean, Indo-Pacific, and South Pacific regions. Examining temperate and tropical species, the main goals of this research have been to identify the optimal environmental factors and farming structures that promote sponge biomass and biosynthesis of the target metabolite. Farming structure tested include those used for bath sponges like threaded rope (e.g., Hadas et al. 2005; de Voogd 2007) and mesh lanterns (e.g., Duckworth et al. 2004). However, one advantage of farming sponges for their metabolites is that final explant shape is not important; thus, additional farming methods are possible.
One new method that has promoted very high growth and survival rates for several sponge species are mesh arrays. Hanging vertically in the water, mesh arrays consist of a mesh tube divided into alternating pockets, each holding one explant (Duckworth and Battershill 2003a). Sponge explants can grow out through the mesh pocket so they are directly exposed to the environment (Fig. 5), maximizing their surface area for feeding and respiration and promoting growth and final size. Explants of Mycale hentscheli (Bergquist and Fromont 1988) experimentally farmed in mesh arrays grew by >3,000% in 8 months (Page et al. 2005). Another new farming structure, developed in the Mediterranean Sea, consists of net material stretched over metal frames, with explants sandwiched between the two layers of netting (van Treeck et al. 2003). One advantage of these net frames is that they can be stacked in various ways on the substrate to maximize farming output. Sponge survival and growth rates were generally high (van Treeck et al. 2003). Another farming method that was tested in the Mediterranean Sea involves placing explants in small plastic containers, which are held upright on horizontal lines (Pronzato 2004). Growth is rapid, and after a few months, the explants grow out of the containers and totally cover the farming structure.
Because the main commercial goal is to supply large volumes of the target metabolite, additional farming techniques are possible such as partially harvesting farmed explants. This technique capitalizes on the high regeneration rates of many sponges and involves removing some biomass (containing metabolite) from each farmed explant, leaving the rest of the explant behind on the farming structure to continue growing. For several sponge species, growth rates of partially harvested explants were similar or higher than non-harvested explants (Duckworth and Battershill 2003b; de Voogd 2007). In a commercial farming venture, sponges would be continually harvested for many years to supply metabolite, reducing the number of farmed sponges needed, thus decreasing infrastructure and costs. The sponges selected for farming should contain high levels of the target metabolite to maximize production and profits.
For farmed sponges, metabolite concentration can vary greatly between neighboring explants (Page et al. 2005). Physical and biological factors such as light intensity and levels of biofouling may influence metabolite biosynthesis in sponges (Thompson et al. 1987; Kreuter et al. 1992; Page et al. 2005; Ferretti et al. 2009). For metabolites biosynthesized by symbiotic microorganisms, changes in environmental factors may also alter microbial populations, influencing metabolite production and yield (Page et al. 2005). Knowing the effect of environmental factors on metabolite biosynthesis or understanding the ecological role of the metabolite could be used to promote metabolite production and total yield. If the ecological role of the target metabolite is to deter predators, for example, it may be possible to mimic predation by cutting the sponge shortly before harvesting to promote metabolite production.
Some sponges farmed for their bioactive metabolites have phenomenal growth rates. Explants of Latrunculia wellingtonensis (Alvarez, Bergquist & Battershill 2002) producing the cytotoxic compounds discorhabdins, for example, showed a five-fold increase on average over 9 months (Duckworth and Battershill 2003b), while M. hentscheli explants producing mycalimides, pateamines, and pelorusides increased 30-fold in size in 8 months (Page et al. 2005). These fast growing species are “fleshy” sponges, which do not have to invest large resources into skeletal support. Fast growth combined with high metabolite biosynthesis suggests that farming sponges to supply metabolites is commercially feasible for some species.
There are currently two research institutes attempting to farm sponges in the sea for mass production of bioactive metabolites. The National Institute of Water and Atmospheric Institute is culturing M. hentscheli in the Marlborough Sounds, New Zealand, to supply peloruside A, a compound with anticancer properties (Handley et al. 2006). Sponges are farmed in mesh arrays that are attached to an existing mussel farm; thus, infrastructure costs are significantly reduced (Handley et al. 2006). During the first year, 3 kg of M. hentscheli was transplanted, producing 70 kg of biomass (Page, personal communication). Most of this biomass was harvested to supply metabolite, but some explants were reseeded back onto the farm, a practice continued in subsequent years until 200 kg was produced. It is estimated that 200 kg of M. hentscheli supplies 2 g of peloruside A (Handley et al. 2006). During scale-up, however, predation by nudibranchs become a serious problem, reducing production; full details of this study will be published soon (Page, personal communication).
