Abstract
Clinical experiences with non-ablative fractional erbium glass laser therapy have demonstrated promising results for dermal remodelling and for the indications of striae, surgical scars and acne scars. So far, molecular effects on human skin following treatment with these laser systems have not been elucidated. Our aim was to investigate laser-induced effects on skin morphology and to analyse molecular effects on gene regulation. Therefore, human three-dimensional (3D) organotypic skin models were irradiated with non-ablative fractional erbium glass laser systems enabling qRT-PCR, microarray and histological studies at same and different time points. A decreased mRNA expression of matrix metalloproteinases (MMPs) 3 and 9 was observed 3 days after treatment. MMP3 also remained downregulated on protein level, whereas the expression of other MMPs like MMP9 was recovered or even upregulated 5 days after irradiation. Inflammatory gene regulatory responses measured by the expression of chemokine (C-X-C motif) ligands (CXCL1, 2, 5, 6) and interleukin expression (IL8) were predominantly reduced. Epidermal differentiation markers such as loricrin, filaggrin-1 and filaggrin-2 were upregulated by both tested laser optics, indicating a potential epidermal involvement. These effects were also shown on protein level in the immunofluorescence analysis. This novel standardised laser-treated human 3D skin model proves useful for monitoring time-dependent ex vivo effects of various laser systems on gene expression and human skin morphology. Our study reveals erbium glass laser-induced regulations of MMP and interleukin expression. We speculate that these alterations on gene expression level could play a role for dermal remodelling, anti-inflammatory effects and increased epidermal differentiation. Our finding may have implications for further understanding of the molecular mechanism of erbium glass laser-induced effects on human skin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For some time, dermatological experiments have been increasingly performed using human three-dimensional (3D) skin equivalents since clinical trials in human skin are laborious and the skin of laboratory animals differs in some biological properties from human skin. Furthermore, clinical trials and animal experiments are rightly subjected to strict surveillance due to ethical reasons. Although human 3D models, established as in vitro test systems, can certainly not meet all the requirements of in vivo conditions, they offer a good alternative and allow for reliable data, hereby avoiding animal experiments or clinical trials in humans. 3D organotypic skin equivalents are also currently applied in several in vitro studies, including dermatotoxicological and pharmacological testing [1–4], and the European Centre for the Validation of Alternative Methods (ECVAM) has approved the use of 3D skin models as an alternative for human clinical tests, e.g. for skin irritation [5]. Recently, we established a novel standardised laser-irradiated in vitro/ex vivo 3D model, using full-thickness human skin equivalents to analyse the effects on wound healing and gene regulation [6].
Non-ablative fractional laser therapy with erbium glass laser systems were successfully used to treat conditions with deeper dermal involvement, such as acne scars [7, 8], mature burn scars [9] and striae distensae [10], which require deep dermal remodelling. Also, for melasma [11], female pattern hair loss [12] and acne vulgaris [13], clinical improvements were described. So far, not much is known about the molecular effects on human skin after treatment with these laser systems.
Our aim was to investigate the effects of two different erbium glass laser systems (XD Microlens and XF Microlens optics from the manufacturer Palomar Medical Technologies, Burlington, MA, USA) on skin morphology and to analyse time-dependent laser-induced molecular effects on gene regulation in vitro in 3D human skin models.
Materials and methods
Isolation of normal human epidermal keratinocytes and normal human dermal fibroblasts
Normal human epidermal keratinocytes (NHEKs) and normal human dermal fibroblasts (NHDFs) were isolated from foreskin obtained from healthy volunteers after cutaneous surgery. After separation of the epidermal sheet from the dermis using dispase (BD Biosciences, Franklin Lakes, NY, USA), trypsin (Lonza, Basel, Switzerland) digestion and neutralising with Trypsin Neutralization Solution (TNS) (Lonza), a single cell suspension of NHDF was generated by incubating the dermis in collagenase 1A (Sigma, Taufkirchen, Germany). This study was conducted according to the Declaration of Helsinki Principles and was approved by the ethical committee of the University Hospital, RWTH Aachen, Germany. A written informed consent was obtained from all participants/participating parents.
