Abstract
Women with temporomandibular disorders (TMD) frequently report pain areas in body regions. This process is associated with central sensitization phenomena, present in chronic pain. The low-level laser therapy (LLLT) has been reported as a therapeutic option for the painful TMD treatment. The aim of this study was to analyze the effect of LLLT on pain intensity (visual analogue scale, VAS), pain sensitivity in orofacial and corporal points (pressure pain threshold, PPT), and on Short Form-McGill Pain Questionnaire (SF-MPQ) indexes of women with myofascial pain (subtype of muscle TMD). Ninety-one women (18–60 years) were included in the study, among which 61 were diagnosed with myofascial pain (Research Diagnostic Criteria for Temporomandibular Disorder—Ia and Ib) and were divided into laser (n = 31) and placebo group (n = 30), and 30 were controls. The LLLT was applied at pre-established points, twice a week, eight sessions (780 nm; masseter and anterior temporal = 5 J/cm2, 20 mW, 10 s; TMJ area = 7.5 J/cm2, 30 mW, 10 s). Pain intensity, pain sensitivity, and the SF-MPQ indexes were measured at the baseline, during laser sessions, and 30 days after treatment. For intra-group comparisons, the Friedman test was performed, and for inter-group, the Mann-Whitney test. Increased pain sensitivity was found in women with myofascial pain when compared to controls (p < 0.05). There was a reduction in pain intensity for both groups after LLLT. The LLLT did not change the PPT for any group (p > 0.05). Active laser and placebo reduced the indexes of sensory, total pain, and VAS, maintaining the results after 30 days; there was a reduction in the affective pain rating index for both groups, with no maintenance after 30 days for placebo, and the present pain intensity decreased in the laser group and did not change in the placebo after LLLT. In conclusion, the LLLT active or placebo are effective in reducing the overall subjective perception of myofascial pain (VAS and SF-MPQ indexes); however, they have no effectiveness in reducing the pain sensitivity in orofacial and corporal points (PPT increase).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Temporomandibular disorder (TMD) is a collective term for a musculoskeletal pain syndrome that affects the masticatory muscles, temporomandibular joint (TMJ), and associated structures [1]. Epidemiological studies have shown that 75% of the population have had at least one TMD sign (incoordination of jaw movements and joint sounds) and 33% at least one symptom (presence of pain in orofacial area and associated structures, limitation in jaw mobility, and difficulty to perform the orofacial functions) [1, 2]. Pain is the main symptom in TMD clinical situation and is usually the reason to search for professional help. The prevalence of painful TMD (orofacial pain) varies between 4 and 10% in the general population, with females during reproductive years at higher risk. Some authors have suggested that the estrogen-induced hyperinflammatory phenotype in women can contribute to central sensitization and might predispose the painful TMD [3].
In addition to pain in the orofacial region, TMD patients often report pain areas in other body regions. This widespread pain condition is associated with the pathophysiological changes in pain processing, mainly the central sensitization phenomena, which is defined as the “increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input” by the International Association for the Study of Pain (IASP). Therefore, they have more predispositions to other painful comorbidities, such as fibromyalgia, back pain, and neck pain, among others [4, 5]. The presence of these tender body points has been associated with an increased risk for the development of TMD and greater difficulty in controlling this condition along with its persistent signs and symptoms [6]. From this perspective, previous studies have shown that these individuals have pressure pain thresholds (PPTs) reduced in corporal points beyond the orofacial area when compared to healthy subjects [4, 6, 7].
One of the methods used to assess pain intensity is the visual analogue scale (VAS), which is one dimensional, reliable, validated and has adequate sensitivity [8, 9]. In addition to the quantitative measures, the evaluation of pain sensitivity through PPT establishment has been increasingly used in the evaluation of patients with TMD signs and symptoms. Many studies have shown that the PPT has wide applicability in clinical practice and research, considering the diagnosis and management of patients with TMD [7, 10, 11]. Criteria for the establishment of the PPT in the masticatory muscles are already well defined, are considered reliable, and are available in the scientific literature [7, 11].
