Abstract
The molecular changes in gene expression following ablative laser treatment of skin lesions, such as atrophic scars and UV-damaged skin, are not completely understood. A standardized in vitro model of human skin, to study the effects of laser treatment on human skin, has been recently developed. Therefore, the aim of the investigation was to examine morphological and molecular changes caused by fractional ablative erbium:YAG laser treatment on an in vitro full-thickness 3D standardized organotypic model of human skin. A fractional ablative erbium:YAG laser was used to irradiate organotypic human 3D models. Laser treatments were performed at four different settings using a variety of stacked pulses with similar cumulative total energy fluence (60 J/cm2). Specimens were harvested at specified time points and real-time PCR (qRT-PCR) and microarray studies were performed. Frozen sections were examined histologically. Three days after erbium:YAG laser treatment, a significantly increased mRNA expression of matrix metalloproteinases and their inhibitors (MMP1, MMP2, MMP3, TIMP1, and TIMP2), chemokines (CXCL1, CXCL2, CXCL5, and CXCL6), and cytokines such as IL6, IL8, and IL24 could be detected. qRT-PCR studies confirmed the enhanced mRNA expression of IL6, IL8, IL24, CXCLs, and MMPs. In contrast, the mRNA expression of epidermal differentiation markers, such as keratin-associated protein 4, filaggrin, filaggrin 2, and loricrin, and antimicrobial peptides (S100A7A, S100A9, and S100A12) as well as CASP14, DSG2, IL18, and IL36β was reduced. Four different settings with similar cumulative doses have been tested (N10%, C10%, E10%, and W25%). These laser treatments resulted in different morphological changes and effects on gene regulations. Longer pulse durations (1000 μs) especially had the strongest impact on gene expression and resulted in an upregulation of genes, such as collagen-1A2, collagen-5A2, and collagen-6A2, as well as FGF2. Histologically, all treatment settings resulted in a complete regeneration of the epidermis 3 days after irradiation. Fractional ablative erbium:YAG laser treatment with a pulse stacking technique resulted in histological alterations and shifts in the expression of various genes related to epidermal differentiation, inflammation, and dermal remodeling depending on the treatment setting applied. A standardized in vitro 3D model of human skin proved to be a useful tool for exploring the effects of various laser settings both on skin morphology and gene expression during wound healing. It provides novel data on the gene expression and microscopic architecture of the exposed skin. This may enhance our understanding of laser treatment at a molecular level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To avoid the need for skin biopsies from patients or the implementation of animal experiments, human organotypic 3D skin equivalents have been established as an appropriate alternative for studies of the human skin [1–4]. In addition, animal skin reveals important morphological and molecular differences compared to human skin, which may limit the application of test results from animal studies to humans. Recently, a standardized human 3D model for the study of morphological and molecular modifications following laser irradiation was described [5].
Fractional treatment with erbium:YAG laser systems has been successfully applied in the treatment of atrophic acne scars [6, 7], photoaged skin [8], or actinic keratoses and in laser-assisted photodynamic therapy [9, 10]. After erbium:YAG laser resurfacing, dermal matrix remodeling including collagen production has been detected [11]. Despite these encouraging clinical data, little is known about the effects of these laser systems at a molecular level [12, 13]. It is not known whether the efficacy of a particular ablative skin rejuvenation treatment depends on the extent of micro-wounding and/or the amount of heat produced. The underlying molecular changes are not fully understood, but have been postulated to be induced by time-dependent changes in heat shock proteins, transforming growth factor β, matrix metalloproteinases (MMPs), hyaluronic acid synthethases, hyaluronidases, and hyaluronic acid (HA), among others [12]. Therefore, the aim of the present study was to improve our understanding of the histological and molecular alterations following ablative fractional treatment using a commercially available erbium:YAG laser system. Of particular interest was the analysis of skin morphology and regulation of gene expression patterns in response to different laser settings.
