Abstract
The aim of this study was to investigate the effects of blue light irradiation on the process of osteogenic differentiation in stem cells. The cells used in this study were derived from human gingival mesenchymal stem cells (hGMSCs), and were treated with 0 (control group), 1, 2, 4 or 6 J/cm2 blue light using blue light-emitting diodes. Cell growth was assessed by the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-Diphenyl-2H-tetrazolium bromide (MTT) cell proliferation assay and osteogenic differentiation was evaluated by monitoring alkaline phosphatase (ALP) activity, alizarin red staining and real-time PCR (RT-PCR). The results of the MTT assay indicated that blue light inhibited hGMSC proliferation, and the ALP and alizarin red results showed that blue light promoted osteogenesis. The expression levels of the osteogenic genes runt-related transcription factor2 (Runx2), collagen type I (Col1) and osteocalcin (OCN) increased significantly (P < 0.05) when cells were irradiated with 2 or 4 J/cm2 of blue light. In conclusion, irradiation with blue light inhibits the proliferation of hGMSC and promotes osteogenic differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary pathogenic mechanism of periodontal disease is the destruction of alveolar bone tissue. Therefore, inhibition of alveolar bone resorption and promotion of regeneration are potential approaches for the treatment of periodontal disease [1]. However, therapeutic techniques that result in complete regeneration are yet to be developed [2]. Tissue regeneration engineering provides a new avenue for research into treatments of periodontal disease, and stem cells are fundamental to this approach. Human gingival mesenchymal stem cells (hGMSCs) are adult odontogenic stem cells which exhibit good stability, colony generation ability and multi-directional differentiation potential [3,4,5,6,7,8]. These cells have attracted the attention of researchers due to their ease of collection, ability to transform into bone cells and high abundance [3,4,5,6]. It has been shown that in vivo transplantation of GMSCs can significantly improve periodontal clinical indicators and promote periodontal regeneration [9]. The promotion of osteogenesis may also be of great interest to researchers.
Directed differentiation of mesenchymal stem cells requires specific culture environments and external stimuli, involving the extracellular matrix, cytokines and physical factors [10, 11]. Studies have shown that low-level laser irradiation (LLLI) promotes cytokine secretion, collagen synthesis and the proliferation and differentiation of osteoblast-like cells, with a concomitant repair of bone defects [12,13,14,15]. With the continuing developments in semiconductor technology, light-emitting diodes (LEDs) have gradually replaced lasers in many research fields. For example, the LED can be used to promote wound healing [16, 17] and bone regeneration [18]. And the study of low-energy LED treatment of mesenchymal stem cells is gradually increasing, such as LED light at a wavelength of 620 nm enhance the proliferation and osteogenic differentiation of hUMSCs during a long culture period [19]. But its effect on the proliferation and osteogenic differentiation of hGMSCs has not yet been investigated. In this study, we irradiate hGMCSs with different energy densities by using low-energy blue LEDs in order to elucidate the effects of light energy on the proliferation and osteogenic differentiation of hGMSCs.
Materials and methods
Cell culture
The study was approved by the Ethics Committee of the Affiliated Hospital of Stomatology Southwest the isolation of hGMSCs (contract grant 20180314001). The eligibility criteria for participants were as follows: all donors were in good general health and exempt from oral and systemic diseases and extraction needed for orthodontic teeth for orthodontic reasons. The samples were repeated four times in the experiments.
hGMSCs were obtained according to the protocol of Laura Tomasello et al. [20]. Clinically healthy gingival tissue was extracted and washed three times with phosphate buffered saline (PBS). Gingiva use was approved by the patient informed consent and the biomedical science research ethics committee of the stomatological hospital of Southwest Medical University. The epithelial tissue was removed, then the remaining tissue was cut into 2 × 2 × 1 mm and mixed 1:1 (v/v) with 3 mg/ml collagenase I (BioSharp Inc., USA) and 4 mg/ml dispase II (Gibco, Carlsbad, CA, USA). The mixture was incubated for 15 min at 37 °C under agitation then centrifuged at 1000 rpm for 5 min. The cells were then seeded into a tissue culture flask and incubated in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone, Shanghai, China) at 37 °C in a humidified atmosphere of 5% CO2. The culture medium was changed twice a week. For subculture, cells were detached with 0.25% trypsin (AMRESCO, Solon, OH, USA) and passaged at a ratio of 1:2 when they had grown to 80–90% confluence. The P2 gingival cells were digested and cells were purified individually. The obtained cells were identified as hGMSCs.