Another instrument widely used to assess pain is the McGill Pain Questionnaire, which was developed by Melzack in 1975 and aims to assess pain in three dimensions: sensory-discriminative, motivational-affective, and cognitive-evaluative [12]. Its first version consisted of 78 descriptors, and in 1987, the same author proposed a reduced version (Short Form-McGill Pain Questionnaire, SF-MPQ) with 15 descriptors (11 sensory and 4 affective), which was translated and validated for Brazilian Portuguese [13, 14]. The use of this questionnaire provides a qualitative assessment of pain, which can complement quantitative measures, such as pain intensity and sensitivity.
Many treatment modalities have been described for the management of TMD. The first choice is always conservative, reversible, and non-invasive, considering the fact that in most cases the TMD is self-limiting and easy controlled [1, 2]. The use of low-level laser therapy (LLLT) has been shown to be an additional option in the TMD treatment, and it is aimed at providing pain relief and the reestablishment of quality of life [15–19]. LLLT promotes analgesic/anti-inflammatory effects and regenerates cells in the tissues in which it is applied, with no reported adverse effects and good acceptance by patients [20].
The results obtained using LLLT are still controversial, as some studies have shown a superior effect to placebo [16, 21–23] and others similar effect to placebo [17, 24]. These differences can be attributed to the great variability of doses, protocols, and application areas that makes it difficult to compare, and some study reviews have shown that there is not enough scientific evidence to indicate predictable results of this therapeutic modality [25, 26].
Several studies have been conducted demonstrating the effectiveness of LLLT in reducing pain at orofacial points, committed by the clinical condition of TMD [16, 21–23, 27]. However, it is unknown what effect this therapy has in corporal points with decreased PPT (distance), which can be sensitized and associated with an imbalance of modulation mechanisms and pain perception. Therefore, the objective of this study was to analyze the effect of LLLT on pain intensity (VAS), pain sensitivity in orofacial and corporal points (PPT), and SF-MPQ indexes of women with myofascial pain.
Materials and methods
Sample
The total sample consisted of 91 women, aged between 18 and 60 years, of whom 61 were diagnosed with myofascial pain, while 30 were controls. This study was developed at the Department of Morphology, Basic Physiology and Pathology of University of São Paulo, and the criteria for inclusion were as follows: female, reporting pain in the facial area lasting at least 3 months, and diagnosed with myofascial pain according to the criteria of the Research Diagnostic Criteria for Temporomandibular Disorder (RDC/TMD—axis I, categories Ia and Ib) [28]. The diagnosis of TMD was established through the revised version of the RDC/TMD (axis I), applied by a single examiner, which was previously trained and calibrated. The evaluations were conducted from October 2014 to December 2015.
Exclusion criteria were follows: women who were in any treatment modality to TMD (interocclusal splints, acupuncture, pharmacological treatment, and others); tumor history; trauma or head and neck surgery; previous diagnosis of fibromyalgia and other painful musculoskeletal syndromes; presence of neurological and psychiatric disorders; women who used prescription drugs, such as anxiolytics, antidepressants, and anticonvulsants; pregnant women; and pacemaker users. During the study, all participants were instructed not to use anti-inflammatory and analgesic drugs, which could interfere with the pain assessment.
The sample comprised only women, as there is a higher prevalence of painful TMD in the female gender and the pain sensitivity (PPT) can vary significantly between men and women with or without TMD [3, 29].
Study design
A double-blind randomized controlled trial was conducted. The sample was divided into three groups: the laser group (n = 31), placebo group (n = 30), and control group (n = 30). Women who fulfilled the criteria described above were randomly assigned by lottery method to receive laser or placebo. The lottery was performed after the initial assessment of patients, 62 papers (31 written tip A and 31 tip B; one patient included at tip B group was excluded in the end of the study because she assumed to have used analgesic drug during the laser sessions) were placed in an envelope and were randomly selected for each patient, in order to avoid directing patients to specific groups. The nomination of the laser tips in A and B was necessary for the study blinding, just like the evaluations/questionnaires applications and laser sessions, which were carried out by different researchers. Researchers and patients were given access to information on laser/placebo tips only after the completion of the study (double blind). The parameters used to investigate the pain were pain intensity (VAS), pain sensitivity (PPT) in orofacial and corporal points, and the SF-MPQ indexes. Participants were evaluated at the following times: baseline (before treatment), T1–T8 (treatment, eight sessions, twice weekly for 4 weeks), and 30 days after LLLT treatment.