Cell culture
Cultivation of NHEK and NHDF was done as previously described [2].
Collagen skin equivalents
Collagen skin equivalents were performed as previously described [1]. For all 3D skin equivalents deployed in this study, NHEK and NHDF originate from a single donor (not pooled material). In brief, to construct the dermis of the skin equivalent, collagen gels were prepared by mixing eight volumes of ice-cold bovine collagen I solution (Vitrogen; Cohesion Technologies, Palo Alto, CA, USA) with one volume of ×10 concentrated Hank’s balanced salt solution (Gibco/Invitrogen). After neutralisation with 1 M NaOH, one volume of NHDF suspended in FCS was added. The final concentration of NHDF in this gel solution was 1 × 105 cells/ml. Four millilitres of this gel solution was poured into each polycarbonate membrane insert (3.0 μm pore size; Corning, NY, USA) and placed in six-well plates. Following complete polymerisation, gels were covered with Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10 % FCS, 100 U/ml penicillin and 100 mg/ml streptomycin and incubated in a humidified atmosphere at 37 °C and 5 % CO2. After 1 to 3 days, approximately 2 × 106 NHEK were seeded on each dermal equivalent. The originated skin equivalents were cultured in equal volumes of DMEM and keratinocyte growth medium with 5 % FCS, 100 U/ml penicillin, 100 mg/ml streptomycin and 50 μg/ml ascorbic acid. After 2 to 4 days of submerged culture, the skin equivalents were lifted to the air–liquid interface and the calcium concentration in the medium was raised to 1.2 mM. Cultivation of the collagen skin models was continued, and medium change was done every other day.
Laser irradiation
3D skin models were irradiated with the non-ablative fractional Erbium glass laser of the Palomar ICON 1540 Fractional Laser System® with 1540-nm XD Microlens or 1540-nm XF Microlens optic (Palomar Medical Technologies, Burlington, MA, USA). 3D models were treated with a single pulse from the 1540-nm XD Microlens [14] at 60 mJ/microbeam, 15 ms using firm compression or with a single pulse from the 1540-nm XF Microlens [15] at 44 mJ/microbeam, 15 ms. During laser irradiation, culture medium was removed and cultures were kept on cold phosphate-buffered saline (PBS). Radiation intensity was kept under equal conditions by fixing the laser head on a tripod. After treatment, the models were cultivated with fresh culture medium and harvested on days 0, 3 or 5 to perform histology and gene expression analysis. An untreated model was maintained as a negative control at any given time. All experiments were repeated twice for every time point.
RNA isolation
Total RNA was isolated using the Nucleo Spin II RNA Mini Kit (Macherey + Nagel, Düren, Germany), according to the manufacturer’s instructions, including on-column digestion of DNA with RNase-free DNase I. The RNA was quantified using photometric measurement (NanoDrop Technologies, Wilmington, DE, USA), and its integrity was analysed on a 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA).
Quantitative reverse transcription polymerase chain reaction
Purified RNA was reverse transcribed with the SS VILO Mastermix (Life Technologies, Carlsbad, CA, USA) according the manufacturer’s instructions. TaqMan experiments were carried out on an ABI Prism 7000 sequence detection system (Applied Biosystems, Weiterstadt, Germany) using Assays-on-Demand gene expression products for human MMP3 (HS00233962_m1), MMP9 (HS00234579_m1), MMP7 (HS01042796_m1), CASP14 (Hs00201637_m1), KRT1 (Hs01549614_g1), KRT17 (Hs00356958_m1), SPINK7 (Hs00261445_m1), CXCL5 (Hs00171085_m1), CXCL6 (Hs00605742_g1), IL8 (Hs00174103_m1), IL36 gamma (Hs00219742_m1), DSC1 (Hs00245189_m1), FLG1 (Hs00856927_g1, FLG2 (Hs00418578_m1) and loricrin (Hs01894962_m1), according to the manufacturer’s recommendations. An Assay-on-Demand product for HPRT (Hs99999909) was used as an internal reference to normalise the target transcripts. All measurements were performed in triplicate in separate reaction wells.