Materials and methods
Isolation and culture of NHEKs and NHDFs
Foreskin specimens were obtained from a 7-year-old male patient undergoing a circumcision surgery. Normal human epidermal keratinocytes (NHEKs) and normal human dermal fibroblasts (NHDFs) were isolated and collected from the same patient. The epidermis was separated from the dermis by digestion with dispase (BD Biosciences, Franklin Lakes, NY). Following trypsin treatment, keratinocytes were isolated (Lonza, Basel, Switzerland) for pH neutralization (TNS; Lonza). By incubating the dermal portion of the biopsy in collagenase 1A (Sigma, Taufkirchen, Germany), a single cell suspension of NHDF was obtained. This study was conducted according to the principles of the Declaration of Helsinki and was approved by the ethics committee of the University Hospital, RWTH Aachen, Germany. Written informed consent was obtained from the participant. Cultivation of NHEKs and NHDFs were performed as previously described [5].
Collagen skin equivalents
Collagen skin equivalents were performed as previously described [1]. In brief, to construct the dermis of the skin equivalent, collagen gels were prepared by mixing eight volumes of ice-cold bovine collagen I solution (Vitrogen; Cohesion Technologies, Palo Alto, CA) with one volume of ×10 concentrated Hank’s balanced salt solution (Gibco/Invitrogen). After neutralization with 1 M NaOH, one volume of NHDF suspended in FCS was added. The final concentration of NHDF in this gel solution was 1 × 105 cells/ml. Four milliliters of this gel solution was poured into each polycarbonate membrane insert (3.0-μm pore size; Corning, NY) and placed in six-well plates. Following complete polymerization, the gels were covered with DMEM containing 10% FCS, 100 U/ml penicillin, and 100 mg/ml streptomycin and were incubated in a humidified atmosphere at 37 °C and 5% CO2. After 1 to 3 days, approximately 2 × 106 NHEK were seeded on each dermal equivalent. The originated skin equivalents were cultured in equal volumes of DMEM and keratinocyte growth medium with 5% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin, and 50 μg/ml ascorbic acid. After 1 to 2 days of submerged culture, the skin equivalents were lifted to the air–liquid interface and the calcium concentration in the medium was raised to 1.2 mM. Cultivation of the collagen skin models was continued and medium change was done every other day.
Laser irradiation
The 3D skin models were irradiated using an ablative fractional erbium:YAG laser (MCL31 Dermablate, Asclepion Laser Technologies GmbH, Jena, Germany). The 3D models were treated with different laser settings—N10% (12 pulses, energy per impulse 0.8 J, fluence per impulse 5 J/cm2, and pulse duration of 300 μs); E10% (six pulses, energy per impulse 1.6 J, fluence per impulse 10 J/cm2, and pulse duration of 300 μs); C10% (15 pulses, energy per impulse 0.6 J, fluence per impulse 4 J/cm2, and pulse duration of 100 μs); and W25% (15 pulses, energy per impulse 2 J, fluence per impulse 4 J/cm2, and pulse duration of 1000 μs)—according to the manufacturer’s recommendations. In the N10%, E10%, and C10% modus, the cover rate was 10% and in the W25% modus was 25%. During laser irradiation, the culture medium was removed and the cultures were kept on cold PBS. Radiation intensity was kept under equal conditions by fixing the laser head on a tripod. After treatment, the models were cultivated with fresh culture medium and harvested on days 0 and 3 for histological analysis and on day 3 for detection of gene expression. An untreated model was maintained as a negative control at any given time. All experiments were repeated twice for every time point.
RNA isolation
Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions, including on-column digestion of DNA with RNase-free DNase I. The RNA was quantified via photometric measurement (NanoDrop Technologies, Wilmington, DE) and its integrity was analyzed on a 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA).