Experimental groups
According to the preliminary experiments and the calculation formula (energy density = power density × irradiation time), cultures were divided into five groups according to the intended energy of LED irradiation: 1 J/cm2–irradiated for 10 s, 2 J/cm2–irradiated for 20 s, 4 J/cm2–irradiated for 40 s, 6 J/cm2–irradiated for 60 s and not irradiated–control group.
Irradiation procedure
A blue light LED (LUX VI; Zhuomuniao, China) with a continuous output and a wavelength of 420–480 nm (1-W output) was used for this study. The distance from the LED to the cell layer was 1 cm (Fig. 1a). The diameter of the light spot on the irradiated cultures was 3.5 cm. At the cell-layer level, the power density was 100 mW/cm2. According to the experimental design, the cells were irradiated consecutively for 10 s, 20 s, 40 s and 60 s every other day, and the first day of irradiation was denoted day 1. The irradiation was done for 28 days, and the corresponding detection was carried out according to the experimental design requirements. Non-irradiated cells were cultured under the same conditions as irradiated cells. All irradiations were done by the same operator.
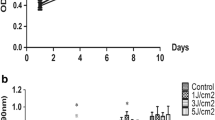
The influence of low-energy blue light on the proliferation of hGMSCs. a The photo that shows the led irradiation for the experiment. b The cell proliferation rate of each experimental group was reduced at every time point on days 3, 5, 7 and 9 compared with the control group (P = 0.000). No significant difference in the rate of proliferation of any experimental group was seen on day 1 (P > 0.05) (*P < 0.05; NS, not statistically significant). Interestingly, the proliferation ability of hGMSCs in the 2 J/cm2 group was weaker than in other experimental groups
Cell proliferation assays
Cell proliferation assays were obtained according to the protocol of Pagin M T et al. [21]. The hGMSCs were seeded into 96-well plates (2.5 × 104 cells/well) with DMEM supplemented with 10% foetal bovine serum (FBS). At the second day, the culture medium was aspirated and replaced with osteogenic differentiation media (ODM; Cyagen Biosciences, Guangzhou, China). The irradiation was done at days 1, 3, 5, 7 and 9. The 3-(4,5-Dimethyl-2-thiazolyl)-2,5-Diphenyl-2H-tetrazolium bromide (MTT) assay was carried out on the first, third, fifth, seventh and ninth days after cells irradiation. At the end of the respective incubation time, the supernatants were discarded and 200 μl of MTT solution [5 mg MTT/phosphate buffered saline (PBS)] was added to each well. After an additional 4-h incubation period, the supernatant was discarded and 200 μl dimethyl sulfoxide was added to solubilise the formazan crystals. The absorbance of each well was measured at 490 nm (Bio-Tek, USA) and the cell growth curve plotted with the average absorbance at each time point. Data were recorded in triplicate.
Alkaline phosphatase activity
Alkaline phosphatase activity was obtained according to the protocol of Jyun-Yi Wu et al. [22]. The purpose of this study was to detect early osteoblast differentiation products.
The hGMSCs were seeded into 35-mm dishes (2 × 104 cells/ml). After the cells were confluent to 50–60%, the medium was changed to ODM and blue light was applied according to the experimental design. The irradiation was done every other day for 14 days. An ALP activity assay was performed at 7 and 14 days. Cells were washed three times with PBS and fixed in 4% paraformaldehyde for 30 min. Paraformaldehyde was removed by aspiration and the cells washed twice with PBS. Following the standard ALP staining protocol, staining reagent was added to the hGMSCs and the cells left to stand for 15 min at 37 °C in the dark. The staining reagent was removed by aspiration, then the cells were washed twice with double distilled water. Subsequently, the cells were observed under a phase-contrast microscope, and the findings were compared with the control group.