Analysis of gene expression using exon expression arrays
Purified mRNA was analysed on the GeneChip Human Gene 2.0 ST array as previously reported [16]. Expression values of each probe set were determined and laser-irradiated samples were compared to laser-irradiated samples following dexpanthenol-treatment probes using the Gene-Spring GX 11.0.2 software (Agilent Technologies, Frankfurt am Main, Germany).
Light microscopy and immunofluorescence
For light microscopy, 4-μm cryosections of skin equivalents were embedded in Tissue Tec OCT and stained with hematoxylin and eosin. Sections were examined using a photomicroscope (DMIL, Leitz, Wetzlar, Germany). For immunofluorescence, 4-μm cryosections were fixed for 10 min in acetone at 4 °C. First antibodies loricrin 1, MMP9 (Abcam, Cambridge, UK), MMP3 (Sigma Aldrich, St. Louis, MO, USA) and filaggrin (AKH1) (Santa Cruz, Dallas, Texas, USA) were diluted with Antibody Diluent (Dako, Glostrup, Denmark) and incubated at room temperature for 1 h. Following the washing steps with PBS, the sections were incubated in fluorochrome-conjugated secondary antibody Alexa Fluor 488 IgG H + L (Molecular Probes, Eugene, Oregon, USA) for 1 h at room temperature. Cell nuclei were stained with DAPI (Applichem, Darmstadt, Germany). After a final washing step, sections were mounted in Fluorescent Mounting Medium (Dako, Glostrup, Denmark) and were coverslipped. The sections were stored in the dark at 4 °C, examined using a photomicroscope (DMIL, Leitz, Wetzlar, Germany) equipped with epifluorescence illumination and were digitally photodocumentated (DISKUS, Hilgers, Königswinter, Germany).
Results
To investigate the effects of non-ablative fractional erbium glass laser systems (1540-nm XD Microlens and 1540-nm XF Microlens optic, Palomar ICON 1540 Fractional Laser System® from the manufacturer Palomar Medical Technologies, Burlington, MA, USA) on skin morphology, full-thickness human 3D skin equivalents were constructed.
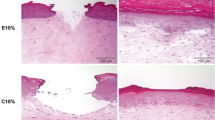
These 3D skin models developed dermal and epidermal structures with stratum corneum, a basal layer and a basal membrane (Fig. 1). The 3D skin models were treated with a single pulse from the fractional non-ablative laser with the 1540-nm XD Microlens optic [14], allowing for deeper dermal remodelling, or the 1540-nm XF Microlens optic [15].
Radiation intensity was kept under equal conditions by fixing the laser head on a tripod, resulting in standardised dermal damages. Figure 1 depicts representative coagulation profiles with a column depth around 1 mm following application of the 1540-nm handpiece with the XD Microlens (Fig. 1a, b) or the XF Microlens (Fig. 1c, d). This laser irradiation model showed clearly defined coagulation of the dermis directly after treatment (Fig. 1a, c). There was no morphologically visible epidermal damage following laser irradiation. The epidermis is nearly not affected morphologically by the laser treatment. Dermal coagulation was nearly totally restored 5 days after irradiation by the XD laser device (Fig. 1b). In the XF device-irradiated samples, the damaged region of collagen coagulation was still visible 5 days after treatment (Fig. 1d).
To our knowledge, there is no data about the molecular effects of erbium glass laser irradiation on human skin.