The marine biotech company Porifarma is developing sponge farms off Bodrum, western Turkey, to supply sponge metabolites and act as biofilters for neighboring fish farms (de Goeij and Osinga, personal communication). Porifarma will farm two sponge species: Dysidea avara (Schmidt 1862), which produces avarol that has antitumor, antibacterial, and antifungal properties, and Chondrosia reniformis (Nardo 1847), which is a good source of collagen that can be converted into nano-particles and used to deliver drugs to the target location. Farming methods vary between species, with D. avara grown on nylon ropes on metal frames and C. reniformis cultured in metal cages. After 1 year, sponge survival was generally high with some explants tripling in size, though farming response varied between species and sites (de Goeij and Osinga, personal communication).
Land-Based Sponge Aquaculture for Metabolite Production
Although farming sponges in land-based systems is comparatively new, over 30 species have been experimentally cultured so far. The majority of the research has occurred in Europe, investigating Mediterranean species. The advantage of land-based sponge culture over sea-based culture is the ability to totally control farming conditions; thus, metabolite biosynthesis could be optimized, providing more reliable production and promoting higher yields. Most of the research examining in vitro sponge culture has focused on feeding requirements, specifically the food types and concentrations that maximize growth. In an earlier review, Osinga et al. (1999b) stated that determining suitable feeding regimes is the key to successful in vitro sponge culture.
Sponges mostly consume small (<10 um) particles like bacteria and microalgae (Reiswig 1971; Stuart and Klumpp 1984; Duckworth et al. 2006). Sponges with symbiotic bacteria may also obtain much of their energy from DOC (Yahel et al. 2003), but this is unlikely for nonsymbiotic species (Ribes et al. 1999). Although sponges may grow on dissolved organic compounds, particularly if marine growth promoters are used (Garcia Camacho et al. 2006b), the likely commercial value of using DOC is to supplement feeding with particulate organic matter. In vitro studies have typically feed bacteria and/or microalgae to sponge explants (e.g., Barthel and Theede 1986; Osinga et al. 1997, 2001; de Garalt et al. 2003; Duckworth et al. 2003; Ferretti 2006) with varying levels of success.
To promote growth, the food type and concentration must exceed the metabolic costs of the sponge (Osinga et al. 1997). Because bacteria contain less carbon (energy) than larger celled microalgae, more bacteria must be consumed to meet metabolic costs (Duckworth and Pomponi 2005). Although sponges can be grown solely on microalgae or bacteria when fed at the right concentration, a mixed diet is best to guarantee that all nutritional requirements (e.g., carbon, fatty acids) are met (Duckworth and Pomponi 2005). Sponge feeding studies have shown that there is not a positive linear relationship between food concentration and sponge growth because high cell concentrations can reduce sponge filtration rates, reducing growth and final size (Osinga et al. 2001; Duckworth et al. 2003). The amount of carbon needed to meet metabolic costs and thus the optimal food concentration will vary therefore between species and habitats. Coral reef sponges, for example, require significantly less bacteria and microalgae to grow in culture than sponges from nutrient-rich environments (Duckworth et al. 2003; Duckworth and Pomponi 2005).
For sponge species that have a siliceous skeletal (spicules), silica must be added in solution to promote growth (Osinga et al. 1997). Additional water quality issues include providing sufficient oxygen and removing waste products. Sponges cultured in tanks produce large volumes of nitrogenous wastes, requiring good biofiltration or regular water exchanges; otherwise, the water will become toxic, killing the cultured sponges. The nitrogenous waste produced by cultured sponges can be recycled to grow microalgae, which in turn can be fed back to the sponges (Osinga et al. 1999b). Without adequate filtration, bioactive metabolites released from cultured sponges could also reach dangerous concentrations. Successful in vitro culture also requires knowledge of the water temperature and pH levels that promote growth and metabolite biosynthesis. Similar to feeding requirements, the optimal environmental conditions for metabolite production are species specific. Generally, sponge species that inhabit stable environments such as coral reef habitats are more likely to suffer physiological stress, resulting in death when culture conditions vary greatly from ambient or natural conditions.
