Abstract
The aim was to analyze the non-specific effects (placebo, spontaneous remission, and regression to the mean) of the low-level laser therapy (LLLT) in women with myofascial pain (painful temporomandibular disorder (TMD)), as well as to differentiate between responders and non-responder clusters to active and placebo LLLT according to the anxiety levels, salivary cortisol, use of oral contraceptives, and premenstrual period. Sixty-four women diagnosed with myofascial pain (Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD)) were included, divided into laser (n = 20), placebo group (n = 21), and 23 controls (without treatment (WT)). The LLLT applied was 780 nm, masseter and temporal = 5 J/cm2 (20 mW–0.5 W/cm2), and TMJ area = 7.5 J/cm2 (30 mW–0.8 W/cm2), eight sessions, twice a week. The pain intensity (visual analogue scale (VAS)), anxiety (Beck Anxiety Inventory), salivary cortisol, and menstrual cycle’s data at the baseline, T1–T8, and 30 days after LLLT (follow-up) were evaluated. The laser group showed 80% of pain reduction, placebo 85%, and WT 43% in T8. Women with severe anxiety and at the premenstrual period did not reduce pain with any LLLT. Active and placebo LLLT had similar effectiveness during the treatment period; however, women with moderate anxiety, cortisol levels above 10 ng/ml, and without contraceptive use maintain analgesia longer with active LLLT than placebo (follow-up 30 days). Women with low levels of anxiety, salivary cortisol below 10 ng/ml, and with contraceptive use showed the higher pain reduction. The analgesia promoted by LLLT in women with myofascial pain is a result of non-specific effects during the treatment period, although active LLLT is more effective in maintaining the analgesia after treatment (30 days) for the cluster of women with moderate anxiety, salivary cortisol above 10 ng/ml, and without contraceptive use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporomandibular disorder (TMD) is a painful musculoskeletal condition, in which the perceiving of signs and symptoms is modulated by emotional and hormonal factors [1, 2]. Women with high levels of stress and anxiety have increased risk of developing myofascial pain, and personal traits of anxiety are strongly associated with the diagnosis of painful TMD [3]. The hormonal fluctuations that occur in menstrual cycle seem to increase the perception of pain in the clinical context of TMD [4]. In addition to pain in the orofacial region, women with TMD often report areas of pain in other body regions. This chronic widespread pain condition is associated with pathophysiological changes in pain processing, mainly to central sensitization phenomena [5].
Low-level laser therapy (LLLT) has been a complementary therapeutic modality that aims for analgesia and mandibular mobility improvement in cases of painful TMD, although there is no scientific consensus about the doses/protocols, and clinical results are still controversial and unpredictable [6]. The biological effects provided by LLLT in applied tissues have been demonstrated in animal models [7, 8], but the results from randomized clinical trials are divergent, as some studies have shown a superior effect of placebo, and others have shown a similar effect of placebo [9,10,11]. With this perspective, it is important to identify which clusters of painful TMD patients are responders and non-responders to LLLT (active and placebo), to correctly indicate this complementary treatment and obtain better clinical outcomes.
The aim of this study was to analyze the non-specific effects (placebo, spontaneous remission, and regression to the mean) of the analgesia promoted by LLLT in women with myofascial pain (painful TMD), as well as to differentiate between responders and non-responder groups to active and placebo LLLT according to the anxiety levels, salivary cortisol, use of oral contraceptives, and premenstrual period.
Methods
The data collected in this research are part of a double-blind randomized controlled trial registered at the Brazilian Registry of Clinical Trials (REBEC) under protocol RBR-2v6ghb. The first part of the results has been previously published [12].
Study design and selection criteria
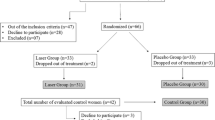
A double-blind randomized controlled trial (parallel) was conducted. Sixty-four women diagnosed with painful TMD, with a mean age of 31.7 ± 5.2 (range from 18 to 40 years), composed a sample for convenience of a patients’ population whom sought health care in the TMD and Orofacial Pain Service at School of Dentistry of Ribeirão Preto/São Paulo University. The eligibility criteria for inclusion were female, reporting pain in the facial area for at least 3 months, and diagnosed with myofascial pain according to the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD—axis I, categories Ia and Ib) [13]. The sample was divided into three groups: laser group (n = 20), placebo group (n = 21), and without treatment group (n = 23). The untreated group allowed the identification of non-specific effects (regression to the mean and spontaneous remission) that could be attributed to the placebo or active LLLT.