Quantitative reverse transcription polymerase chain reaction
Purified RNA was reversed-transcribed using the SS VILO Mastermix (Life Technologies) according to the manufacturer’s instructions. TaqMan experiments were carried out on an ABI Prism 7000 sequence detection system (Applied Biosystems, Weiterstadt, Germany) using Assays-on-Demand gene expression products for FLG (Hs00856927_g1), FLG2 (Hs00418578_m1), COL1A1 (Hs00164004_m1), COL1A2 (Hs00164099_m1), COL6A3 (Hs00915125_m1), CXCL1 (HS00236937_m1), CXCL2 (Hs00601975_m1), CXCL5 (Hs00171085_m1), CXCL6 (Hs00605742_g1), CXCL10 (Hs00171042_m1), CXCL11 (Hs00171138_m1), HSPA1B/HSPA1A (Hs00271244_s1), HSPB3 (Hs00272204_s1), HPRT (Hs99999909_m1), IL8 (Hs00174103_m1), IL18 (Hs99999040_m1), IL20 (Hs00218888_m1), IL36 beta (Hs00758166_m1), keratin-associated protein 4 (KRT4; Hs00970607_g1), KRT13 (Hs00357961_g1), KRT14 (Hs00606019_gH), KRT16 (Hs00955088_g1), KRT19 (Hs00761767_s1), LOR (Hs01894962_m1), MIF (Hs00236988_g1), MMP2 (Hs00234422_m1), TIMP1 (Hs99999139_m1), TIMP1_2 (Hs00171558_m1), and TIMP2 (Hs00234278_m1), according to the manufacturer’s recommendations. An Assays-on-Demand product for HPRT (Hs99999909) was used as an internal reference to normalize the target transcripts. All measurements were performed in triplicate in separate reaction wells.
Analysis of gene expression using exon expression arrays
Purified mRNA was analyzed on the GeneChip Human Gene 2.0 ST array, as previously reported [10], using the Gene-SpringGX 13.0 software (Agilent Technologies, Frankfurt am Main, Germany).
Light microscopy
For light microscopy, 4-μm cryosections of skin equivalents were embedded in Tissue Tec OCT and stained with hematoxylin and eosin. Sections were examined using a photomicroscope (DMIL, Leitz, Wetzlar, Germany).
Results
To investigate the effects of fractional ablative laser treatment on skin morphology and gene regulation, we recently developed a full-thickness human 3D skin equivalent [5, 14]. These 3D skin models contain dermal and epidermal structures with a functionally active stratum corneum and basal membrane.
In this study, we treated 3D skin models with a fractional erbium:YAG laser at different settings, according to the manufacturer’s recommendations, applying similar cumulative doses (60 J), a similar pattern size (13 × 13 mm), and a similar number of spots (169). The settings varied in the energy and fluence per pulse, the pulse duration, and the cover rate.
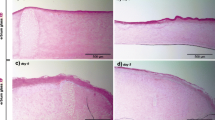
Fractional ablative erbium:YAG laser treatment with multiple stacked pulses applying the N10% setting led to a partial ablation of the epidermal part of the skin model (Fig. 1a). A complete reconstitution of the epidermal equivalent and stratum corneum could be detected 3 days after the initial treatment (Fig. 1). Microarray analysis from these tissues (day 3) revealed upregulation of IL8, TIMP1, and CXCL5 and downregulation of KRT4, KRT13, KRT19, and filaggrin (Fig. 2). These effects were confirmed via independent quantitative reverse transcription PCR (qRT-PCR); additionally, a strong upregulation of CXCL-2 and CXCL-6, HSPB3 (Fig. 3), as well as MMP2 (Fig. 5), a moderate upregulation of MIF (Fig. 3), and a significant downregulation of loricrin mRNA expression could be detected (Fig. 4).