On days 7 and 14, the cells were digested, washed three times with PBS and 500 μl of 1% TritonX-100 added to each dish. Dishes were incubated at 37 °C for 40 min. Lysis buffer was added according to the instructions in the ALP activity kit (Nanjing Jiancheng Biological Engineering Institute, Nanjing, China). The total protein concentration in the cell lysate was measured using the bicinchoninic (BCA) assay (Lipulai, Beijing, China) and the activity of ALP per gram protein was calculated.
Alizarin red detection activity
According to previous report [22], the purpose of this study was to detect late osteoblast differentiation products. The irradiation was done every other day for 28 days.
On day 28, cells which were in the petri dish were washed three times with PBS. Paraformaldehyde (4%) was added and the cells incubated for 30 min Paraformaldehyde was removed and cells were washed twice with PBS. A 0.2% solution of alizarin red dye (Beijing Solarbio Science & Technology Co., Beijing, China) was added and the cells allowed to stand for 15 min. The staining reagent was removed, cells washed twice with double distilled water and imaged using an optical microscope. To quantify the staining, cells were destained by adding 5 ml of cetylpyridinium chloride solution [5 mg cetylpyridinium chloride/double distilled water] (Beijing Solarbio Science & Technology Co., Beijing, China) and incubating at room temperature for 30 min. The supernatant was pipetted into a 96-well plate and the absorbance of each sample measured at 562 nm (Bio-Tek, USA). Cetylpyridinium chloride solution was used as the blank.
Real-time polymerase chain reaction analysis
According to the effect of light on the proliferation of hGMSCs and osteogenic differentiation, the analysis showed that the osteogenic differentiation of hGMSCs under the irradiation of 2 J/cm2 and 4 J/cm2 was stronger than other kinds of light energy. Therefore, 2 J/cm2 and 4 J/cm2 light energy were selected for the next stage of RT-PCR.
Total RNA extraction was carried out using the Trizol kit (Beijing Baiao Science & Technology Co., Beijing, China) as per the manufacturer’s instructions on day 7. Real-time PCR reactions were performed with the QuantiTect SYBR Green PCR kit (Qiagen, Hilden, Germany) and an IcycleriQ Multi-color Real-time PCR Detection System (Bio-Rad). The expression of genes was calculated by the method of 2-ΔΔCT as described previously. The primers for specific genes were shown in Table 1. Real-time polymerase chain reaction (RT-PCR) was performed to analyse the expression levels of osteogenic-related gene type I (COLI), osteocalcin (OCN) and Runx2 in each group of cells. The PCR reaction system and conditions used were strictly in accordance with the product specifications.
Statistical analysis
All statistical calculations were performed with SPSS13.0 statistical software. The Bonferroni Correction was used between cell proliferation groups and P < 0.05 showed statistically significant differences within the groups. The ALP and alizarin red detection activity were analysed by one-way ANOVA. The results of RT-PCR were compared using the two sample and paired design t tests. The ANOVA and t tests were evaluated at the 95% confidence level (P < 0.05).
Results
Inhibition of cell growth following blue light irradiation
The growth curve was plotted for each group of cells using the results of the MTT assay (Fig. 1b). Each experimental group showed a reduced rate of proliferation at every time point on days 3, 5, 7 and 9 (P = 0.000 < 0.05). There was no significant difference in the rate of proliferation between irradiated groups (P > 0.05), but the cell proliferation rate in the 2 J/cm2 group at 3, 5, 7 and 9 days was slower than that in other groups.
Effect of blue light irradiation on osteogenesis
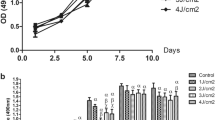
The results of ALP staining on days 7 and 14 are shown in Fig. 2a. The levels of ALP were higher in every experimental group than in the control group. The groups that were irradiated with 2 J/cm2 and 4 J/cm2 of blue light showed similar levels of ALP, which were higher than other groups. As Fig. 2a show, the levels of ALP were higher on day 14 than day 7.