Women who fulfilled the criteria previously described randomly assigned to receive treatment (laser or placebo) or not. The randomization was made by lottery method (simple) after the initial assessment: papers written tip A and B were placed in an envelope and were randomly selected for each patient to avoid directing patients to specific groups. The laser tips were also named A and B to enable study blinding, and the evaluations/questionnaires, applications, and different evaluators carried out laser sessions. The tips were identical, and both have emitted a guiding light (visible) and a beep; however, the placebo tip did not emit the laser light. The device setting for adjustment of the energy density, time, and power was made equally for both tips; the equipment used in the study made it possible to adjust the dose, but in the placebo tip, no laser radiation was emitted. The person responsible for setting the device was not involved in the data collection or analysis. Researchers and patients were given access to information on the laser/placebo tips only after the completion of the study (double blind). Participants were evaluated at the following times: baseline (before treatment), T1–T8 (treatment, eight sessions, twice weekly for 4 weeks), and 30 days after LLLT treatment (follow-up); the patients that did not receive treatment were followed for the same period and assessments moments.
We excluded women who were receiving any treatment modality for TMD; had a tumor or trauma history; previous diagnosis of fibromyalgia or other painful musculoskeletal syndromes; neurological and psychiatric disorders; took anxiolytics, antidepressants, and anticonvulsants; or were pregnant. During the study, all participants were instructed not to use antiinflammatory and analgesic drugs, which could interfere with the pain assessment. The evaluations were conducted from October 2014 to December 2015. This study was approved by the Ethics Committee in Research of the School of Dentistry of Ribeirão Preto (under protocol 33658114.7.0000.5419).
Low-level laser therapy
A GaAlAs laser (Twin Laser, MMOptics, São Carlos, São Paulo, Brazil) was used after calibration by the manufacturer. The laser application was performed at predetermined points: the masseter (three points: upper, middle, and lower), the anterior temporalis (three points: upper, middle, and lower), and the TMJ region (four points forming a cross and one central point). The LLLT was performed in two sessions per week for four consecutive weeks, totaling eight sessions. The continuous emission mode was used, in direct contact with the patient’s skin, with the tip perpendicular to the irradiated area. The irradiation parameters used were wavelength = 780 nm; distance between the points = 1 cm, spot area = 0.034 cm2, masseter and anterior temporal: energy density = 5 J/cm2 (20 mW; 10 s–0.5 W/cm2); and for the TMJ area = 7.5 J/cm2 (30 mW; 10 s–0.8 W/cm2).
Each placebo group member received applications with a tip, similar to an active laser tip however emitting only a guide light (wavelength = 630 nm, 0 J/cm2, non-therapeutic light) and an audible signal. During the laser sessions, researchers and patients in both groups used protective goggles and obeyed the biosafety standards. The control group received no treatment with LLLT, and the participants were assessed using the same parameters described in a single session.
Pain assessment, anxiety, and salivary cortisol
The pain intensity was measured by means of a visual analogue scale (VAS), considering the point 0 (left) as no pain and 10 (right) as the worst pain imaginable by the patient. Each volunteer was instructed to mark what point best represented the intensity of her pain.
To determine the anxiety levels, the Beck Anxiety Inventory (BAI) was applied. This questionnaire is composed of 21 multiple-choice questions, with four possible answers (Likert scale): no (zero), mild (score 1), moderately (2), and severely (4). The total score represents the perceived anxiety level: 0–15 (low), 16–25 (moderate), and 26–63 (severe).