In the E10% setting, ablation of the epidermal and upper dermal parts of the skin model was observed (Fig. 1b). A complete reconstitution of the epidermal equivalent and stratum corneum could be detected after 3 days of subsequent cell culture. Microarray analysis from these tissues (day 3) revealed upregulation of TIMP1, CXCL1, CXCL2, CXCL5, CXCL6, MMP2, KRT14, and IL8 and downregulation of IL36β, IL18, filaggrin 1 and 2, and loricrin (Fig. 2.). These effects were confirmed using independent RT-qPCR, which, in addition, revealed a significant downregulation of KRT4, KRT13, KRT14, and KRT19 (Fig. 4) and a moderate upregulation of COL6A2 (Fig. 5).
In the C10% setting, extensive ablation of the epidermis and laminar upper dermal parts of the skin model was observed (Fig. 1c). Complete reconstitution of the dermal and epidermal parts could be detected 3 days after initial treatment. A microarray analysis showed upregulation of CXCL2 and CXCL5 and downregulation of KRT13, KRT14, KRT19, IL18, and IL36β (Fig. 2). These effects were confirmed using an independent qRT-PCR analysis, and in addition, a strong upregulation of IL8, MIF, MMP2, CXCL6, and caspase 14 (CASP14; Figs. 3 and 5), as well as a significant downregulation of loricrin and KRT4, was observed (Fig. 4).
The most distinctive effects on the epidermal and dermal structures were seen directly after laser treatment that used the W25% parameters. Fractional ablative erbium:YAG laser treatment led to partial ablation of the epidermal and extensive dermal parts of the skin model (Fig. 1c). However, complete reconstitution of the epidermal equivalent and stratum corneum could also be detected 3 days after treatment, as detected after treatment using the other laser settings. Microarray analysis from these tissues (day 3) revealed upregulation of IL8, CXCL1, CXCL2, CXCL5, CXCL6, CXCL12, TIMP2, IL24, MMP-1, MMP-2, and MMP-3, IL6, COL6A2, and FGF2, as well as downregulation of loricrin, filaggrin 1 and 2, KRT4, KRT13, IL18, IL36β, CASP14, S100A9, S100A12, and DSG2 (Fig. 2). These effects were confirmed using independent qRT-PCR analysis, and in addition, a strong upregulation of TIMP1 and MIF (Figs. 3 and 5), as well as downregulation of KRT13, KRT14, and KRT19, could be detected (Fig. 4).
Discussion
Erbium:YAG laser systems are successfully used in treating lesions having epidermal and dermal involvement, including atrophic acne scars, verruca vulgaris, and precancerous lesions like actinic keratosis. Erbium:YAG laser irradiation has been discouraged in lesions having substantial acute inflammation or hypertrophic scars.
Still, the molecular mechanisms of wound healing after laser treatment and a particular ablative skin rejuvenation treatment are not completely understood. It has been postulated that wound contraction of atrophic scars, for example, depends on the extent of micro-wounding and/or the amount of heat produced. Furthermore, it has been postulated that there are time-dependent gene regulatory changes of heat shock proteins, transforming growth factor β, MMPs, hyaluronic acid synthethases, hyaluronidases, and HA, among others [12].
MEDLINE search did not reveal preceding reports on ablative fractional erbium:YAG laser treatment using in vitro 3D human skin models. This model provides a collagen pattern that closely resembles intact human skin. Therefore, it is ideally suited for examining the effects of laser irradiation, particularly of the deep reticular layer, as has been histologically validated [5].
Interestingly, the erbium:YAG laser treatment in settings E10% and W25% led to significantly more gene regulation than the other settings. This is obvious because these settings also affect the deeper dermal tissue layers. However, the gene regulatory effects of epidermal proteins were similar in all settings. Laser irradiation resulted in decreased gene expression for markers of epidermal differentiation, such as loricrin and filaggrin 1 and 2. These markers participate in terminal formation of keratinocytes and development of the skin barrier [13]. Also, the expression of CASP14, which plays an important role in epidermal differentiation, was found to be reduced [15, 16]. Similarly, there were decreased amounts of KRT4, KRT13, KRT14, and KRT19, as well as S100A7A, S100A9, and S100A12. It has been shown that these antimicrobial peptides enhance the differentiation of keratinocytes and strengthen the epidermal tight junction barrier [17]. Besides these epidermal effects, antimicrobial peptides such as S100A7 also show antifibrotic effects and are markedly decreased in scar tissue and keloids [18]. In our study, we observed a decrease of these antimicrobial peptides after fractional erbium:YAG laser irradiation, which may be disadvantageous in treating keloids.
