Abstract
Low-level laser therapy (LLLT) has been widely used in the treatment of the stomatognathic system dysfunction; however, its biological effect remains poorly understood. This study evaluated the effect of LLLT (GaAlAs, 780 nm, 20 J/cm², 40 mW) on masseter muscle of HRS/J mice after different numbers of laser irradiations (three, six, and ten) for 20 s in alternate days. Three experimental groups were defined according to the number of laser irradiations and three control groups (n = 5) were used. On the third day after the last irradiation, all animals were killed and the masseter muscle was removed and processed for the following analysis: (a) transmission electron microscopy, (b) zymography, (c) immunohistochemistry for vascular endothelial growth factor (VEGF) and VEGFR-2. The results showed: (a) with six laser applications, a dilation of T tubules, and sarcoplasmic reticulum cistern, increased pinocytosed vesicles in the endothelium; with ten laser applications, few pinocytic vesicles in the endothelium and condensed mitochondria. (b) Under the conditions of this study, the synthesis of other matrix metalloproteinases was not observed, only the MMP-2 and -9. (c) After ten laser irradiations, immunostaining was observed only for VEGFR-2. We conclude that after six laser applications, ultrastructural changes may facilitate the Ca+2 transfer to cytosol and increase the fluid transport from one surface to another. The ultrastructural changes and no immunostaining for VEGF with ten applications may decrease the metabolic activity as well as damage the angiogenic process, suggesting that an effective number of laser applications may be less than ten, associating to this therapy a better cost–benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In medical practice, lasers are widely used for treatment of patients with temporomandibular disorders, involving the joints and masticatory muscles, with pain expression that radiates to the face, neck, and shoulders. The laser treatment has been successful due to its anti-inflammatory effect [1], its analgesic and muscular relax actions [2], ability to reduce fatigue during tetanic contractions [3], improves the skeletal muscle performance and decrease the skeletal muscle damage [4], delays muscle fatigue and decreases the blood lactate post-exercise in rat model [5]. In addition, laser reduces electromyographic activity and muscular symptoms [6], increases the bite force, and decreases the orofacial pain [7, 8]. Despite these studies demonstrating the efficacy of low-level laser therapy (LLLT), the biological effects on specific tissues are not well understood.
Non-surgical lasers use portions of the visible and invisible spectrum, with wavelengths between 700 and 980 nm, emitting pulsed or continuous wave [9], whose power reaches up to hundreds of milliwatts (mW). Therefore, an enormous variation in these used parameters was found in literature. For example, irradiation with He–Ne laser (632.8 nm) energy density of 7.2 J/cm2 has a deleterious effect on the keratinocytes differentiation [10]. On the other hand, the LLLT with 15 mW output power and 3.8 J/cm2 of energy density for 15 s, induced apoptosis during the tissue healing after three applications [11]. Moreover, it was observed that the 585 nm laser may be more harmful than the 595 nm in vascular tissue of normal human skin [12]. The changes on instrument type, semiconductor, wavelength, intensity, exposure time, and duration of LLLT used in different published studies make comparisons difficult. Each wavelength has different type of interactions, depending if the target is muscle or joint tissues [13, 14], considering that each one has different characteristics [15]. According to Rizzi et al. [16], LLLT decreased the iNOS protein and blocked the NF-kB activation in injured gastrocnemius muscle.
Several previous studies analyzed the changes of muscle fibers in malocclusion induction by histoenzymologic methods, which identify the fibers according to the speed of fiber contraction and oxidative capacity [17, 18]. However, a fewer number of studies analyzed the laser effect on muscular ultrastructure in experimental animals [19].
The matrix metalloproteinases (MMPs) are a family of zinc endopeptidases capable of degrading protein structures such as collagen, elastin, and other proteins present in the extracellular matrix [20, 21]. These enzymes are primarily responsible for remodeling, degradation, or modification of the extracellular matrix [21]. Under physiological conditions, activated MMPs are controlled by tissue inhibitors of metalloproteinases and are usually expressed at low levels. However, the levels of some MMPs may be raised rapidly when the tissue is subjected to ultraviolet radiation (UVR) [22, 23] during inflammation, wound healing, and progression of cancer [24, 25]. However scarce studies are found involving laser on the extracellular matrix of muscle by zymography, which is an important method for MMPs detection.