Effect of blue LED on osteogenic differentiation of hGMSCs. a Alkaline phosphatase staining on days 7 and 14, and alizarin red staining on day 28. b Analysis of ALP activity on day 7. The largest effects were seen in hGMSCs irradiated at doses of 2 and 4 J/cm2, in which activity increased to 251% and 162% of the control respectively (**P < 0.01). Analysis of ALP activity on day 14. All groups showed increased activity following irradiation, with 1 J/cm2, 2 J/cm2, 4 J/cm2 and 6 J/cm2 showing levels of 175%, 251%, 254% and 161% of the control group respectively (**P < 0.01). Analysis of mineral nodule formation on day 28. Increased staining intensity was seen in all groups of irradiated hGMSCs, with 1 J/cm2, 2 J/cm2, 4 J/cm2 and 6 J/cm2 resulting in staining intensities of 112%, 124%, 122% and 156% of the control group respectively (**P < 0.01)
On day 7, the ALP activity had increased for every experimental group in Fig. 2b. Specifically, after the cells had been exposed to 1, 2, 4 or 6 J/cm2 of blue light, ALP activities were equivalent to 106%, 251%, 162% and 105% of the control group activities respectively. On day 14, the ALP activity of all experimental groups had increased further in Fig. 2b. Specifically, the ALP activities of cells that had been exposed to 1, 2, 4 or 6 J/cm2 of blue light had increased to 175%, 251%, 254% and 161% of the control group activities respectively.
Mineralisation of nodules in response irradiation
Samples stained with alizarin red on day 28 are shown in Fig. 2a. Following irradiation with 6 J/cm2 blue light, the number of mineralised nodules within the cells was higher than in other experimental groups, but differences in staining intensity could not be clearly distinguished.
The results of the alizarin red enzyme standard assay are shown in Fig. 2b. Irradiation promoted the mineralisation of hGMSCs, with statistically significant differences seen between all experimental groups and the control group (P = 0.000 < 0.05). Following irradiation with 1, 2, 4 or 6 J/cm2 blue light, the alizarin red activities were equivalent to 112%, 124%, 122% and 156% of the control respectively.
Changes in gene expression following irradiation
The effects of irradiation on total RNA were analysed by gel electrophoresis and the results are shown in Fig. 3a. It can be seen from the figure that the extracted RNA has good integrity, which can be further studied. The relative expression levels of COL-1, Runx2 and OCN are shown in Fig. 3b. Cells that were exposed to 2 J/cm2 and 4 J/cm2 of blue light showed significant increases in the expression of all three genes (P = 0.008 < 0.05). The degree of upregulation was similar for both the 2 J/cm2 and 4 J/cm2 groups, with no significant difference between the two (P > 0.05).
Discussion
Light-emitting diodes are an economical, energy-saving and safe alternative to LLLIs [23]. Among the available LEDs, red LEDs have been utilised in clinical and scientific research [24,25,26], but information on the use of blue LEDs is relatively scarce. It has been reported that greater effects were seen on the proliferation and osteogenic differentiation of epithelial and amniotic fluid–derived mesenchymal stem cells when blue LED irradiation was applied compared with red LED irradiation [27, 28]. This indicates that the use of blue LEDs could affect the proliferation and differentiation of stem cells.
Low-energy blue LEDs can induce various biological effects in cell systems [27, 29, 30]. In clinical treatment of the oral cavity, blue light-emitting curing lights is small and precise, and the operator can set the specific irradiation time of the instrument, making it an ideal experimental system. Low-energy lasers have various biological effects on cells and tissues. When the energy density is in the range of 1–4 J/cm2, it will have an effective photobiological effect on osteoblasts and mesenchymal stem cells [31, 32], with no negative effect on normal nuclei [33, 34]. Previous study reported that 660-nm LED irradiation could promote cell proliferation and osteogenic differentiation of human periodontal ligament cells with doses of 1, 2 or 4 J/cm2 [22]. In this study, treatment with low-energy blue LEDs (420–480 nm) showed an inhibitory effect on the proliferation of hGMSCs. The reason why the two results differ needs further investigation. Based on the antagonism of stem cell proliferation and differentiation [35], it is therefore speculated that low-energy blue LED promotes the differentiation of hGMSCs.
We hypothesise that at the point when hGMSCs are induced to differentiate into osteoblasts, the effects of blue LED irradiation have already occurred. The blue light affects cells when growth is at its most vigorous. The results of this study support those of other scholars including Liebmann [27] and Higuchi [30]. Previous studies have found that blue light has an inhibitory effect on the proliferation of epithelial cells and amniotic fluid–derived mesenchymal stem cells, but the magnitude of the inhibition and energy dependence is not known. This study found that irradiation with light of various energies inhibited the proliferation of hGMSCs, but the most significant effect was seen using 2 J/cm2 light energy. From this, the specific mechanism of low-energy blue light regulation of proliferation and osteogenic differentiation of hGMSCs can be elucidated.