It is known that there is a link between skin barrier function and inflammation. There is evidence that cytokines also affect the evolution of keratinocytes and the skin barrier [2, 14, 19]. Thus, changes in chemokine and interleukin expression, as seen in the 3D skin model employed in this study, may also modify epidermal formation. Laser treatment resulted in the upregulated expression of the chemokines CXCL1, CXCL2, CXCL5, and CXCL6. These are known to attract and activate neutrophilic granulocytes during acute granulocytic inflammation. There was also increased expression of inflammatory markers, such as IL6, IL8, and IL24. In contrast, IL18 and IL36β were downregulated. Formation of hypertrophic scars has been associated with inflammation and its cellular mediators. For example, fibroblasts of hypertrophic scar tissue contain increased levels of IL6, IL8, fibroblast growth factor, and monocyte chemotactic protein-1 compared to fibroblasts of normal skin [20, 21]. In contrast, lesions of embryonic tissues show no scar formation, little or no inflammatory infiltration, and decreased expression of IL6 and IL8 [22].
In addition, there was upregulation of the aforementioned markers of epidermal development following irradiation of deeper layers of the corium using the E10% and W25% laser settings. Thus, a more pronounced superficial impact was expected following laser treatment in the N10% modus. This is consistent with the decreased disruption of the dermoepidermal junction and deeper penetration associated with repetitive stimulation (stacking mode) using the N10%, E10%, and W25% settings. This suggests that epidermal micro-injuries also occur after dermal laser remodeling.
The W25% setting was the only setting showing the strongest effect on dermal gene regulation. MMPs participate in the restructuring of the extracellular matrix. At first, it was thought that their main role was the elimination of damaged structural extracellular matrix proteins such as collagen [23, 24]. MMP3 causes fibroblast contraction and shrinkage of wounds [25]. More recent data suggest that MMPs also participate in the inflammatory component and rebuilding of the epidermal lining associated with wound healing. Increased MMP gene expression has been demonstrated in human wound healing in vivo and in explant models of human skin as a result of non-ablative fractional photothermolysis irradiation [13], as well as ablative laser treatment [12]. Thus, MMP1 expression was upregulated in mature burn scars 48 h after fractional CO2 laser treatment [26]. There was clinical improvement, histological remodeling of the collagen architecture, and biochemical changes associated with clinical improvement [27]. Melerzanov et al. demonstrated decreased expression of MMP1, MMP2, and MMP12 in monolayers of the keratinocyte cell line HaCaT at various time points after laser irradiation [28]. In contrast, in the present study, MMP1, MMP2, and MMP3 mRNAs were increased after laser treatment. This may be explained by a fine-tuning effect of erbium:YAG laser treatment on MMP expression.
Furthermore, we observed upregulatory effects on dermal proteins like FGF2 and collagens. This is consistent with our unpublished results using CO2 laser systems showing stronger dermal involvement. This effect might result from thermal effects or longer pulse durations. We suggest that the inflammatory response caused by the erbium:YAG laser may stimulate the formation of new collagen in atrophic scars and other non-inflammatory lesions, which may be beneficial in treating these lesions. In contrast, this effect is most likely counterproductive in hypertrophic scars, keloids, and inflammatory diseases. In this study, the observed differences in gene expression for different energy fluences suggest that non-laser treatments (e.g., medical skin needling) may have different clinical effects. Gene expression in animal experiments using skin microneedling showed increased expression of various growth factors such as TGFß1-3, FGF, EGF, VEGF, and TNF-α, as well as upregulation of collagen I and downregulation of collagen III. Clinically, an increase of epidermal thickness was achieved [29].