Vascular endothelial growth factor (VEGF) is a glycoprotein that belongs to the family gene for vascular growth factors, involved in angiogenesis [26]; it is a protein located in the subsarcolemmal region of skeletal muscle fibers, in vascular smooth muscle and the wall of capillaries, being of the last mentioned structure, one factor that has an effect on endothelial cell proliferation [27]. VEGF acts at capillaries neoformation from pre-existing blood vessels, and there are five types of VEGF in mammals: VEGF-A, VEGF-B, VEGF-C, VEGF-D, and PlGF (placental growth factor). Among its receptors, the VEGFR-1 and the R-2 are receptors for VEGF-A, and it is believed that the binding of VEGF-A receptor VEGFR-2 is the major signaling pathway for angiogenesis [28]. However, recent data show that VEGF exerts multiple non-angiogenic effects in various cell types including neurons, skeletal muscle, and heart cells. One therapy that results in the formation of new blood vessels, by angiogenic gene action, seems to be a promising strategy [29]. In this context, the family of VEGF and VEGFR-2 may be considered an important molecular tool to analyze the effect of laser on the muscle.
Currently, despite the increasing use of phototherapy in the medical field, there are a small number of basic researches using LLLT. Literature shows the need for further studies using animal models, considering that the wavelengths, dosages, and appropriate conditions of laser irradiation are not well established [30], and phototherapy is a possible alternative treatment to reduce the need for invasive procedures [31]. Thus, to help clinicians in understanding the biological effect of lasers in clinical practice and improve the cost benefits associated with this treatment, this study examined the effect of three different laser applications (three, six, and ten) on masseter muscle of HRS/J hairless mouse, using the usually recommended parameters [32] for muscle pain and temporomandibular disorder therapy.
Materials and methods
Laser
The Twin Laser (MM Optics; São Carlos, São Paulo, Brazil) was used, 780 nm, with a semiconductor gallium–aluminum–arsenide, and the assessment of output power was made before this experiment research using this apparel by the manufacturer.
Animals
Male mice, strain HRS/J (hairless), 3 months old, weighing about 35 g, obtained from the vivarium of the Faculty of Dentistry of Ribeirão Preto, University of São Paulo were divided into groups according to the number of laser irradiations (three, six and ten). Each group was composed by control (n = 5) and irradiated (n = 5) animals. The animals were kept in polyethylene boxes under conditions of controlled room temperature between 24 and 25° C and 12 h of light per day. They received food and water ad libitum. All study procedures were approved by the Local Ethics Committee (number: 08.1.290.53.9) in accordance with international laws for animals use.
Experimental procedures
Animals of the experimental group were anesthetized with halothane and the left masseter muscle was irradiated with energy density of 20 J/cm2 (continuous wave, 40 mW, 20s, 0.04 cm2 of spot area) in alternate days [32]. The laser irradiation was performed in direct contact with the skin, on one point (Energy: 0.8 J/point, power density: 1 W/cm2) in the middle region of the left masseter muscle. The control group also received the same treatment but without irradiation. Seventy-two hours after the last application [33] the animals were anesthetized with intramuscular injection of xylazine (10 mg/kg) and ketamine (75 mg/kg) and the masseter muscle was dissected for transmission electron microscopy, immunohistochemistry and zymography analyses.
Samples preparation for transmission electron microscopy (TEM)
On the third day after the last irradiation, animals were anesthetized and the left masseter muscle was excised, reduced to small fragments, and immersed in modified Karnovsky solution containing 2.5 % glutaraldehyde and 2 % paraformaldehyde in sodium phosphate buffer 0.1 M, pH 7.4 [34]. The fragments, post-fixed in a solution of osmium tetroxide at 4° C, were immersed in 5 % uranyl acetate aqueous, dehydrated in an ascending series of alcohol, treated with propylene oxide, infiltrated by mixture of pure resin and propylene oxide (1:1), and embedded in Spurr resin to complete polymerization. After identifying the interest area, in thick sections (300 nm), ultrathin sections (55 nm) were obtained, collected on 200 mesh networks, contrasted with uranyl acetate and lead citrate solutions [34], and examined using a transmission electron microscope Jeol 1010 (TEM).