Assessment of pain
Pain intensity was measured by means of a VAS, which features a straight line of 10 cm, where the numbers are arranged in a gradual and increasing order, considering the point 0 (left) as no pain and 10 (right) as the worst pain imaginable by the patient. Each volunteer was instructed to mark what point best represented the intensity of her pain. The researchers then measured in centimeters the distance between the zero point and that marked by the patient, which represented the intensity of the patient’s pain at the time of the evaluation [8, 30].
Pain sensitivity was determined by the PPT, measured using a digital compression algometry IDDK model (Kratos, Cotia, São Paulo, Brazil), with a tip measuring 1 cm2 of diameter and an application rate of 0.5 kg/s. Each volunteer was instructed to press a lock button attached to the equipment, which was placed in her hands when she felt pressure becoming pain. Then, this value was noted by the researchers and represented the pain sensitivity at that moment. The PPT was measured bilaterally at the following points: the masseter, anterior temporal, and TMJ region (orofacial points); the occipital region (insertion of suboccipital muscle); the trapezoid (the midpoint of the upper area); the supraspinal region (above the scapula and around the medial area); the lower neck (the anterior portion of intertransversarii spaces between C5 and C7); the region of the lateral epicondyle (2 cm distal to the epicondyle); the knee (at the fat pad, knee medial line); the gluteus (the upper outer quadrant of the buttock, gluteus medius muscle); and the trochanter (posterior to its prominence). The determination of these corporal points was based on the tender points established as diagnostic criteria for fibromyalgia by the American College of Rheumatology [31]. It is important to consider that the presence of a previous diagnosis of fibromyalgia was an exclusion criterion for our sample, i.e., the location of tender points was only one reference used, considering possible areas of pain.
The evaluation of the sensory and affective dimensions of pain was performed by applying the SF-MPQ version translated and adapted to Brazilian Portuguese [13]. It consists of five indexes: sensory pain rating index (S-PRI), affective pain rating index (A-PRI), total pain rating index (T-PRI), VAS (evaluative overall intensity of total pain experience), and present pain intensity (PPI). The S-PRI consists of 11 descriptors about sensory pain experience and the A-PRI of four affective descriptors, these descriptors are measured based on a Likert scale of 0–3 the pain intensity at the time, with the scale featuring 0 (none), 1 (mild), 2 (moderate), and 3 (severe). The evaluative overall intensity of total pain experience consists of a VAS scale, similar to that previously described. Also, in the PPI are presented six words that describe patient’s experience: no pain, mild, discomforting, distressing, horrible, and excruciating. Each participant was instructed to point out the one that best described her pain at the time of the interview.
Laser irradiation parameters
A GaAlAs laser (Twin Laser, MMOptics, São Carlos, São Paulo, Brazil) was used after review and calibration by the manufacturer. The laser application was performed at predetermined points: the masseter (three points: upper, middle, and lower), the anterior temporal (three points: upper, middle, and lower), and the TMJ region (four points forming a cross and one central point) [18]. The LLLT was performed in two sessions per week for four consecutive weeks, totaling eight sessions [26, 27]. The LLLT was applied in the continuous emission mode, in direct contact with the patient’s skin, with the tip perpendicular to the irradiated area. The irradiation parameters used were as follows: wavelength = 780 nm (near infrared); distance between the points of application = 1 cm; spot area = 0.034 cm2; for masseter and anterior temporal: energy density = 5 J/cm2, laser optical power = 20 mW, and time per point = 10 s; and for the TMJ area: energy density = 7.5 J/cm2, laser optical power = 30 mW, and time per point = 10 s.
Each placebo group member received applications with a tip, similar to active laser tip but emitting only a guide light and an audible signal. Thus, it was not possible to identify the tips, which were named A and B. The identification of active and placebo tips was performed only after completion of the study. During the laser sessions, researchers and patients in both groups used protective goggles and obeyed the biosafety standards. The control group received no treatment with LLLT, and the participants were assessed using the same parameters described in a single session.