Generally, in vitro testing in academic research has somewhat obscured the difficulties involved. Good cell culture practices have been proposed, but are rarely applied [30, 31]. Guidance for Good Laboratory Practices to extend in vitro studies for regulatory work has been developed, but its application in practice is not completely clear. Also, there are some fundamental problems as to the artificial, non-physiological conditions cells are maintained in. Blood electrolyte concentrations, extracellular matrix, or the extent of cell contacts are diverse in in vivo situations. Cell densities are less than 1% of the tissue situation, which impairs intracellular signaling. Most cell layers are representing only one cell type with no cell–cell interactions, often monoclonal in origin and further degenerated during maintenance, and no adnexal structures which are also involved in wound healing. Culture conditions are not homeostatic due to continuous depletion of nutrients, sudden exchange of media, accumulation of waste products, and insufficient oxygen supply. The lack of biotransformation capabilities is probably the best-known limitation [32]. In our in vitro study, the thickness of the epidermis is approximately 100 μm, which corresponds to the thickness of the epidermis on the forehead or cheeks [33].
Wound healing in general is a dynamic process that passes in three phases that overlap in time: inflammation, tissue formation, and tissue remodeling [34]. Studies analyzing wound healing use mainly mechanical methods of wound induction, which are laborious and difficult to standardize [35]. In the previously published work of Ferraq et al. [35], Er:YAG laser treatment of human skin in vivo achieved a rapid standardized epidermal ablation, which was similar in terms of epidermal regeneration and barrier formation to our results. In our experiments, we used a fractional Er:YAG laser. Hereby, we were able to set multiple standardized injuries with defined dimensions in human full-thickness 3D skin equivalents, enabling the investigation of wound healing on the molecular and histological level. This ex vivo laser model using human skin allows the generation of different time courses to analyze the gene regulation both on the RNA and protein levels at the same time points. These time course analyses are due to ethical reasons hardly possible in clinical in vivo studies in humans. The induced epidermal wounds were uniform in size and rapidly obtained as compared to the in vivo wounds induced by Er:YAG laser treatment [35]. Human skin models were found to be appropriate for in vitro wound healing analysis. After 1 week, re-epithelization of the laser-treated human skin model was completed in this skin model type, similar to epidermal regeneration in humans in vivo [36]. Therefore, this 3D skin model could also serve as a model to analyze the impact of different laser systems on skin physiology and morphology. Molecular mechanisms resulting in the proliferative effect of, e.g., pantothenate, were previously investigated by global gene expression analysis (microarray analysis) in cultured human dermal fibroblasts and in a clinical trial in vivo [5, 37, 38]. Correlations between data retrieved from 3D skin models and human skin in vivo have been demonstrated [5, 37, 38]. A limitation of this simplified human in vitro 3D skin model, which contains only two cell types—keratinocytes and fibroblasts—is not able to meet all the complex requirements of in vivo conditions. However, the observed gene regulatory effects can be specifically attributed to keratinocytes and fibroblasts.
In summary, the human 3D model system used in the present study may be useful in studying physiology, morphology, and gene expression after treatment via different laser systems ex vivo. It permits histologic examination and evaluation of altered gene expression on the RNA and protein levels. The fully developed corium permits examination of the effects of non-ablative laser irradiation on the collagen structure in the deeper layers of the skin. Data can be obtained in a standardized way using a direct comparison of the different laser systems at defined time points after irradiation and the time course of each individual lesion can be documented. Finally, and perhaps most importantly, this model greatly reduces the need for studies in humans and animals.