Qualitative analysis of proteinases (MMP) by zymography
The substrate zymography included in sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) was used to detect gelatinase activity of whether the masseter muscle irradiated or not. Masseter muscle dissected and frozen in dry ice (1:4 w/w dilution) was homogenized in 50 mM Tris–HCl (pH 7.4) containing 10 mM CaCl2 and 1 % cocktail of protease inhibitors in a “Turrax TE-102” (Turratec, São Paulo, Brazil). The total of homogenate muscle was centrifuged at 12,000×g and used to measure the levels of protein by the Lowry method [35]. Aliquots of supernatant (50 mL) were mixed with 100 mM Tris–HCl (pH 7.4) containing 4 % SDS, 20 % glycerol, and 0.001 % bromophenol blue. Proteolytic activity was assessed qualitatively, comparing the irradiated and control animals.
Immunohistochemistry for vascular endothelial growth factor and receptor-2 for vascular endothelial growth factor
Transversal histological sections, 10 μm thick, were obtained from muscle rapidly frozen in isopentane, cooled in liquid nitrogen, using the Leica cryostat (CM1850, Germany).
To inhibit nonspecific binding, slides were incubated with PBS-BSA (2 %) in an acrylic tank. The slides were incubated with primary antibody diluted in PBS-BSA (2 %), washed, and endogenous peroxidase activity was blocked by immersion in a container with 30 % H2O2. The sample tissues were covered by drops of yellow link DAKO, transferred to an acrylic tub, and then covered by red link. After washing and drying, each slide was covered by the developer solution (Dako kit), and counter-stained by hematoxylin and mounted in Entellan. The images were captured using a Leica IM50 program on a computer connected to a Leica DC 300F camera adapted to the Leica microscope DMLB2.
Result
Ultrastructure of the masseter muscle
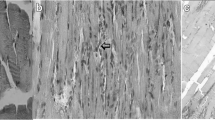
Under transmission electron microscopic analysis, muscular fibers with invaginations of the sarcolemma and dilated cisternae of sarcoplasmic reticulum (Fig. 1a) in relation to other groups was observed in the group with six laser irradiations. The group submitted to ten laser irradiations showed the mitochondrial cristae to be condensed and with dilated T tubules and sarcoplasmic reticulum cisternae (Fig. 1d). In relation to endothelial cells, the group with six irradiations (Fig. 1b) showed pinocytosis and macropinocytose vesicles, as well as cytoplasmic protrusions and endothelial cell budding, whose characteristics were more relevant than in groups with no irradiation (Fig. 1c) and ten irradiations (Fig. 1e).
a Irradiated muscle (6) with dilated cisternae of sarcoplasmic reticulum (arrow). Magnification ×20,000. b Irradiated endothelial cell (6) with numerous pinocytosis (arrow) and macropinocytose vesicles (thick arrow) and endothelial cell budding (curved arrow). Magnification ×20,000. c Non irradiated muscle with normal T tubules and cisterns of sarcoplasmic reticulum (arrow). Magnification ×20,000. d Irradiated muscle (10) with condensed mitochondrial cristae (arrow) and dilated T tubules and cisternae of sarcoplasmic reticulum (thin arrow). Magnification ×20,000. e Irradiated (10) capillary (*) with thin wall and few pinocytic vesicles. Magnification ×20,000
Zymography
The representative data of two separate experiments with five animals showed that the LLLT did not change the proteinase activity profile of masseter muscle in this experimental animal model (Fig. 2). However, analyzing the electrophoretic profiles corresponding to irradiated groups, i.e., which received ten laser irradiations, it is possible to observe lower intensity of the bands with proteolytic activity when compared with the control group (Fig. 2, T3).
Electrophoretic profile of the proteolytic activity in masseter muscle of hairless mice. C1, 2, and 3 correspond to non irradiated groups. T1, 2, and 3 correspond to groups with three, six, and ten irradiations (20 J/cm2), respectively. The data are representative of two separate experiments (n = 5)
Immunohistochemistry for VEGF and VEGFR-2
VEGF immunostaining was diffusely found in connective tissue of the perimysium and endomysium, whose pattern was similar between the control and treated groups with three and six laser irradiations (Fig. 3a, b, c and d), however, without immunoidentification in the group that received ten laser irradiations (Fig. 3e and f). Figure 3g shows the negative control (VEGF) of irradiated masseter muscle.