To the dosages of salivary cortisol, the data collection was conducted between 7 and 10 a.m., with a precleaning of the oral cavity by brushing and rinsing with abundant water [14]. Approximately 1 ml of saliva was collected, stored in the Salivette® tube (Genese LTDA, São Paulo, Brazil), and kept in a freezer −20 °C. The patients were instructed to put the cotton roller in the mouth floor area below the tongue or chew it for 3 min. The measurements of salivary cortisol were performed by an external laboratory (Laboratório Especializado em Análises Clínicas (LEAC) LTDA, São Paulo, Brazil). The used kit was the KAPDB290 (CTS Salivar-150810-DiaSource). The methodology of analysis was ELISA (sensitivity of 1.0 ng/ml). Between 7 and 10 a.m., the salivary cortisol considered normal might vary according to some studies: below 6.4–8.2 ng/ml [15,16,17]. In the present study, the reference value adopted was 10 ng/ml, based on the statistical analysis of the sample, which pointed this value as being a cutoff point among women with painful TMD who presented higher or less pain intensity.
Contraceptive use and premenstrual period
The data relative to the contraceptive use (type, administration, continuous use or with breaks) or not, date of last menstruation, and premenstrual period (considered as 5 days before the first day of menstruation) was also collected. These variables were monitored weekly during the study period and patient’s follow-up.
Assessment moments
Participants were evaluated at the following times: baseline (before treatment), T1–T8 (treatment, eight sessions, twice weekly for 4 weeks), and 30 days after LLLT treatment. The pain intensity (VAS), anxiety levels, the data relative to the contraceptive use, and premenstrual period were assessed every session; the salivary cortisol was measured at the baseline, T2, T4, T8, and 30 days (follow-up).
Statistical analysis
Initially, the data showed a non-parametric distribution (Shapiro-Wilk test); then, a logarithmic transformation was performed; and the Tukey-Kramer test for multiple comparisons was used for groups with different sizes (p < 0.05).
Results
Table 1 shows the results relative to the sample composition, mean age, TMD pain duration (month), TMD classification (RDC/TMD), contraceptive use, salivary cortisol, and anxiety levels. There was a small variation in the mean age between the groups, which ranged from 29 to 32 years. The mean duration of TMD pain was higher for the placebo group (75 months) and lower for the without treatment group (53 months). Regarding to the TMD classification, the laser and placebo groups had a higher percentage of myofascial pain diagnoses associated with joint dislocation and degenerative disease (45 and 53%, respectively), whereas the without treatment group showed a slight superiority of diagnoses of myofascial pain alone (46%) (Table 1).
In general, most of the women have used contraceptives: 70% for the laser group, 61% for placebo, and 73% for the without treatment group. Among these, oral contraceptives were the most used, either continuously or with breaks. Regarding salivary cortisol and anxiety, the largest portion of the sample had dosages above 10 ng/ml and moderate anxiety level for all groups (Table 1).
The laser and placebo groups showed reduction of pain intensity from the third laser session (T3) and maintained the results after 30 days of reevaluation with the same effectiveness. Compared to the baseline, the laser group showed 80% of pain reduction and the placebo 85% after the end of treatment (T8). The group of women who did not receive any treatment also showed a significant reduction in pain intensity at the following moments: T6, T8, and 30 days, whereas in T8, there was a 43% reduction of pain in comparison to the baseline (Fig. 1).
Women with severe anxiety levels did not respond to active or placebo LLLT, because they did not report reduction in pain intensity during the treatment or reevaluation period. Women with moderate anxiety had a reduction in pain only in the last laser sessions (from T5 to laser and T4 to placebo), and at the reevaluation assessment (30 days after LLLT), the placebo group returned to pain levels similar to the baseline, while the laser group maintained the results of analgesia. Low-anxiety women responded more effectively to active and placebo LLLT, as they showed reduction of pain in the first laser sessions and maintained the results even after therapy was finished (Fig. 2).
Mean and error bars of the pain intensity (VAS) during study assessments (baseline, T1–T8, and 30 days) for the subgroups: severe, moderate, and low anxiety (groups laser [I] and placebo [II]). The asterisks indicate statistical difference in comparison to the baseline for each group, intra-group analysis (Tukey-Kramer, p < 0.05)
Regarding the salivary cortisol, women who had dosages below 10 ng/ml had lower pain intensity at baseline and responded similarly to active and placebo LLLT, while women who had salivary cortisol above 10 ng/ml reported higher pain scores, greater delay in analgesia response (from T6), and better response to active LLLT, which promoted higher reduction of pain and maintained the results after 30 days (Fig. 3).