References
Neis MM, Wendel A, Wiederholt T, Marquardt Y, Joussen S, Baron JM, Merk HF (2010) Expression and induction of cytochrome p450 isoenzymes in human skin equivalents. Skin Pharmacol Physiol 23:29–39
Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, Luscher B, Baron JM (2012) IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 129:426–433
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69–70:1–18
Mathes SH, Ruffner H, Graf-Hausner U (2014) The use of skin models in drug development. Adv Drug Deliv Rev 69–70:81–102
Marquardt Y, Amann PM, Heise R, Czaja K, Steiner T, Merk HF, Skazik‐Voogt C, Baron JM (2015) Characterization of a novel standardized human three‐dimensional skin wound healing model using non‐sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med 47:257–265
Sardana K, Manjhi M, Garg VK, Sagar V (2014) Which type of atrophic acne scar (ice‐pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg 40:288–300
Lee SJ, Kang JM, Chung WS, Kim YK, Kim HS (2014) Ablative non-fractional lasers for atrophic facial acne scars: a new modality of erbium:YAG laser resurfacing in Asians. Lasers Med Sci 29:615–619
Latowsky BC, Abbasi N, Dover JS, Arndt KA, Kaminer MS, Rohrer TE, Macgregor JL, Wesley NO, Durfee MA, Tahan SR (2012) A randomized, controlled trial of four ablative fractionated lasers for photoaging: a quadrant study. Dermatol Surg 38:1477–1489
Choi SH, Kim KH, Song KH (2015) Efficacy of ablative fractional laser-assisted photodynamic therapy with short-incubation time for the treatment of facial and scalp actinic keratosis: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol 29:1598–1605
Ko DY, Jeon SY, Kim KH, Song KH (2014) Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol 28:1529–1539
Orringer JS, Rittié L, Hamilton T, Karimipour DJ, Voorhees JJ, Fisher GJ (2011) Intraepidermal erbium:YAG laser resurfacing: impact on the dermal matrix. J Am Acad Dermatol 64(1):119–128
Helbig D, Paasch U (2011) Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Skin Res Technol 17:119–128
Orringer JS, Rittie L, Baker D, Voorhees JJ, Fisher G (2010) Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol 163:757–768
Amann PM, Marquardt Y, Steiner T, Hölzle F, Skazik-Voogt C, Heise R, Baron JM (2016) Effects of non-ablative fractional erbium glass laser treatment on gene regulation in human three-dimensional skin models. Lasers Med Sci 31(3):397–404
Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Investig Dermatol 115(6):1148–1151
Hoste E, Kemperman P, Devos M, Denecker G, Kezic S, Yau N, Gilbert B, Lippens S, De Groote P, Roelandt R, Van Damme P, Gevaert K, Presland RB, Takahara H, Puppels G, Caspers P, Vandenabeele P, Declercq W (2011) Caspase-14 is required for filaggrin degradation to natural moisturizing factors in the skin. J Investig Dermatol 131(11):2233–2241
Hattori F, Kiatsurayanon C, Okumura K, Ogawa H, Ikeda S, Okamoto K, Niyonsaba F (2014) The antimicrobial protein S100A7/psoriasin enhances the expression of keratinocyte differentiation markers and strengthens the skin’s tight junction barrier. Br J Dermatol 171:742–753
Gauglitz GG, Bureik D, Zwicker S, Ruzicka T, Wolf R (2015) The antimicrobial peptides psoriasin (S100A7) and koebnerisin (S100A15) suppress extracellular matrix production and proliferation of human fibroblasts. Skin Pharmacol Physiol 28:115–123
Hvid M, Johansen C, Deleuran B, Kemp K, Deleuran M, Vestergaard C (2011) Regulation of caspase 14 expression in keratinocytes by inflammatory cytokines—a possible link between reduced skin barrier function and inflammation? Exp Dermatol 20(8):633–636
Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, Tredget EE (2011) Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 226:1265–1273