Masseter muscle, VEGF immunostaining. a Control for the group that received three laser applications, ×40 magnification. b Control for the group that received six laser applications, Magnification ×40. c control for the group that received ten laser applications, ×40 magnification. d Three laser applications, magnification ×40. e Six laser applications, magnification ×40. f Ten laser applications with weaker immunostaining, magnification ×40. g Negative control, VEGF of irradiated masseter muscle, magnification ×40
Immunohistochemical expression of VEGFR-2 showed immunoidentification more localized in vascular endothelial cells, whose expression pattern was similar between the control and irradiated with three, six, and ten applications (Fig. 4a, b, c, d, e, f). Figure 4g shows the negative control (VEGFR-2) of irradiated masseter muscle.
Masseter muscle, VEGFR-2 immunoidentification. a Control for the group that received three laser applications, magnification ×40. b Control for the group that received six laser applications, magnification ×40. c Control for the group that received ten laser applications, magnification ×40. d Three laser irradiations group, magnification ×40. e Six laser irradiations group, magnification ×40. f Ten laser irradiations group, magnification ×40. g Negative control, VEGFR-2 of irradiated masseter muscle, magnification ×20
Discussion
The most relevant ultrastructural alterations of muscle fibers demonstrated in the treated group at six and ten laser irradiations, dilated invaginations of the sarcolemma (T-tubule) and sarcoplasmic reticulum cisterns. Considering that this organelle stores Ca+2 at high concentrations, these characteristics suggest that the low laser therapy interfere with the transfer mechanism of Ca+2 into the cytosol. With regard to the ultrastructure of mitochondria, the group that received ten laser irradiations showed a condensation mitochondrial cristae, whose characteristics may reveal that the number of radiation interfere in metabolic activity of the muscle fiber. These data strengthen the studies of Rizzi et al. [36] in which the increased metabolic activity revealed by the reaction of succinate dehydrogenase was evident by the number of six laser applications, but was lower with ten applications. It is well established that the chain of mitochondrial electron transport is photosensitive to red and infrared light [37], being activated by LLLT [38, 39]. The literature described that a muscle using laser irradiation with an energy density of 5 and 10 J/cm2 for seven consecutive days, showed an increase in the space between the outer and inner membrane of mitochondria and also dilated mitochondrial cristae [19]. With respect to endothelial cells, the group with six laser irradiations showed pinocytosis vesicles and macropinocytose, as well as cytoplasmic protrusions and endothelial cells budding, more relevant than to the groups with three and ten irradiations. The relationship of these vesicles is clear; with the ability of the cell membrane include fluids, transporting them from one surface to another, in both directions, suggesting that six irradiations stimulated the activity of endothelial cells. Although the molecular mechanisms involved in these biological effects are still little known, laser therapy improves the microcirculation [40]. Ten laser irradiations under the experimental conditions of this study caused undesirable damage on the ultrastructural morphology of mitochondria and endothelial cells, which may impair the metabolic activity of the muscle fiber. These data corroborate to the studies in which ten applications of laser (20 J/cm2) altered the relative area of the basal and granulosum layers of the epithelium [25, 41]. Thus, studies aiming to find a number of the most effective laser irradiation will be very important, which may be smaller than the one used in this study. The present research evaluated the effects of laser irradiation on proteinases activity in masseter muscle of HRS/J mice. Using zymographic methods as it was used in the present study, acute exposure to ultraviolet radiation caused a dose-dependent induction (0.96 to 2.87 J/cm2) in the activity of MMP-2 (72 kDa of gelatinase) and MMP-9 (92 kDa of gelatinase) in the skin of hairless mice [42]. LLLT did not cause significant changes in proteinase activity profile on masseter muscle of HRS/J mice, different from the effect caused by the exposure to ultraviolet radiation. However, analyzing the electrophoretic profiles corresponding to the group that received ten laser irradiations, we found lower intensity of the bands indicating proteolytic activity, when compared with the control group. It is known that under normal conditions, MMP activity is required for tissue remodeling, but altered MMP activity has been reported in disease. When the masseter muscle of rats were irradiated with different doses of LLLT (0, 0.5, 1, 2.5, 5, 20 J/cm2), the groups irradiated with the highest doses revealed increased activity of MMP-2; and in all groups exposed to different energy densities of laser irradiation, MMP-9 activity [43] increased. The band of low intensity showed by zymography method suggests that ten laser applications may increase the risks for altered activity of MMP on healthy muscle. Thus, future studies using different parameters are needed aiming to clarify this issue.