Because of the relationship between sponge biomass and water quality, most in vitro culture studies have grown small explants and/or used few replicates compared to sea-based culture experiments (e.g., Belarbi et al. 2003; Nickel and Brümmer 2003; Osinga et al. 2003; Garcia Camacho et al. 2006b). For some studies, replicate number was too low to run statistical tests; thus, differences between culture treatments could not be statistically determined (e.g., Osinga et al. 2001; Belarbi et al. 2003; Garcia Camacho et al. 2006a). In addition, some in vitro culture studies have experimentally farmed sponges for only a few weeks or months (e.g., Nickel et al. 2001; Osinga et al. 2001; Duckworth et al. 2003), often less farming time than sea-based culture studies. These experimental restraints have hampered the commercialization of in vitro sponge culture for drug products.
Growth of in vitro cultured sponges varies greatly between studies, resulting from both interspecific differences in growth potential and the experimental nature of in vitro sponge culture. Some studies, however, have reported phenomenal growth rates: Explants of Crambe crambe (Schmidt 1862) grew by 1,380% of their initial weight in 22–45 days (Belarbi et al. 2003), while Pseudosuberites (aff.) andrewsi grew by 730% in 54 days (Osinga et al. 1999a). Although comparable to growth rates recorded from some sea-based studies, initial explant size and replicate number was low for these in vitro studies, and thus, results have to be treated cautiously. For many sponges cultured in tanks on land, growth rates are high initially and then stabilize after a few weeks (Osinga et al. 1999a; Duckworth et al. 2003; Garcia Camacho et al. 2006a). This growth pattern likely results from several factors, including food limitation and behavioral response to being cut into explants. Constant high growth rates are needed for in vitro culture to be commercially viable.
Most in vitro culture studies to date have focused on sponge growth and survival; however, some studies have also examined the effect of culture conditions on metabolite biosynthesis. Concentration of the antitumor metabolite stevensine in Axinella corrugata (George and Wilson 1919), for example, doubled from 200 to 400 mg g DW−1 when the sponge was fed food cells at three times the natural food concentration (Duckworth et al. 2003). Final size of A. corrugata was also greatest at this food concentration, indicating that both growth and metabolite biosynthesis can be maximized under the same culture conditions. Aplysina (Verongia) aerophoba (Nardo 1843) produced 0.13 mg g WW−1 of the cytotoxic metabolite aeroplysinin-1 when cultured under light for 10 days, but only 0.02 mg g WW−1 when grown in the dark (Kreuter et al. 1992). It is therefore possible to manipulate both biological and physical conditions to maximize production of the target metabolite.
Although some studies have reported good farming responses, there are many hurdles to overcome before in vitro sponge culture can be considered a commercially viable method of metabolite supply. Long-term studies involving many sponge replicates that showed rapid continuous growth combined with high metabolite biosynthesis are lacking.
Commercial Sponge Culture
Comparing various culture methods to supply drug products, Sipkema et al. (2005) determined that sea-based sponge culture is often economically cheaper than in vitro or land-based culture. For example, production costs of the anticancer compound halichondrin B from Lissodendoryx sp. farmed on land is approximately double the costs of sea-based culture (Sipkema et al. 2005). Land-based culture of Acanthella cavernosa (Dendy 1922) was also considered not economically viable (Mendola 2003). These findings agree with seafood aquaculture, where the additional costs associated with land-based culture such as supplying sufficient food and maintaining good water quality can prevent economical viability. Production costs for sea-based culture can be very low, particularly for small-scale bath sponge culture where farm set-up and operational costs (excluding labor) are a few thousand dollars (Adams et al. 1995; MacMillan 1996). Sea-based culture is currently the only commercially viable method used to produce either bath sponges or bioactive metabolites for drug production and will likely remain so for the foreseeable future. Commercial culture of bath sponges is probably most feasible in tropical regions because of comparatively higher growth rates than temperate bath sponges. Commercial culture of sponges for bioactive metabolites, although currently limited to two temperate locations, is likely profitable in both temperate and tropical regions. The potential of disease outbreaks, as experienced in the past (Smith 1941), will always threaten sea-based culture but good management techniques will reduce the threat. Commercial farming operations currently rely on cutting sponges to generate explants to farm, but seeding farming structures with sexually produced larvae has great potential (de Caralt et al. 2007). Another existing development is using sponge farms as biofilters in controlling pelagic populations of microbes.