Sideek MA, Teia A, Kopecki Z, Cowin AJ, Gibson MA (2016) Co-localization of LTBP-2 with FGF-2 in fibrotic human keloid and hypertrophic scar. J Mol Histol 47(1):35–45
Mofikoya BO, Adeyemo WL, Ugburo AO (2012) An overview of biological basis of pathologic scarring. Niger Postgrad Med J 19:40–45
Shah JM, Omar E, Pai DR, Sood S (2012) Cellular events and biomarkers of wound healing. Indian J Plast Surg 45:220–228
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40:1334–1347
Bullard KM, Mudgett J, Scheuenstuhl H, Hunt TK, Banda MJ (1999) Stromelysin-1-deficient fibroblasts display impaired contraction in vitro. J Surg Res 84:31–34
Qu L, Liu A, Zhou L, He C, Grossman PH, Moy RL, Mi QS, Ozog D (2012) Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 44:517–524
Ozog DM, Liu A, Chaffins ML, Ormsby AH, Fincher EF, Chipps LK, Mi QS, Grossman PH, Pui JC, Moy RL (2013) Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol 149:50–57
Melerzanov A, Lavrov A, Sakania L, Korsunskaya I, Petersen E, Sobolev V (2014) Effects of laser radiation on MMP gene expression in keratinocytes. Prime J 4:39
Zeitter S, Sikora Z, Jahn S, Stahl F, Strauß S, Lazaridis A, Reimers K, Vogt PM, Aust MC (2014) Microneedling: matching the results of medical needling and repetitive treatments to maximize potential for skin regeneration. Burns 40(5):966–973
Coecke S, Balls M, Bowe G, Davis J, Gstraunthaler G, Hartung T, Hay R, Merten O-W, Price A, Schechtman L, Stacey G, Stokes W (2005) Guidance on good cell culture practice. Altern Lab Anim 33:261–287
Hartung T (2007) Food for thought … on cell culture. Altern Anim Exp 24:143–147
Coecke S, Ahr H, Blaauboer BJ, Bremer S, Casati S, Castell J, Combes R, Corvi R, Crespi CL, Cunningham ML (2006) Metabolism: a bottleneck in in vitro toxicological test development. Altern Lab Anim 34:49–84
Sasaki G, Travis HM, Tucker B (2009) Fractional CO2 laser resurfacing of photoaged facial and non-facial skin: histologic and clinical results and side effects. J Cosmet Laser Ther 11:190–201
Singer AJ, Clark RA (1999) Cutaneous wound healing. N Engl J Med 341(10):738–746 (Review)
Ferraq Y, Black DR, Theunis J, Mordon S (2012) Superficial wounding model for epidermal barrier repair studies: comparison of erbium:YAG laser and the suction blister method. Lasers Surg Med 44(7):525–532
Kottner J, Hillmann K, Fimmel S, Seité S, Blume-Peytavi U (2013) Characterisation of epidermal regeneration in vivo: a 60-day follow-up study. J Wound Care 22(8):395–400
Wiederholt T, Heise R, Skazik C, Marquardt Y, Joussen S, Erdmann K, Schröder H, Merk HF, Baron JM (2009) Calcium pantothenate modulates gene expression in proliferating human dermal fibroblasts. Exp Dermatol 18(11):969–978
Heise R, Skazik C, Marquardt Y, Czaja K, Sebastian K, Kurschat P, Gan L, Denecke B, Ekanayake-Bohlig S, Wilhelm KP, Merk HF, Baron JM (2012) Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol 25:241–248
Acknowledgements
These studies were supported in part by a grant from Asclepion Laser Technologies, Jena, Germany.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding source
None.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the ethical committee of the University Hospital, RWTH Aachen, Germany.
Informed consent
A written informed consent was obtained from all participants/participating parents.
Rights and permissions
About this article
Cite this article
Schmitt, L., Amann, P.M., Marquardt, Y. et al. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med Sci 32, 805–814 (2017). https://doi.org/10.1007/s10103-017-2175-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-017-2175-0