Statistical analysis
The data presented non-parametric distribution, so they were expressed as median and interquartile ranges. For an intra-group comparison of the median values of pain intensity, pain sensibility (PPT), and SF-MPQ indexes, the Friedman test of variance by ranks was performed. For inter-group comparisons between TMD patients and the controls (PPT), the Mann-Whitney test was used. For all tests, a significance level of 5% was considered.
Results
Sample and TMD diagnosis
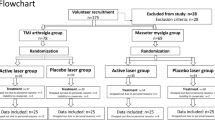
Initially, 148 women were evaluated, of which 66 fulfilled the criteria of research and were proposed for participation. Then, the sample was randomized and divided into three groups: laser (n = 33), placebo (n = 33) and control (n = 42). Thirty-one patients of the laser group, 30 of the placebo group, and 30 controls completed all phases of the study. The exclusion reasons are described in Fig. 1.
The average age of the laser group was 38.45 (±12.56), the placebo group 38.87 (±10.88), and the control group 38.67 (±11.18). Considering the patients with TMD, established by RDC/TMD (n = 61), and those who completed all phases of the study, 36 (59%) had myofascial pain (Ia), 14 (22%) myofascial pain with limited opening (Ib), and 11 (19%) myofascial pain associated with disk displacement or arthralgia, arthritis, and arthrosis (I/II and I/III).
Pain intensity (VAS)
There was a decrease in pain intensity (p < 0.05) for both groups (laser and placebo) when comparing the last session of treatment with the baseline (Fig. 2), for the laser group from T5 and the placebo group from T4. In the evaluation of 30 days after the treatment completion, the results regarding the perception of pain intensity were still decreased for both groups, with statistical significance (p < 0.05).
Median (horizontal line), square interquartile range (bar), and minimum and maximum (whiskers) values of pain intensity reported at the baseline, T1–T8 (laser sessions), and 30 days after treatment for the laser and placebo groups. Significant difference compared to baseline (Friedman test, considering p < 0.05) is indicated by the asterisk
Pressure pain threshold
The median values and interquartile range of the PPTs in orofacial and corporal points, considering TMD patients and controls, are shown in Table 1. When comparing these inter-group values at the baseline, a high statistical difference was found between TMD and controls for most of the sites, except for the right lateral epicondyle region (p = 0.08), indicating that TMD women not only have lower PPTs at orofacial sites but also decreased pain sensitivity in a generalized manner. This difference persisted after laser sessions (Table 1) in the inter-group comparison at T8, suggesting that the active laser or placebo was not able to change the pain sensitivity of TMD patients who have PPT decreased when compared to controls. Pain sensitivity was also analyzed intra-group along the laser sessions to observe if there was variance among the baseline, T4, T8, and 30 days after the treatment completion (Table 2). No differences were observed among the PPTs at the LLLT treatment times evaluated for both groups (p > 0.05) on any site, reinforcing the findings in the inter-group comparison.
SF-MPQ indexes
The SF-MPQ descriptors that had higher mean values reported in the perception of TMD patients were as follows: aching, heavy, tender, and tiring-exhausting. The intra-group analysis obtained from the SF-MPQ also points little or no difference between the laser and placebo groups, which followed the same trend: a reduced perception of pain during treatment when compared to the baseline (Fig. 3). The S-PRI, T-PRI, and VAS were similar between groups, and the perception of pain relief was maintained after 30 days for active laser and placebo. This maintenance of the treatment effect after 30 days was not observed for the placebo group in the A-PRI. Regarding the PPI, the laser group showed a significant reduction from the T6 and maintained this improvement even after 30 days, and the placebo group did not show any change for this index.