In the present study, the VEGF immunostaining patterns were similar between the control and treated groups with three and six laser irradiations, but no immunostaining was found in the group with ten laser irradiations. VEGF is an essential mediator for angiogenesis in skeletal muscle [44]. Some studies show positive results between laser application and VEGF expression in various tissues and experimental conditions, as culture of odontoblast-like cells [45], psoriatic plaques [46], neuropathic pain after chronic constrictive lesion [47], recovery of bone defects in rats with osteoporosis [48], healing of burns [49], and post-infarct hearts in rats [50]. Researches showing immunoreactivity for VEGF and VEGFR-2 antibodies in muscle are scarce in the literature; however, these factors had a positive identification on the masseter muscle when the rats were irradiated with different doses of LLLI (0, 0.5, 1, 2.5, 5, 20 J/cm2) [51]. After ten laser radiations in this research, as it was described by Dias et al. using highest energy density, no VEGF immunoidentification was found in mouse muscle, suggesting that angiogenesis may be impaired when compared to control groups and treated groups with three and six irradiations.
The patterns for VEGFR-2 immunostaining were similar between the control and treated groups with three, six, and ten irradiations. VEGFR-2 is mainly expressed in vascular endothelial cells, and it has strong tyrosine kinase activity, acting as a transducer for the primary differentiation and proliferation of positive endothelial cell precursor [52]. Thus, the similar VEGFR-2 expression between the groups suggests in the present research that the differentiation and proliferation of endothelial precursor cell was not modified by different number of laser irradiations.
In this experimental condition, the ultrastructural and molecular effects of the low-level laser therapy were evident. Although the large number of laser irradiations stimulated the muscle fiber activities, some characteristics suggested that the actual number of laser irradiations may be less than ten. Then, to associate the better cost–benefit to this therapy, besides the effectiveness and safety, future studies that correlate molecular effects and laser irradiations that are lower than ten irradiations are necessary.
References
Reis SR, Medrado AP, Marchionni AM, Figueira C, Fracassi LD, Knop LA (2008) Effect of 670-nm laser therapy and dexamethasone on tissue repair: a histological and ultrastructural study. Photomed Laser Surg 26:307–313
Núñez SC, Garcez AS, Suzuki SS, Ribeiro MS (2006) Management of mouth opening in patients with temporomandibular disorders through low-level laser therapy and transcutaneous electrical neural stimulation. Photomed Laser Surg 24:45–49
Lopes-Martins RA, Marcos RL, Leonardo PS, Prianti AC, Muscará MN, Aimbire F, Frigo L, Iversen VV, Bjordal JM (2006) Effect of low-level laser (Ga–Al–As 655 nm) on skeletal muscle fatigue induced by electrical stimulation in rats. J Appl Physiol 101:283–288
de Almeida P, Lopes-Martins R, Tomazoni SS, Silva JA, de Carvalho PET, Bjordal JM, Leal Junior EC (2011) Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem Photobiol 87:1159–1163
Leal Junior EC, Lopes-Martins RA, de Almeida P, Ramos L, Iversen VV, Bjordal JM (2010) Effect of low-level laser therapy (GaAs 904 nm) in skeletal muscle fatigue and biochemical markers of muscle damage in rats. Eur J Appl Physiol 108:1083–1088
Shinozaki S, Ohnishi H, Hama K, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Mashima H, Sugano K (2008) Indian hedgehog promotes the migration of rat activated pancreatic stellate cells by increasing membrane type-1 matrix metalloproteinase on the plasma membrane. J Cell Physiol 216:38–46
de Medeiros JS, Vieira GF, Nishimura PY (2005) Laser application effects on the bite strength of the masseter muscle, as an orofacial pain treatment. Photomed Laser Surg 23:373–376