Even though numerous studies stretching over 100 years have shown that sponges can grow quickly and survive well when cultured in good environmental conditions using appropriate farming methods, commercial sea-based culture is still largely in its infancy. Small-scale farms for production of bath sponges or metabolite have highlighted both its potential and limitations. Although commercial farming for sponge metabolites is potentially more lucrative, it has several challenges that bath sponge culture does not have like high costs of metabolite extraction and alternative sources of supply (e.g., chemical synthesis). Because bath sponges are easily farmed using simple technology, with minimal processing and transport costs, bath sponge culture is ideal for underdeveloped coastal communities. It is likely that the greatest potential of commercial bath sponge aquaculture is for coastal indigenous communities, providing an alternative and environmentally friendly source of income (Fig. 6).
References
Adams C, Stevely J, Sweat D (1995) Economic feasibility of small-scale sponge farming in Pohnpei, Federated States of Micronesia. J World Aquac Soc 26:132–142
Ayling AL (1983) Growth and regeneration rates in thinly encrusting Demospongiae from temperate waters. Biol Bull 165:343–352
Barthel D, Theede H (1986) A new method for the culture of marine sponges and its application for experimental studies. Ophelia 25:75–82
Belarbi EH, Dominguez MR, Carcia MCC, Gómez AC, Camacho G, Grima EM (2003) Cultivation of explants of the marine sponge Crambe crambe in closed systems. Biomolecular Engineering 20:333–337
Bergquist PR (1978) Sponges. University of California Press, Berkeley
Blunt JW, Copp BR, Hu WP, Munro MHG, Northcote PT, Prinsep MR (2009) Marine natural products: review. Nat Prod Rep 26:170–244
Cheshire AC, Butler AJ, Westphalen G, Rowland B, Steveson J, Wilkinson CR (1995) Preliminary study of the distribution and photophysiology of the temperate phototrophic sponge Cymbastela sp. from South Australia. Mar Freshw Res 46:1211–1216
Corriero G, Longo C, Mercurio M, Marzano CN, Lembo G, Spedicato MT (2004) Rearing performance of Spongia officinalis on suspended ropes off the Southern Italian Coast (Central Mediterranean Sea). Aquaculture 238:195–205
Crawshay LR (1939) Studies in the market sponges. I. Growth from the planted cutting. J Mar Biol Assoc UK 23:553–574
de Caralt S, Otjens H, Uriz MJ, Wijffels RH (2007) Cultivation of sponge larvae: settlement, survival, and growth of juveniles. Mar Biotechnol 9:592–605
de Garalt S, Agell G, Uriz MJ (2003) Long-term culture of sponge explants: conditions enhancing survival and growth, and assessment of bioactivity. Biomolecular Engineering 20:339–347
de Voogd NJ (2007) The mariculture potential of the Indonesian reef-dwelling sponge Callyspongia (Euplacella) biru: growth, survival and bioactive compounds. Aquaculture 262:54–64
Dubios R (1914) Spongiculture par essaimage. IX Congres International de Zoologie de Monaco, Obertur, Rennes, pp 659–660
Duckworth AR (2003) Effect of wound size on the growth and regeneration of two temperate subtidal sponges. J Exp Mar Biol Ecol 287:139–153
Duckworth AR, Battershill CN (2003a) Developing farming structures for production of biologically active sponge metabolites. Aquaculture 217:139–156
Duckworth AR, Battershill CN (2003b) Sponge aquaculture for the production of biologically active metabolites: the influence of farming protocols and the environment. Aquaculture 221:311–329
Duckworth AR, Pomponi SA (2005) Relative importance of bacteria, microalgae and yeast for growth of the sponge Halichondria melanadocia (De Laubenfels, 1936): a laboratory study. J Exp Mar Biol Ecol 323:151–159