Median (horizontal line), square interquartile range (bar), and minimum and maximum (whiskers) values of S-PRI, A-PRI, T-PRI, PPI-VAS, and evaluative overall intensity of total pain experience (SF-MPQ indexes) at the following times: baseline, T2, T4, T6, T8, and 30 days after treatment for the laser and placebo groups. Significant difference compared to baseline (Friedman test, considering p < 0.05) is indicated by the asterisk
Discussion
The main results of this study were as follows: (1) increased pain sensitivity (PPT) in orofacial and corporal points of women with TMD when compared to controls, the LLLT (active or placebo) did not change this pain sensitivity; (2) reduction in pain intensity (VAS) for both groups (active laser and placebo); (3) SF-MPQ indexes S-PRI, T-PRI, and VAS decreased with maintenance of the results after 30 days of treatment completion for both groups. The A-PRI was decreased for both groups, but there was no maintenance of the results after 30 days for the placebo group; the PPI was decreased for the laser group and did not change for the placebo.
LLLT is a non-pharmacological therapeutic modality that is easy to apply, safe, and affordable. A meta-analysis published in 2015 provides the best current evidence about the LLLT effectiveness in the treatment of TMD. The study demonstrated that LLLT has a limited effectiveness in reducing pain in patients with TMD, but it promotes significant improvement in the functional aspects involving the jaw mobility, such as mouth opening and chewing [26]. Other studies have shown similar results, which point to an improvement in the orofacial functions of the masticatory muscles when treated with LLLT [18, 19, 32].
The LLLT effectiveness is more pronounced when using the infrared laser associated with the application protocols involving higher irradiation levels (energy density and/or power density), the greater number of sessions, and the frequency of application [27]. It is important to point out that there is no consensus in the scientific literature about the doses and protocols of LLLT for the TMD treatment that complicates standardization of the research and the comparison of results.
The LLLT has proven to have anti-inflammatory and analgesic effects (with a reduction of the orofacial allodynia and hyperalgesia) when applied in specific regions, such as the inflamed TMJs of rats [33]. However, these effects are promoted only in the applied areas, so it would be necessary prior to the palpation of the muscles and joints to determine the points of greatest pain and to map the locations where LLLT should be applied. Some studies use this technique and apply the laser at the points of the greatest pain [15, 19, 23], while other studies apply it at predetermined points [18, 22]. In our study, LLLT was applied at predetermined points, which may have influenced the results, especially in pain sensitivity (PPT), although Sancakli et al. (2015) conducted a study in which they compared the laser application location (at predetermined points vs. points of the greatest pain) and did not find differences [32].
The LLLT did not change the pain, measured by VAS, because the reduction of pain occurred for both groups, laser and placebo. Many factors can influence the perception of pain intensity by the patient: previous painful experiences, psychological factors (anxiety, depression, addiction), and demographic (educational level, type of work, marital status) [6]. Among these, the emotional factors have gained more prominence in scientific research, as they are associated cyclically with a painful experience: pain is influenced by emotional factors and the behavior can change by the presence of pain, as demonstrated in animal models [33, 34]. Pain intensity is probably the most evaluated parameter in TMD patients, either in research or in clinical practice, but the use of this tool can cause an overestimation or underestimation of pain [35].
These results could also be explained by the placebo effect, which arguably promotes analgesia by the release of endogenous opioids and the activation of the descending neural pathways of the pain modulation, which are influenced by many factors: memory, belief, hope of being treated, learning, professional-patient relationship, and socio-cultural context, among others [36, 37]. The use of a modern technology equipment, such as the laser, may have amplified the placebo effect and influenced the magnitude of the results.
The investigation into pain sensitivity, established by the PPT, has been increasingly used in researches because it allows for an objective assessment of the pain threshold in a specific muscle or joint location [11]. Asymptomatic individuals have high PPT levels, while individuals suffering with myofascial pain syndromes have low values. This difference in pain sensitivity in local and distant points can be explained by central and peripheral sensitization processes, which lead to the increased excitability of nociceptors [11, 38]. From this perspective, previous studies have shown that these individuals have reduced PPTs at other corporal points beyond the masticatory muscles compared to healthy subjects, confirming the results obtained in our study [4, 6, 38].
The LLLT has not changed the pain sensitivity in both groups, active laser and placebo, considering the orofacial and corporal points evaluated. These results contradict previous studies that showed that laser therapy was able to increase the PPT of the masticatory muscles in TMD patients [22, 32]. However, these studies did not define the sex in their samples, unlike in our study, which used only women, who differ in the pain modulation process when compared to men [29].