Emshoff R, Bösch R, Pümpel E, Schöning H, Strobl H (2008) Low-level laser therapy for treatment of temporomandibular joint pain: a double-blind and placebo-controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105:452–456
Airaksinen O, Kolari PJ, Hietanen M, von Nandelstradh P, Põntinen PJ (1993) Low power lasers in physical therapy: measurement of optical output power of devices. Acupunct Electrother Res 18:9–16
Haas AF, Isseroff RR, Wheeland RG, Rood PA, Graves PJ (1990) Low-energy helium-neon laser irradiation increases the motility of cultured human keratinocytes. J Invest Dermatol 94:822–826
Rocha Júnior AM, Vieira BJ, de Andrade LC, Aarestrup FM (2009) Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed Laser Surg 27:303–307
Pikkula BM, Chang DW, Nelson JS, Anvari B (2005) Comparison of 585 and 595 nm laser-induced vascular response of normal in vivo human skin. Lasers Surg Med 36:117–123
Karu T, Pyatibrat L, Kalendo G (1995) Irradiation with He–Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–223
Karu TI (2008) Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol 84:1091–1099
Shefer G, Barash I, Oron U, Halevy O (2003) Low-energy laser irradiation enhances de novo protein synthesis via its effects on translation-regulatory proteins in skeletal muscle myoblasts. Biochim Biophys Acta 1593:131–139
Rizzi CF, Mauriz JL, Freitas Corrêa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J (2006) Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med 38:704–713
Maxwell L, Carlson D, McNamara JJ, Faulkner J (1981) Adaptation of the masseter and temporalis muscles following alteration in length, with or without surgical detachment. Anat Rec 200:127–137
Kitagawa Y, Mitera K, Ogasawara T, Nojyo Y, Miyauchi K, Sano K (2004) Alterations in enzyme histochemical characteristics of the masseter muscle caused by long-term soft diet in growing rabbits. Oral Dis 10:271–276
Iyomasa DM, Garavelo I, Iyomasa MM, Watanabe IS, Issa JP (2009) Ultrastructural analysis of the low level laser therapy effects on the lesioned anterior tibial muscle in the gerbil. Micron 40:413–418
Kossodo S, Wong WR, Simon G, Kochevar IE (2004) Effects of UVR and UVR-induced cytokines on production of extracellular matrix proteins and proteases by dermal fibroblasts cultured in collagen gels%. Photochem Photobiol 79:86–93
Lahmann C, Young AR, Wittern KP, Bergemann J (2001) Induction of mRNA for matrix metalloproteinase 1 and tissue inhibitor of metalloproteinases 1 in human skin in vivo by solar simulated radiation. Photochem Photobiol 73:657–663
Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ (1997) Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 337:1419–1428
Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ (1996) Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379:335–339
Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17:463–516
Zucker S, Pei D, Cao J, Lopez-Otin C (2003) Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol 54:1–74
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9:777–794
Hudlicka O, Brown MD (2009) Adaptation of skeletal muscle microvasculature to increased or decreased blood flow: role of shear stress, nitric oxide and vascular endothelial growth factor. J Vasc Res 46:504–512
Yen P, Finley SD, Engel-Stefanini MO, Popel AS (2011) A two-compartment model of VEGF distribution in the mouse. PLoS One 6:e27514
Giacca M, Zacchigna S (2012) VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond. Gene Ther 19:622–629
Gao F, Chen XL, Wei P, Gao HJ, Liu YX (2001) Expression of matrix metalloproteinase-2, tissue inhibitors of metalloproteinase-1, -3 at the implantation site of rhesus monkey during the early stage of pregnancy. Endocrine 16:47–54
Wu EC, Wong BJ (2008) Lasers and optical technologies in facial plastic surgery. Arch Facial Plast Surg 10:381–390