Duckworth AR, Wolff CW (2007) Bath sponge aquaculture in Torres Strait, Australia: effect of explant size, farming method and the environment on culture success. Aquaculture 271:188–195
Duckworth AR, Battershill CN, Bergquist PR (1997) Influence of explant procedures and environmental factors on culture success of three sponges. Aquaculture 156:251–267
Duckworth AR, Battershill CN, Schiel DR (2004) Effects of depth and water flow on growth, survival and bioactivity of two temperate sponges cultured in different seasons. Aquaculture 242:237–250
Duckworth AR, Wolff C, Evans-Illidge E (2007) Developing methods for commercially farming bath sponges in tropical Australia. In: Custódio MR, Hajdu E, Lôbo-Hajdu G, Muricy G (eds) Porifera Research: Biodiversity, Innovation and Sustainability. Rio de Janeiro Museu Nacional, pp 297–302
Duckworth AR, Samples GA, Wright AE, Pomponi SA (2003) In vitro culture of the tropical sponge Axinella corrugata (Demospongiae): effect of food cell concentration on growth, clearance rate, and biosynthesis of stevensine. Mar Biotechnol 5:519–527
Duckworth AR, Brück WM, Janda KE, Pitts TP, McCarthy PJ (2006) Retention efficiencies of the coral reef sponges Aplysina lacunosa, Callyspongia vaginalis and Niphates digitalis determined by Coulter counter and plate culture analysis. Mar Biol Res 2:243–248
FAO (2004) Collation, analysis and dissemination of global and regional fishery statistics. Food and Agricuture Organisation, Fishery Information, Data and Statistics Unit, Rome
Ferretti C (2006) Aquaculture of two Mediterranean sponge species for bioactive molecules production. Dipartimento per lo Studio del Territorio e delle sue Risorse
Ferretti C, Vacca S, de Ciucis C, Marengo B, Duckworth AR, Manconi R, Pronzato R, Domenicotti C (2009) Growth dynamics and bioactivity variation of the Mediterranean demosponges Agelas oroides (Agelasida, Agelasidae) and Petrosia ficiformis (Haplosclerida, Petrosiidae). Marine Ecology (in press)
Gaino E, Pronzato R (1989) Ultrastructural evidence of bacterial damage to Spongia officinalis fibres (Porifera, Demospongiae). Dis Aquat Org 6:67–74
Garcia Camacho E, Chileh T, Cerón García MC, Sánchez Mirón A, Belarbi EH, Chisti Y, Molina Crima E (2006a) A bioreaction-diffusion model for growth of marine sponge explants in bioreactors. Appl Microbiol Biotechnol 73:525–532
Garcia Camacho F, Chileh T, Cerón García MC, Sánchez Mirón A, Belarbi EH, Contreras Gómez A, Molina Crima E (2006b) Sustained growth of explants from Mediterranean sponge Crambe crambe cultured in vitro with enriched RPMI 1640. Biotechnol Prog 22:781–790
Hadas E, Shpigel M, Ilan M (2005) Sea ranching of the marine sponge Negombata magnifica (Demospongiae, Latrunculiidae) as a first step for latrunculin B mass production. Aquaculture 244:159–169
Handley SJ, Kelly S, Kelly M (2003) Non-destructive video image analysis method for measuring growth in sponge farming: preliminary results from the New Zealand bath-sponge Spongia (Heterofibria) manipulatus. NZ J Mar Freshwat Res 37:613–621
Handley SJ, Page MJ, Northcote PT (2006) Anti-cancer sponge: the race is on for aquaculture supply. Water Atmos 14:14–15
Hummel H, Sepers ABJ, de Wolf L, Melissen FW (1988) Bacterial growth on the marine sponge Halichondria panicea induced by reduced waterflow rate. Mar Ecol Prog Ser 42:195–198
Jokiel PL (1980) Solar ultraviolet radiation and coral reef epifauna. Science 207:1069–1071
Kelly M, Handley SJ, Page MJ, Butterfield P, Hartill B, Kelly S (2004) Aquaculture trials of the New Zealand bath-sponge Spongia (Heterofibria) manipulatus using lanterns. NZ J Mar Freshwat Res 38:231–241
Kreuter MH, Robitzki AR, Chang S, Steffen R, Michaelis M, Kljajic Z, Bachmann M, Schröder HC, Müller WEG (1992) Production of the cytostatic agent aeroplysinin by the sponge Verongia aerophoba in in vitro culture. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 101:183–187