The reduction in pain intensity and no changes in pain sensitivity in women with TMD can be explained by the fact that the intensity of reported pain (VAS) is related to a general perception of the painful experience, which is the result of a complex combination of physical and emotional factors. Since the evaluation of pain sensitivity (PPT) is a much more objective parameter, which considers a specific site at the time of the examination, it is less influenced by impressions and personal interpretations. Although both evaluations represent subjective perceptions, there seems to be no correlation between pain intensity and sensitivity, the hypothesis that the high pain intensity is related to the increased pain sensitivity has not been demonstrated in a previous study, agreeing to the results obtained in ours [39].
Lauche et al. (2014) also stated that the assessment of pain intensity and sensitivity are parameters that should not be analyzed together: the PPT is measured in a restricted area, while the perception of pain intensity is related to a wider area, involving various anatomical structures [40]. The PPT is a compression stimulus that becomes painful from a certain threshold, and the pain intensity reflects the patient’s opinion about a painful experience; the PPT and VAS are influenced by emotional factors differently; and the relationship between the pain intensity and the PPT does not occur in a linear way due to the chronic characteristics of pain, such as central sensitization [30, 40].
It should be considered that the women in this study had pain in the orofacial region for at least 3 months, so it is plausible that these painful experiences presented chronicity characteristics with central sensitization and failure in pain modulation pathways that may have contributed to the persistence of the pain syndrome and no PPT modification [41]. Other studies have obtained results indicating significant improvement in scores of VAS and PPT values with minor changes, showing that perhaps the change in pain sensitivity is a process more difficult to occur [42]. Slade et al. (2014) stated that PPT is a floating variable, which can be changed, but it should not be used as a predictive factor for the development or maintenance of a painful TMD [43]. Greenspan et al. (2013) demonstrated that increased pain sensitivity contributes modestly to the risk of developing TMD, i.e., the PPT modification does not guarantee the absence of perceived pain by patients with TMD [44].
The SF-MPQ evaluates pain in quantitative and qualitative dimensions, which allows an assessment more accurate of this experience, because usually the pain is evaluated only based on quantitative variables. The results of this study from the analysis of pain descriptors indicate that, considering the sensory dimension, the pain from TMD manifests as a heavy and aching pain that worsens or is elicited by palpation (tender) and the affective dimension as a tiring-exhausting pain. Both descriptions are related to a musculoskeletal painful condition, characteristic of the TMD. Cao et al. (2008) found very similar results, but in the affective dimension, the descriptor “sickening” was also widely reported [45].
The comparison of the SF-MPQ indexes of sensory, affective, total pain, and VAS (evaluative overall intensity of total pain experience) during laser sessions followed the same trend for the laser and placebo groups, these indexes have reduced when compared to the baseline. The PPI for the laser group showed better results when compared to the placebo. The SF-MPQ indexes are composed by the subjective perception of pain experience, based on the last days and on the moment of assessment. Although the groups showed in general similar results to the SF-MPQ indexes, the PPI showed differences between laser and placebo, which did not occur in the evaluation of pain intensity, and both evaluations are related to the pain in the moment of assessment. This can be explained by the fact that PPI is a qualitative assessment, while the VAS is a visual-numerical evaluation (quantitative), in which the individual quantifies the pain intensity. The qualitative analysis is always more difficult to assess than the quantitative because it demands a better understanding of the scale and descriptors by the patient [13, 14].
In this sense, the most subjective variables evaluated in this study (VAS and SF-MPQ indexes) that represent the overall perception of the pain experience were reduced in laser and placebo groups. Also, the PPT, which is considered a more objective variable and is related to neurophysiological processes of the pain chronicity, did not change. It can be argued that the subjective perception of painful experiences after treatment did not differ between patients who composed the laser and the placebo groups, so the effectiveness of LLLT in pain control was not superior to the placebo effect.
In conclusion, active LLLT and placebo reduced the overall perception of pain experience, measured by pain intensity (VAS) and SF-MPQ indexes, but were not able to change the pain sensitivity (PPT) in orofacial and corporal points of women with myofascial pain. Thus, pain control promoted by LLLT was not superior to the placebo effect since both groups had similar results. The reduction in pain intensity and SF-MPQ indexes was not accompanied by an increased PPT after LLLT, suggesting that there is no correlation between these variables and the PPT cannot be considered a single parameter in the evaluation of the improvement of pain perception in temporomandibular disorder.