Mean and error bars of the pain intensity (VAS) during study assessments (baseline, T1–T8, and 30 days) for the subgroups: salivary cortisol above 10 ng/ml (gray) and under 10 ng/ml (black) (groups laser and placebo). The asterisks indicate statistical difference in comparison to the baseline for each group, intra-group analysis (Tukey-Kramer, p < 0.05)
Similarly, women using contraceptives had lower baseline pain scores and effectively responded to active and placebo LLLT. However, women who did not use contraceptives reported greater pain intensity throughout the study period, but responded better to active LLLT, which promoted higher analgesia and maintenance of re-evaluation results, although the placebo group also had pain remission at certain times during the treatment period (T3, T6, and T8) (Fig. 4).
Mean and error bars of the pain intensity (VAS) during study assessments (baseline, T1–T8, and 30 days) for the subgroups: without contraceptive use (gray) and with contraceptive use (black) (groups laser and placebo). The asterisks indicate statistical difference in comparison to the baseline for each group, intra-group analysis (Tukey-Kramer, p < 0.05)
Women who were in the premenstrual period at the time of evaluation did not report reduction in pain intensity for both the active and placebo groups at time T4, T8, and 30 days. Women who were not in the premenstrual period had a significant reduction in pain scores for all moments assessed, regardless of active or placebo therapy (Fig. 5).
Mean (bar) and standard deviation (whisker) of the pain intensity (VAS) during the assessments: baseline, T4, T8, and 30 days for the subgroups: premenstrual and not premenstrual periods (groups laser [I] and placebo [II]). Number of women in each assessment: laser (11 with contraceptive use with breaks and 6 without contraceptive use, total: 17)—premenstrual (baseline—6, T4—5, T8—7, 30 days—7) and not premenstrual (baseline—11, T4—12, T8—10, 30 days—10); placebo (9 with contraceptive use with breaks and 7 without contraceptive use, total: 16)—premenstrual (baseline—4, T4—5, T8—6, 30 days—6) and not premenstrual (baseline—12, T4—11, T8—10, 30 days—10). The asterisks indicate statistical difference in comparison to the baseline for each group, intra-group analysis (Tukey-Kramer, p < 0.05)
Discussion
The placebo effect is defined as beneficial health outcomes that are not related to the relatively direct biological effects of an intervention and can be elicited by an inert agent [18, 19]. Every modality of therapeutic intervention is associated with beneficial cognitive effects, such as reducing stress, and modulatory mechanisms of the mesolimbic and mesocortical areas related to the expectation of a cure/improvement are the most described [18, 19]. The brain mechanisms activated by placebo are very similar to those activated by drugs, for example. The patient’s psychosocial context during any kind of therapy (the ritual of the therapy act) may change the biochemical and neural circuitry, leading to clinical results that are quite satisfactory, even without any therapeutic intervention [20, 21].
The pain intensity reported is the subjective graduation of a global experience, which is influenced by cognitive, motivational, cultural, and other factors. The factors that underlie the placebo effect are as significant in the perception of pain relief as the biological effect of the laser, because they lead to similar clinical results [18, 19]. The placebo response is closely related to the reward expectation and pain relief because of the anticipatory anxiety, which means that placebo and anxiety are very close in clinical practice [20]. In our study, the placebo effect was probably higher because LLLT is a therapeutic modality that involves expensive equipment and technology, leading to more expectation. Another important point to be discussed is that the guide light emitted by the non-therapeutic tip may amplify the placebo effect because of the visualization of the light, and might have some therapeutic effect, even if not indicated by the manufacturer.
The analgesia promoted by LLLT is a result of non-specific effects during the intervention period, since the group that did not receive treatment also showed reduction of pain, indicating spontaneous remission of signs/symptoms and regression to the mean, besides the placebo group presented the same effectiveness than the active laser group. This “get-better-anyway effect” phenomenon occurs in clinical trials, whose variable of interest is pain, because at the beginning of the study, patients usually show maximum pain intensity scores, since this is an inclusion criterion, and over time, there is a tendency for natural regression of pain, characteristic of painful TMD [22, 23].