After the differentiation of mesenchymal stem cells begins, bone signature proteins begin to be expressed [35, 36], including the enzymes ALP, OCN, Runx2 and COL-1. In this study, according to Fig. 2, the expression of ALP was significantly higher in the 2 J/cm2 and 4 J/cm2 groups than other experimental groups or the control group. This indicates that blue LED can promote early osteogenic differentiation of hGMSCs.
To our knowledge, the effects of blue light on the formation of mineralised nodules of hGMSCs in the late stages of osteogenic differentiation have not been studied. This experiment provides a preliminary study in this direction. Through quantitative analysis of alizarin red staining, it was found that blue light promotes the osteogenic differentiation of hGMSCs, and irradiation with 2 J/cm2 and 4 J/cm2 promoted the mineralisation of hGMSCs, specifically the early stages of mineral nodule formation. The significant promotion of mineralisation seen following irradiation with 6 J/cm2 suggests that blue LEDs emit an appropriate range of light energy, and that the gradual increase of energy can promote the formation of late mineralised nodules.
Ozawa [32] suggested that light-induced osteogenesis plays a role in early differentiation. So in this study, the osteogenesis-related genes were detected by RT-PCR on day 7. The three genes COL-1, Runx2 and OCN are markers of mesenchymal stem cell maturation and osteoblasts [35, 37]. The high levels of ALP expression and activity on day 7 in the 2 J/cm2 and 4 J/cm2 groups suggested that blue light induced changes in gene expression in early osteogenic differentiation. Therefore, the expression levels of the three other marker enzymes (COL-1, Runx2 and OCN) were also analysed in the 2 J/cm2 and 4 J/cm2 groups. Expression levels of all three genes were significantly increased after irradiation, indicating that blue LED treatment promotes the early differentiation of hGMSCs. Our results revealed that COL-1 is an early osteogenic differentiation gene and its expression level was the highest of the three genes studied on day 7. Runx2, as an osteogenic gene expressed during the middle period of osteogenic differentiation, had a lower expression level than COL-1. Expression levels of OCN, a late osteogenic gene, were lower than the former two in all conditions. The experimental data are consistent with the osteogenic gene expression seen during the differentiation of mesenchymal stem cells into osteoblasts [38].
The promotion of osteogenic differentiation of hGMSCs by blue light may provide an avenue for accelerating this differentiation. At the genetic level, the increased expression of osteogenic genes indicates that light irradiation enhanced the osteogenic potential of hGMSCs. An understanding of this phenomenon is beneficial to the development of regenerative therapeutics and may enable the complete restoration of alveolar bone tissue. However, the use of blue LEDs combined with hGMSCs as seed cells in periodontal tissue regeneration still requires further study.
In summary, the proliferation of hGMSCs was analysed under different light energy irradiation. The ALP staining and activity analyses highlighted that blue light promotes the early osteogenic differentiation of hGMSCs, while alizarin red analysis revealed the impact of various energies of blue light on mineralisation ability. The expression of COL-1, Runx2 and OCN genes were detected by RT-PCR, and the effects of low-energy LED blue light on the proliferation and osteogenic differentiation of hGMSCs were preliminary explored. However, further exploration into the specific mechanism through which LED blue light affects the proliferation and osteogenic differentiation of hGMSCs. Published studies have shown that the effect of red LED irradiation on cells is mainly through the resonance with intracellular mitochondria, which alters the biological properties of cells [39]. Therefore, the influence of blue light on the secretion of cytokines and function of mitochondria requires further study.
However, the specific mechanism of low-energy LED blue light on gingival mesenchymal stem cell proliferation and osteogenic differentiation is still unclear. Further studies are necessary to clarify this matter.
Conclusion
Low-energy blue LED at 1, 2, 4 and 6 J/cm2 inhibits the proliferation of hGMSCs and promotes osteogenic differentiation. 2 J/cm2 and 4 J/cm2 light energy can significantly promote early osteogenic differentiation, and 6 J/cm2 light energy promotes advanced osteogenic differentiation.