References
De Leeuw R, Klasser GD (2013) Orofacial pain: guidelines for assessment, diagnosis and management. Quintessense: Chicago
Scrivani SJ, Keith DA, Kaban LB (2008) Temporomandibular disorders. N Engl J Med 359(25):2693–705
Ribeiro Da Silva MC, Fillingim RB, Wallet SM (2016) Estrogen-induced monocytic response correlates with temporomandibular disorder pain: a case control study. J Dent Res. doi: 0022034516678599
Aaron LA, Buchwald D (2003) Chronic diffuse musculoskeletal pain, fibromyalgia and co-morbid unexplained clinical conditions. Best Pract Res Clin Rheumatol 17:563–574
Fernández-de-las-Peñas C, Galán-del-Río F, Fernández-Carnero J, Pesquera J, Arendt-Nielsen L, Svensson P (2009) Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: evidence of impairment in central nociceptive processing. J Pain 10(11):1170–8
Lim PF et al (2010) Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain 26:116–120
Da Cunha CO et al (2014) Determination of a pressure pain threshold cut-off value for the diagnosis of temporomandibular joint arthralgia. J Oral Rehabil 41(5):323–329
Carlsson AM (1983) Assessment of chronic pain, aspects of the reliability and validity of the visual analogue scale. Pain 16:87–101
Price DD et al (1994) A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain 56:217–226
Ohrbach R, Gale EM (1989) Pressure pain threshold, clinical assessment, and differential diagnosis: reliability and validity in patients with myogenic pain. Pain 39:157–169
Santos Silva RS et al (2005) Pressure pain threshold in the detection of masticatory myofascial pain: an algometer-based study. J Orofac Pain 19(4):318–324
Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1:277–99
Menezes Costa L da C et al (2011) The Brazilian-Portuguese versions of the McGill Pain Questionnaire were reproducible, valid, and responsive in patients with musculoskeletal pain. J Clin Epidemiol 64(8):903-912.
Ferreira KA, de Andrade DC, Teixeira MJ (2013) Development and validation of a Brazilian version of the short-form McGill pain questionnaire (SF-MPQ). Pain Manag Nurs 14(4):210–219
Cetiner S, Kahraman SA, Yücetaş S (2006) Evaluation of low-level laser therapy in the treatment of temporomandibular disorders. Photomed Laser Surg 24(5):637–641
Fikácková H et al (2007) Effectiveness of low-level laser therapy in temporomandibular joint disorders: a placebo-controlled study. Photomed Laser Surg 25(4):297–303
Emshoff R et al (2008) Low-level laser therapy for treatment of temporomandibular joint pain: a double-blind and placebo-controlled trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 105(4):452–456
Melchior MO et al (2013) Does low intensity laser therapy reduce pain and change orofacial myofunctional conditions? Cranio 31(2):133–139
Ahrari F et al (2014) The efficacy of low-level laser therapy for the treatment of myogenous temporomandibular joint disorder. Lasers Med Sci 29(2):551–557
Gao X, Xing D (2009) Molecular mechanisms of cell proliferation induced by low power laser irradiation. J Biomed Sci 16:1–16
Carrasco TG et al (2008) Low intensity laser therapy in temporomandibular disorder: a phase II double-blind study. Cranio 26(4):274–281
Da Silva MAMR et al (2012) Low level laser therapy as na adjunctive technique in the management of temporomandibular disorders. Cranio 30(4):264–71
De Moraes Maia ML et al (2014) Evaluation of low-level laser therapy effectiveness on the pain and masticatory performance of patients with myofascial pain. Lasers Med Sci 29(1):29–35
Da Cunha LA et al (2008) Efficacy of low-level laser therapy in the treatment of temporomandibular disorder. Int Dent J 58(4):213–217
Petrucci A et al (2011) Effectiveness of low-level laser therapy in temporomandibular disorders: a systematic review and meta-analysis. J Orofac Pain 25(4):298–307
Chen J et al (2015) Efficacy of low-level laser therapy in the treatment of TMDs: a meta-analysis of 14 randomised controlled trials. J Oral Rehabil 42(4):291–299
De Moraes Maia ML et al (2012) Effect of low-level laser therapy on pain levels in patients with temporomandibular disorders: a systematic review. J Appl Oral Sci 20(6):594–602