In the present study, it was also shown that the application of active and placebo LLLT is more effective than no intervention at all, as other studies involving chronic pain have already found similar results: reduction of 30 to 40% of initial pain without any treatment and above 68–90% with treatment [24,25,26]. According to the Initiative on Methods, Measurement and Pain Assessment in Clinical Trials (IMMPACT), a 30% of pain reduction represent the minimum threshold expected to be considered an effect of moderate clinical importance, while a 50% of reduction represent an important change [27]. Within this perspective, there are groups of responders and non-responders to certain therapies for chronic pain, so it is important to identify clusters that respond to LLLT to correctly indicate this complementary therapy.
Consequently, the results of this study demonstrated that women with severe anxiety are non-responders to active or placebo LLLT, while women with moderate anxiety also responded to active and placebo LLLT during the treatment period, and after 30 days of therapy’s finalization, they responded better to active LLLT. The group of women with low anxiety scores was the one who responded best to LLLT, both active and placebo, without distinction. The perception of TMD signs and symptoms is significantly modulated by emotional factors, which are associated with pain multidimensionality. Women with high levels of anxiety show more risk of developing myofascial pain, and personal traits of anxiety are strongly associated with the diagnosis of painful TMD [1, 3].
Emotional factors such as pain catastrophizing and anxiety can influence the expression of the pain control process, increasing nociceptive sensitization [1, 2]. Anxiety has been identified as a predictor of pain perception, while other factors such as sex, depression, stress, somatosensory amplification, age, and weight appear to have no direct relationship with the referred pain intensity levels; however, they could influence the nociceptive sensitization [1, 2]. The presence of painful TMD leads to a greater predisposition to anxiety [3], and more anxious individuals are more susceptible to the perception of pain in TMD [1].
The relationship between pain and emotional factors, for example, anxiety and stress, is intrinsic, since they contribute to a higher nociceptive perception and less activation of endogenous mechanisms of pain modulation [2]. Considering that pain is a subjective experience, in the presence of anxiety and stress, the perception and interpretation of pain information can be amplified. Furthermore, the pain may have an emotional commitment that leads to social isolation, kinesiophobia, anxiety, stress, and depression [2, 3, 25, 28]. In the case of TMD, the high psychological distress (mood, anxiety, depression, stress response, somatization) and state of pain amplification (neuroendocrine and autonomic function, impaired pain regulation, proinflammatory state) directly influence the perception of pain symptomatology, in addition to the genetic/epigenetic factors and environmental contributions [29].
The results also showed that women with lower levels of salivary cortisol (below 10 ng/ml) are more responsive to active and placebo LLLT than women with cortisol above 10 ng/ml. There is an association between pain sensitivity, endogenous levels of salivary cortisol, and psychological stress (perceived), which may influence the magnitude of response to certain types of therapy [28, 30]. Although, it is relevant to consider that psychological stress may not be directly related to the salivary cortisol dosage. A recent study demonstrated heterogeneity in the sample of TMD patients: some had hypercortisolemia, while others had no differences in cortisol dosage when compared to controls [31].
Women with contraceptive use, especially those with continuous use, suffer less hormonal changes during the menstrual cycle, as the sudden decrease in estradiol and increase in progesterone during ovulation increase the TMJ nociceptive sensitivity, which predisposes women who do not use contraception to show higher levels of pain, regardless of whether they belong to the laser or placebo group [32]. However, there is no consensus in the scientific literature about the association between estrogen levels and TMD [33].
Hormonal fluctuations may generate, in addition to higher nociceptive sensitivity, a greater difficulty in responding to painful TMD therapies. The results of this study demonstrated that women who use contraceptives are more responsive to active and placebo LLLT than women who do not use, confirming this hypothesis. Among women who did not use contraceptives, the active laser group maintained analgesia longer than placebo, which returned to pain after 30 days.