References
Chen FM, Zhang J, Zhang M, An Y, Chen F, Wu ZF (2010) A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 31(31):7892–7927
Chen FM, Jin Y (2010) Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng B Rev 16(2):219–255
Fournier BP, Ferre FC, Couty L, Lataillade JJ, Gourven M, Naveau A, Coulomb B, Lafont A, Gogly B (2010) Multipotent progenitor cells in gingival connective tissue. Tissue Eng A 16(9):2891
Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD (2011) Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells 29(11):1849–1860
Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, Mishra GC, Wani MR (2010) Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun 393(3):377
Wang F, Yu M, Yan X, Wen Y, Zeng Q, Yue W, Yang P, Pei X (2011) Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev 20(12):2093
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM (2013) Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 34(29):7033–7047
Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, Nguyen AL, Kwon CW, Le AD (2010) Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 28(10):1856–1868
Fawzy El-Sayed KM, Paris S, Becker ST, Neuschl M, De Buhr W, Sälzer S, Wulff A, Elrefai M, Darhous MS, El-Masry M, Wiltfan J, Dörfer CE (2012) Periodontal regeneration employing gingival margin-derived stem/progenitor cells: an animal study. J Clin Periodontol 39(9):861–870
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689
Liu ZJ, Zhuge Y, Velazquez OC (2009) Trafficking and differentiation of mesenchymal stem cells. J Cell Biochem 106(6):984–991
Byrnes KR, Wu X, Waynant RW, Ilev IK, Anders JJ (2005) Low power laser irradiation alters gene expression of olfactory ensheathing cells in vitro. Lasers Surg Med 37(2):161–171
Kipshidze N, Nikolaychik V, Keelan MH, Shankar LR, Khanna A, Kornowski R, Leon M, Moses J (2001) Low-power helium: Neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro. Lasers Surg Med 28(4):355–364
Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A (2014) Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J Lasers Med Sci 5(4):163–170
Oliveira FA, Matos AA, Santesso MR, Tokuhara CK, Leite AL, Bagnato VS, Machado MA, Peres-Buzalaf C, Oliveira RC (2016) Low intensity lasers differently induce primary human osteoblast proliferation and differentiation. J Photochem Photobiol B Biol 163:14–21
Teuschl A, Balmayor ER, Redl H, Van GM, Dungel P (2015) Phototherapy with led light modulates healing processes in an in vitro scratch-wound model using 3 different cell types. Dermatol Surg 41(2):261–268
Gao Y, Zhang Y, Lu Y, Wang Y, Kou X, Lou Y (2016) Tob1 deficiency enhances the effect of bone marrow-derived mesenchymal stem cells on tendon-bone healing in a rat rotator cuff repair model. Cell Physiol Biochem 38(1):319–329
omür D, Sindel A, Serap Toru H, Yüce Esra Ay S, Tozogllu S (2016) The comparison of the efficacy of blue light-emitting diode light and 980-nm low-level laser light on bone regeneration. J Craniofac Surg 27(8):2185–2189
Yang D, Yi W, Wang E, Wang M (2016) Effects of light-emitting diode irradiation on the osteogenesis of human umbilical cord mesenchymal stem cells in vitro. Sci Rep 6:37370
Tomasello L, Mauceri R, Coppola A, Pitrone M, Pizzo G, Campisi G, Pizzolanti G, Giordano C (2017) Mesenchymal stem cells derived from inflamed dental pulpal and gingival tissue: a potential application for bone formation. Stem Cell Res Ther 8(1):179
Pagin MT, de Oliveira FA, Oliveira RC, Rezende MLRD, Greghi SLA, Damante CA (2014) Laser and light-emitting diode effects on pre-osteoblast growth and differentiation. Lasers Med Sci 29(1):55–59
Jyun-Yi W, Chia-Hsin C, Li-Yin Y, Ming-Long Y, Chun-Chan T, Yan-Hsiung W (2013) Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cellsviacyclic adenosine monophosphate. Int J Oral Sci 5(2):85–91