Lauckner G (1980) Diseases of Porifera. In: Kinne O (ed) Diseases of marine animals. Wiley, Chichester, pp 139–165
Leichter JJ, Witman JD (1997) Water flow over subtidal rock walls: relation to distributions and growth rates of sessile suspension feeders in the Gulf of Maine. J Exp Mar Biol Ecol 209:293–307
Louden D, Whalan S, Evans-Illidge E, Wolff C, de Nys R (2007) An assessment of the aquaculture potential of the tropical sponges Rhopaloeides odorabile and Coscinoderma sp. Aquaculture 270:57–67
MacMillan SM (1996) Starting a successful commercial sponge aquaculture farm. Center for Tropical and Subtropical Aquaculture, University of Hawaii, Honolulu
Mendola D (2003) Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: process developments and economics. Biomolecular Engineering 20:441–458
Milanese M, Sarà M, Manconi R, Ben Abdalla A, Pronzato R (2008) Commercial sponge fishing in Libya: historical records, present status and perspectives. Fish Res 89:90–96
Milanese M, Chelossi E, Manconi R, Sarà M, Sidri M, Pronzato R (2003) The marine sponge Chondrilla nucula Schmidt, 1862 as an elective candidate for bioremediation in integrated aquaculture. Biomolecular Engineering 20:363–368
Moore HF (1910) A practical method of sponge culture. Bulletin of the United States Bureau of Fisheries 28(1908, Pt. 1):545–585
Müller WEG, Wimmer W, Schatton W, Böhm M, Batel R, Filic Z (1999) Initiation of an aquaculture of sponges for the sustainable production of bioactive metabolites in open systems: example, Geodia cydonium. Mar Biotechnol 1:569–579
Müller WEG, Grebenjuk VA, Le Pennec G, Schröder HC, Brümmer F, Hentschel U, Müller IM, Breter HJ (2004) Sustainable production of bioactive compounds by sponges-cell culture and gene cluster approach: a review. Mar Biotechnol 6:105–117
Nickel M, Brümmer F (2003) In vitro sponge fragment culture of Chondrosia reniformis (Nardo, 1847). J Biotechnol 100:147–159
Nickel M, Leininger S, Proll G, Brümmer F (2001) Comparative studies on two potential methods for the biotechnological production of sponge biomass. J Biotechnol 92:169–178
OEA (2004) Aquaculture profile for Pohnpei Federated States of Micronesia. Office of Economic Affairs, State of Pohnpei
Osinga R, Tramper J, Wijffels RH (1999a) Cultivation of marine sponges. Mar Biotechnol 1:509–532
Osinga R, Planas Muela E, Tramper J, Wijffels RH (1997) In vitro cultivation of four marine sponge species. Determination of the nutritional demands. In: Le Gal Y, Muller-Feuga A (eds) Marine microorganisms for industry. Ifremer, France, pp 121–127
Osinga R, Belarbi EH, Grima EM, Tramper J, Wijffels RH (2003) Progress towards a controlled culture of the marine sponge Pseudosubertes andrewsi in a bioreactor. J Biotechnol 100:141–146
Osinga R, de Beukelaer P, Meijer EM, Tramper J, Wijffels RH (1999b) Growth of the sponge Pseudosuberites (aff.) andrewsi in a closed system. J Biotechnol 70:155–161
Osinga R, Kleijn R, Groenendijk E, Neiink P, Tramper J, Wijffels RH (2001) Development of in vivo sponge cultures: particle feeding by the tropical sponge Pseudosuberites aff. andrewsi. Mar Biotechnol 3:544–554
Page MJ, Northcote PT, Webb VL, Mackey S, Handley SJ (2005) Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida). Aquaculture 250:256–269
Palumbi SR (1984) Tactics of acclimation: morphological changes of sponges in an unpredictable environment. Science 295:685–687
Pile AJ, Patterson MR, Witman JD (1996) In situ grazing on plankton <10 µm by the boreal sponge Mycale lingua. Mar Ecol Prog Ser 141:95–102
Pomponi SA (2006) Biology of the Porifera: cell culture. Can J Zool 84:167–174
Pronzato R (1999) Sponge-fishing, disease and farming in the Mediterranean Sea. Aquatic Conservation: Marine and Freshwater Ecosystems 9:485–493