The premenstrual period seems to be critical to the pain perception, as women with myofascial pain in this phase of the menstrual cycle showed higher pain intensity and sensitivity. Other studies have shown that in the perimenstrual phase, the pain sensitivity is lower compared to the follicular/ovulatory phases, and this could predispose women to feeling more pain [4]. The sudden drop in progesterone in the late luteal phase of the menstrual cycle may contribute to the development of premenstrual symptoms, including anxiety and cutaneous hyperalgesia, as a result of an increase in the FOS protein in the periaqueductal gray (PAG) [34]. In this sense, the results of our study demonstrated that women in the premenstrual period are non-responders to LLLT, both active or placebo. Therefore, women who report experiencing premenstrual symptoms or are in this phase of the cycle when they seek professional help for painful TMD probably will not have analgesia response with the LLLT.
Such findings should guide the clinical practice of professionals that use LLLT in women with painful TMD, who should be conscientious that the effectiveness is a result of non-specific effects during the intervention period and that some clusters (women with moderate anxiety, cortisol levels above 10 ng/ml, and the non-use of contraceptives) maintains analgesia longer when they receive active laser rather than placebo (reevaluation after 30 days), although during laser sessions, there are no differences in effectiveness. Besides, there are clusters of women that are non-responders to LLLT (severe anxiety and who are in the premenstrual period). In agreement with our results, the main indications of LLLT are women with low levels of anxiety, salivary cortisol below 10 ng/ml, and who use contraceptives without distinction between active and placebo LLLT.
The limitations of this study were the non-use of a protocol/questionnaire that evaluate the perceived emotional stress in complementation to the salivary cortisol dosage; the follow-up time of the results obtained with LLLT that could be extended beyond 1 month; and the menstrual cycle assessments that should be performed at all stages of the cycle, not only at the premenstrual cycle, depending on a sample with no contraceptive use.
Conclusion
The analgesia promoted by LLLT in women with myofascial pain (painful TMD) is a result of non-specific effects (placebo, spontaneous remission, and regression to the mean). Active and placebo LLLT have similar effectiveness during the treatment period, although active LLLT is more effective in maintaining the analgesia after treatment (30 days of revaluation) for the subgroup of women with moderate anxiety, salivary cortisol above 10 ng/ml, and without contraceptive use. Women with severe anxiety or who are in the premenstrual period are non-responders to active or placebo LLLT. The most respondent clusters are women with low levels of anxiety, salivary cortisol below 10 ng/ml, and who use oral contraceptives, without distinction between active and placebo LLLT.
References
Dıraçoglu D et al (2008) Temporomandibular dysfunction and risk factors for anxiety and depression. J Back Musculoskelet Rehabil 29(3):487–491
Nahman-Averbuch H et al (2016) Relationship between personality traits and endogenous analgesia: the role of harm avoidance. Pain Pract 16(1):38–45
Reissmann DR et al (2014) Temporomandibular disorder pain is related to the general disposition to be anxious. J Oral Facial Pain Headache 28(4):322–330
Drobek W, Schoenaers J, De Laat A (2002) Hormone-dependent fluctuations of pressure pain threshold and tactile threshold of the temporalis and masseter muscle. J Oral Rehabil 29(11):1042–1051
Lim PF et al (2010) Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain 26(2):116–120
Chen J et al (2015) Efficacy of low-level laser therapy in the treatment of TMDs: a meta-analysis of 14 randomised controlled trials. J Oral Rehabil 42(4):291–299
Iyomasa MM et al (2013) Zymographic and ultrastructural evaluations after low-level laser irradiation on masseter muscle of HRS/J strain mice. Lasers Med Sci 28(3):777–783
Silveira PC et al (2013) Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med Sci 28(2):431–436
de Melchior MO et al (2013) Does low intensity laser therapy reduce pain and change orofacial myofunctional conditions? Cranio 31(2):133–139
De Moraes Maia ML et al (2014) Evaluation of low-level laser therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med Sci 29(1):29–35