Whelan HT, Connelly JF, Hodgson BD, Barbeau L, Post AC, Bullard G, Buchmann EV, Kane M, Whelan NT, Warwick A, Margolis D (2002) NASA light-emitting diodes for the prevention of oral mucositis in pediatric bone marrow transplant patients. J Clin Laser Med Surg 20(6):319–324
Peng F, Wu H, Zheng Y, Xu X, Yu J (2012) The effect of noncoherent red light irradiation on proliferation and osteogenic differentiation of bone marrow mesenchymal stem cells. Lasers Med Sci 27(3):645–653
Li WT, Chen CW, Huang PY (2013) Effects of low level light irradiation on the migration of mesenchymal stem cells derived from rat bone marrow. Conf Proc IEEE Eng Med Biol Soc 2013:4121–4124
Gao Y, Zhang Y, Lu Y, Wang Y, Kou X, Lou Y, Kang Y (2016) TOB1 deficiency enhances the effect of bone marrow-derived mesenchymal stem cells on tendon-bone healing in a rat rotator cuff repair model. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 38(1):319–329
Liebmann J, Born M, Kolb-Bachofen V (2010) Blue-light irradiation regulates proliferation and differentiation in human skin cells. J Investig Dermatol 130(1):259–269
Higuchi A, Shen PY, Zhao JK, Chen CW, Ling QD, Chen H, Wang HC, Bing JT, Hsu ST (2011) Osteoblast differentiation of amniotic fluid-derived stem cells irradiated with visible light. Tissue Eng A 17(17):2593–2602
Mikami R, Mizutani K, Aoki A, Tamura Y, Aoki K, Izumi Y (2017) Low-level ultrahigh-frequency and ultrashort-pulse blue laser irradiation enhances osteoblast extracellular calcification by upregulating proliferation and differentiation via transient receptor potential vanilloid 1. Lasers Surg Med 50(4):340–352
Higuchi A, Shen PY, Zhao JK, Chen CW, Ling QD, Chen H, Wang HC, Bing JT, Hsu ST (2011) Osteoblast differentiation of amniotic fluid-derived stem cells irradiated with visible light. Tissue Eng A 17(21–22):2593–2602
Hou JF, Zhang H, Yuan X, Li J, Wei YJ, Hu SS (2008) In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med 40(10):726–733
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22(4):347–354
Liu TCY, Duan R, Yin PJ, Li Y, Li SL (2000) Membrane mechanism of low-intensity laser biostimulation on a cell. In: Biomedical Photonics and Optoelectronic Imaging pp 186–192
Liu TCY, Jiao JL, Xu XY, Liu XG, Deng SX, Liu SH (2005) Photobiomodulation: phenomenology and its mechanism. Proceedings of SPIE. The Int Soc Opt Eng 5630:185–191
Owen TA, Aronow M, Shalhoub V, Barone LM, Wilming L, Tassinari MS, Kennedy MB, Pockwinse S, Lian JB, Stein GS (1990) Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol 143(3):420–430
Wang D, Christensen K, Chawla K, Xiao G, Kresbach PH, Franceschi RT (1999) Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res 14(6):893–903
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Wang J, Qiang Z, Zhu Q, Song S, Shi L, Ma C, Tan X, Yin D, Niu Z (2013) Temporal expression of Pelp1 during proliferation and osteogenic differentiation of rat bone marrow mesenchymal stem cells. PLoS One 8(10):e75477
Sutherland JC (2002) Biological effects of polychromatic light. Photochem Photobiol 76(2):164–170
Funding
This work was supported by the Luzhou Municipal People’s Government-Southwest Medical University science and technology strategic cooperation projects of China (no. 2017LZXNYD-T03), Luzhou Municipal Science and Technology Bureau of China (no. 2016-R-70(13/24)). The reagents of this study were supported by these funds that all came from Southwest Medical University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study were in accordance with the Ethics Committee of the Affiliated Hospital of Stomatology Southwest Medical University Certificate (contract grant 20180314001) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 2871 kb)
Rights and permissions
About this article
Cite this article
Zhu, T., Wu, Y., Zhou, X. et al. Irradiation by blue light-emitting diode enhances osteogenic differentiation in gingival mesenchymal stem cells in vitro. Lasers Med Sci 34, 1473–1481 (2019). https://doi.org/10.1007/s10103-019-02750-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-019-02750-3