Meireles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL (2008) Effectiveness of laser photobiomodulation at 660 or 780 nanometers on the repair of third-degree burns in diabetic rats. Photomed Laser Surg 26:47–54
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Watanabe I, Yamada E (1983) The fine structure of lamellated nerve endings found in the rat gingiva. Arch Histol Jpn 46:173–182
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Rizzi É, Issa JP, Dias FJ, Leão JC, Regalo SC, Siéssere S, Watanabe IS, Iyomasa MM (2010) Low-level laser intensity application in masseter muscle for treatment purposes. Photomed Laser Surg 28(Suppl 2):S31–S35
Tafur J, Mills PJ (2008) Low-intensity light therapy: exploring the role of redox mechanisms. Photomed Laser Surg 26:323–328
Silveira PC, Streck EL, Pinho RA (2007) Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B 86:279–282
Wilden L, Karthein R (1998) Import of radiation phenomena of electrons and therapeutic low-level laser in regard to the mitochondrial energy transfer. J Clin Laser Med Surg 16:159–165
Tullberg M, Alstergren PJ, Ernberg MM (2003) Effects of low-power laser exposure on masseter muscle pain and microcirculation. Pain 105:89–96
Leão JC, Issa JP, Pitol DL, Rizzi EC, Dias FJ, Siéssere S, Regalo SC, Iyomasa MM (2011) Histomorphological and angiogenic analyzes of skin epithelium after low laser irradiation in hairless mice. Anat Rec (Hoboken) 294:1592–1600
Vicentini FT, Simi TR, Del Ciampo JO, Wolga NO, Pitol DL, Iyomasa MM, Bentley MV, Fonseca MJ (2008) Quercetin in w/o microemulsion: in vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur J Pharm Biopharm 69:948–957
Dias FJ, Issa JP, Vicentini FT, Fonseca MJ, Leão JC, Siéssere S, Regalo SC, Iyomasa MM (2011) Effects of low-level laser therapy on the oxidative metabolism and matrix proteins in the rat masseter muscle. Photomed Laser Surg 29:677–684
Audet GN, Meek TH, Garland T, Olfert IM (2011) Expression of angiogenic regulators and skeletal muscle capillarity in selectively bred high aerobic capacity mice. Exp Physiol 96:1138–1150
Pereira LB, Chimello DT, Wimmers Ferreira MR, Bachmann L, Rosa AL, Bombonato-Prado KF (2012) Low-level laser therapy influences mouse odontoblast-like cell response in vitro. Photomed Laser Surg 30:206–213
Rácz E, de Leeuw J, Baerveldt EM, Kant M, Neumann HA, van der Fits L, Prens EP (2010) Cellular and molecular effects of pulsed dye laser and local narrow-band UVB therapy in psoriasis. Lasers Surg Med 42:201–210
Hsieh YL, Chou LW, Chang PL, Yang CC, Kao MJ, Hong CZ (2012) Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury-possible involvements in hypoxia-inducible factor 1α (HIF-1α). J Comp Neurol. In press
Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Peitl O, Zanotto ED, Parizotto NA (2011) Biosilicate® and low-level laser therapy improve bone repair in osteoporotic rats. J Tissue Eng Regen Med 5:229–237
Renno AC, Iwama AM, Shima P, Fernandes KR, Carvalho JG, De Oliveira P, Ribeiro DA (2011) Effect of low-level laser therapy (660 nm) on the healing of second-degree skin burns in rats. J Cosmet Laser Ther 13:237–242
Tuby H, Maltz L, Oron U (2006) Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med 38:682–688
Dias FJ, Issa JP, Barbosa AP, de Vasconcelos PB, Watanabe IS, Mizusakiiyomasa M (2012) Effects of low-level laser irradiation in ultrastructural morphology, and immunoexpression of VEGF and VEGFR-2 of rat masseter muscle. Micron 43:237–244
Shibuya M (2006) Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): a dual regulator for angiogenesis. Angiogenesis 9:225–230, discussion 231
Acknowledgment
We are grateful to FAPESP for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iyomasa, M.M., Rizzi, E.C., Leão, J.C. et al. Zymographic and ultrastructural evaluations after low-level laser irradiation on masseter muscle of HRS/J strain mice. Lasers Med Sci 28, 777–783 (2013). https://doi.org/10.1007/s10103-012-1156-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1156-6