Sancakli E et al (2015) Early results of low-level laser application for masticatory muscle pain: a double-blind randomized clinical study. BMC Oral Health 15(1):131
Magri LV et al (2017) Effectiveness of low-level laser therapy on pain intensity, pressure pain threshold, and SF-MPQ indexes of women with myofascial pain. Lasers Med Sci 32(2):419–428
Schiffman EL (2010) The research diagnostic criteria for temporomandibular disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain 24(1):63–78
Hill CM, Walker RV (2001) Salivary cortisol determination and self-rating scales in the assessment of stress in patients undergoing the extraction of wisdom teeth. Br Dent J 191:513–515
Kobayashi H et al (2017) Diurnal changes in distribution characteristics of salivary cortisol and immunoglobulin a concentrations. Int J Environ Res Public Health 31:14(9)
Smyth J et al (1997) Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology 22:89–105
Kirschbaum C et al (1999) Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 61:154–162
Oken BS (2008) Placebo effects: clinical aspects and neurobiology. Brain 131(11):2812–2823
Colagiuri B et al (2015) The placebo effect: from concepts to genes. Neuroscience 307:171–190
Jakovljevic M (2014) The placebo-nocebo response: controversies and challenges from clinical and research perspective. Eur Neuropsychopharmacol 24(3):333–341
Frisaldi E, Piedimonte A, Benedetti F (2015) Placebo and nocebo effects: a complex interplay between psychological factors and neurochemical networks. Am J Clin Hypn 57(3):267–284
Morton V, Torgerson DJ (2003) Effect of regression to the mean on decision making in health care. BMJ 326(7398):1083–1084
Hróbjartsson A, Gøtzsche PC (2010) Placebo interventions for all clinical conditions. Cochrane Database Syst Ver 1:CD003974
Ferreira ML et al (2013) The smallest worthwhile effect of nonsteroidal anti-inflammatory drugs and physiotherapy for chronic low back pain: a benefit-harm trade-off study. J Clin Epidemiol 66(12):1397–1404
O'Brien EM et al (2010) Patient-centered perspective on treatment outcomes in chronic pain. Pain Med 11(1):6–15
Zeppieri G Jr et al (2012) Preliminary results of patient-defined success criteria for individuals with musculoskeletal pain in outpatient physical therapy settings. Arch Phys Med Rehabil 93(3):434–440
Dworkin RH et al (2008) Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 9(2):105–121
Jo KB et al (2016) Association of pain intensity, pain-related disability, and depression with hypothalamus-pituitary-adrenal axis function in female patients with chronic temporomandibular disorders. Psychoneuroendocrinology 69:106–115
Slade GD et al (2016) Painful temporomandibular disorder: decade of discovery from OPPERA studies. J Dent Res 95(10):1084–1092
Godfrey KM et al (2014) Salivary cortisol and cold pain sensitivity in female twins. Ann Behav Med 47(2):180–188
Salameh E et al (2015) Investigation of the relationship between psychosocial stress and temporomandibular disorder in adults by measuring salivary cortisol concentration: a case-control study. J Indian Prosthodont Soc 15(2):148–152
Vilanova LS et al (2015) Hormonal fluctuations intensify temporomandibular disorder pain without impairing masticatory function. Int J Prosthodont 8(1):72–74
Berger M et al (2015) Association between estrogen levels and temporomandibular disorders: a systematic literature review. Prz Menopauzalny 14(4):260–270
Devall AJ, Lovick TA (2010) Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats. Neuropsychopharmacology 35(5):1174–1185
Acknowledgments
This study was financially supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were performed in accordance with the guidelines of the Brazilian Ethics Committee on Human Research. This study was conducted after approval by the Ethics Committee of the School of Dentistry of Ribeirão Preto (under protocol 33658114.7.0000.5419). All subjects were informed about the study and signed a consent form. Patients who composed the placebo group and did not have pain reduction after the study completion were invited to receive treatment with LLLT, in the same parameters of the active laser group.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Magri, L.V., Carvalho, V.A., Rodrigues, F.C.C. et al. Non-specific effects and clusters of women with painful TMD responders and non-responders to LLLT: double-blind randomized clinical trial. Lasers Med Sci 33, 385–392 (2018). https://doi.org/10.1007/s10103-017-2406-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2406-4