Schiffman EL et al (2010) The Research Diagnostic Criteria for Temporomandibular Disorders. V: methods used to establish and validate revised Axis I diagnostic algorithms. J Orofac Pain 24:63–78
Bragdon EE et al (2002) Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain 96(3):227–237
Fishbain DA, Lewis JE, Gao J (2012) Is there significant correlation between self-reported low back pain visual analogic scores and low back pain scores determined by pressure pain induction matching? Pain Pract 13:358–363
Wolfe F et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum 33:160–172
Sancakli E et al (2015) Early results of low-level laser application for masticatory muscle pain: a double-blind randomized clinical study. BMC Oral Health 15(1):131–136
Desiderá AC et al (2015) Laser therapy reduces gelatinolytic activity in the rat trigeminal ganglion during temporomandibular joint inflammation. Oral Dis 21(5):652–658
Do Nascimento GC, Leite-Panissi CR (2014) Time-dependent analysis of nociception and anxiety-like behavior in rats submitted to persistent inflammation of the temporomandibular joint. Physiol Behav 125:1–7
Lund I et al (2005) Lack of interchangeability between visual analogue and verbal rating pain scales: a cross sectional description of pain etiology groups. BMC Med Res Methodol 5:31–36
Chouchou F, Lavigne GJ (2014) Placebo analgesia and sleep. Pathol Biol 62(5):270–275
Gourion D, Mouchabac S (2016) Placebo effect: clinical, biological and therapeutical involvements in depression. Encéphale 42:24–30
Vedolin GM et al (2009) The impact of stress and anxiety on the pressure pain threshold of myofascial pain patients. J Oral Rehabil 36(5):313–321
Sanches ML et al (2015) Correlation between pressure pain threshold and pain intensity in patients with temporomandibular disorders who are compliant or non-compliant with conservative treatment. Oral Surg Oral Med Oral Pathol Oral Radiol 120(4):459–468
Lauche R et al (2014) Neck pain intensity does not predict pressure pain hyperalgesia: re-analysis of seven randomized controlled trials. J Rehabil Med 46(6):553–560
Latremoliere A, Woolf CJ (2009) Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 10(9):895–926
Drobek W, De Laat A, Schoenaers J (2001) Tactile threshold and pressure pain threshold during treatment of orofacial pain: an explorative study. Clin Oral Investig 5(3):185–193
Slade GD et al (2014) Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain 55(10):2134–2143
Greenspan JD et al (2013) Pain sensitivity and autonomic factors associated with development of TMD: the OPPERA prospective cohort study. J Pain 14:63–74
Cao Y, Chen HM, Fu KY (2008) Investigation on clinical pain features in temporomandibular disorders. Zhonghua Kou Qiang Yi Xue Za Zhi 43(5):293–295
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding source
This study was financially supported by São Paulo Research Foundation (FAPESP) and Coordination for the Improvement of Higher Education Personnel (CAPES).
Ethical approval and informed consent
All procedures were performed in accordance with the guidelines of the Brazilian Ethics Committee on Human Research. This study was conducted after approval by the Ethics Committee of the School of Dentistry of Ribeirão Preto (under protocol: 33658114.7.0000.5419). All subjects were informed about the study and signed a consent form (approved by the Ethics Committee). Patients who composed the placebo group and did not have pain reduction after the study completion were invited to receive treatment with LLLT, in the same parameters of the active laser group.
Rights and permissions
About this article
Cite this article
Magri, L.V., Carvalho, V.A., Rodrigues, F.C.C. et al. Effectiveness of low-level laser therapy on pain intensity, pressure pain threshold, and SF-MPQ indexes of women with myofascial pain. Lasers Med Sci 32, 419–428 (2017). https://doi.org/10.1007/s10103-016-2138-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2138-x