Pronzato R (2004) A climber sponge. Bollettino Dei Musei E Degli Istituti Biologici Dell'Universita Di Genova 68:549–552
Pronzato R, Manconi R (2008) Mediterranean commercial sponges: over 5000 years of natural history and cultural heritage. Marine Ecol 29:146–166
Pronzato R, Bavestrello G, Cerrano C, Magnino G, Manconi R, Pantelis J, Sarà M, Sidri M (1999) Sponge farming in the Mediterranean Sea: new perspectives. Mem Queensl Mus 44:485–491
Reiswig HM (1971) Particle feeding in natural populations of three marine demosponges. Biol Bull 141:568–591
Ribes M, Coma R, Gili JM (1999) Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar Ecol Prog Ser 176:179–190
Schmitz FJ, Bowden BF, Toth SI (1993) Antitumor and cytotoxic compounds from marine organisms. In: Attaway DH, Zaborsky OR (eds) Marine biotechnology. Pharmaceutical and bioactive natural products. Plenum, New York, pp 197–308
Sebens KP (1987) The ecology of indeterminate growth in animals. Annu Rev Ecol Syst 18:371–407
Sipkema D, Osinga R, Schatton W, Mendola D, Tramper J, Wijffels RH (2005) Large-scale production of pharmaceuticals by marine sponges: sea, cell, or synthesis. Biotechnol Bioeng 90:201–222
Smith FGW (1941) Sponge disease in British Honduras, and its transmission by water currents. Ecology 22:415–421
Storr JF (1957) The sponge industry of Florida. State of Florida, Board of Conservation, Educational Series No. 9
Storr JF (1964) Ecology of the Gulf of Mexico commercial sponges and its relation to the fishery. United States Fish and Wildlife Service, Special Scientific Report-Fisheries No. 466
Stuart V, Klumpp DW (1984) Evidence for food-resource partitioning by kelp-bed filter feeders. Mar Ecol Prog Ser 16:27–37
Thompson JE, Murphy PT, Bergquist PR, Evans EA (1987) Environmentally induced variation in diterpene composition of the marine sponge Rhopaloeides odorabile. Biochem Syst Ecol 15:595–606
Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, de Voogd NJ, Alvarez B, Hajdu E, Pisera AB, Vacelet J, Manconi R, Schoenberg C, Janussen D, Tabachnick KR, Klautau M (2008) World Porifera database. Available online at http://www.marinespecies.org/porifera. Consulted on 2009-05-13
van Treeck P, Eisinger M, Müller J, Paster M, Schuhmacher H (2003) Mariculture trials with Mediterranean sponge species: the exploitation of an old natural resource with sustainable and novel methods. Aquaculture 218:439–455
Verdenal B, Vacelet J (1990) Sponge culture on vertical ropes in the Northwestern Mediterranean Sea. In: Rützler K (ed) New perspectives in sponge biology. Smithsonian Institution Press, Washington DC, pp 416–424
Vogel S (1974) Current-induced flow through the sponge, Halichondria. Biol Bull 147:443–456
Webster NS (2007) Sponge disease: a global threat? Environ Microbiol 9:1363–1375
Wilkinson CR, Vacelet J (1979) Transplantation of marine sponges to different conditions of light and current. J Exp Mar Biol Ecol 37:91–104
Yahel G, Sharp JH, Marie D, Häse C, Genin A (2003) In situ feeding and element removal in the symbiont-bearing sponge Theonella swinhoei: bulk DOC is the major carbon source. Limnol Oceanogr 48:141–149
Acknowledgments
I would like to thank the many people who have supported my research into farming sponges, from providing guidance to helping out in the field. Specifically, I thank Chris Battershill, Dame Patricia Bergquist, Chris Woods, Pete Notman, Shirley Pomponi, Elizabeth Evans-Illidge, Carsten Wolff, John Morris, and Samson Lowatta. I also thank two anonymous reviewers for their helpful comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duckworth, A. Farming Sponges to Supply Bioactive Metabolites and Bath Sponges: A Review. Mar Biotechnol 11, 669–679 (2009). https://doi.org/10.1007/s10126-009-9213-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-009-9213-2