To analyse possible laser-mediated stimulatory effects on skin remodelling, we investigated the influence on gene expression in these laser-irradiated 3D skin models using an Affymetix gene array and quantitative reverse transcription polymerase chain reaction (qRT-PCR). In particular, gene array analysis showed a downregulation of MMP3 and other matrix metalloproteinases (MMPs) 3 days after laser treatment (Fig. 2). Consistently, expression of tissue inhibitor of metalloproteinase 3 (TIMP3) was moderately upregulated on day 3 (Fig. 2c, XF device). After 5 days, MMP7, MMP9 and MMP11 were moderately upregulated. Filaggrin 1, filaggrin 2 and loricrin upregulation was observed after 5 days; this upregulation was stronger in the XF laser device. Expression of caspase 14, which is also involved in keratinocyte terminal differentiation [17], was reduced following irradiation with both devices, respectively. Inflammatory response on gene level, measured using IL-8 expression, was reduced after 3 days (XF device) and 5 days (XF and XD devices). Reduction also occurred with the expression of IL6 and chemokines such as CXCL1, CXCL2, CXCL5, CXCL6 and CXCL10. Desmocollein 1 (DSC1), which is required for epidermal cell adhesion and desmosome formation, was also upregulated by both devices, but to a larger extent with the XD device. These data were confirmed by qRT-PCR (data not shown). It should be underlined that the upregulation of the epidermal differentiation markers filaggrin 1, filaggrin 2 and loricrin, as well as the keratinocyte adhesion gene DSC1, is increased compared to the XD device. Furthermore, immunofluorescence staining revealed that loricrin and filaggrin 1 protein expression is clearly enhanced 5 days after laser irradiation mainly in the basal epidermal layers compared to the untreated control models (Fig. 3). Consistent with its expression on mRNA level, MMP3 protein expression is reduced after erbium glass laser treatment with both devices, respectively. Similar to the mRNA results, laser treatment increased MMP9 protein expression (XF > XD) compared to the untreated control model.
Gene expression in laser-irradiated 3D skin models (microarray analysis) XD optic laser-irradiated 3D skin equivalents (a, b) were compared to XF optic laser-irradiated 3D skin equivalents (c, d). 3D skin models were harvested 3 or 5 days after laser treatment, and gene expression was measured using the Affymetrix® Gene Chip Human Exon 1.0 ST array
Epidermal differentiation markers were upregulated whereas MMP expression was regulated differentially. An immunofluorescence examination of loricrin, filaggrin 1, MMP3 and MMP9 was performed in 3D skin equivalents 5 days after XD or XF optic laser irradiation. 3D models were counterstained with DAPI
Discussion
Non-ablative fractional laser therapy using erbium glass laser systems was successfully used to treat conditions with deeper dermal involvement, such as acne or mature burn scars, which require deep dermal remodelling. These procedures are considered minimally invasive with a high safety profile [18].
To our knowledge, this is the first report demonstrating erbium glass laser treatment in an in vitro 3D human skin model. Previously, erbium glass laser systems were evaluated in animal models [19] or in humans [20]. Consistent with previous reports [14, 15], the efficacy of fractional non-ablative erbium glass laser treatments on deeper penetration and minimised epidermal involvement was also demonstrated in our 3D human skin models. Due to the full-developed dermal structure, collagen skin models offer good experimental possibilities for testing non-ablative laser systems, particularly on deeper dermal layers. The histological examination of these in vitro 3D human skin models revealed similar results compared to the histological analysis of human skin following erbium glass fractional laser treatment in vivo [20].
A deep penetration with reduced involvement at the dermal/epidermal junction has potential advantages, particularly for conditions that may require deeper remodelling [14]. However, we observed an upregulation of epidermal differentiation markers, such as loricrin, filaggrin-1 and filaggrin-2, following laser irradiation. Filaggrin-1 and loricrin were also upregulated on protein level. Caspase 14, which is involved in keratinocyte terminal differentiation and is important for the formation of the skin barrier [17], was downregulated. These gene regulations indicate a potential involvement of the epidermis and an improved epidermal differentiation and expression of important skin barrier proteins; although, no clear epidermal injuries were histologically detectable. Since inflammatory mediators influence epidermal keratinocyte differentiation and skin barrier function [2, 21], the observed chemokine and interleukin regulation in our 3D skin models may be implicated in epidermal differentiation. Furthermore, we observed a higher regulation of these epidermal differentiation markers after treatment with the XF laser device compared to the XD device. The XF optic houses a micro-lens array that separates a single large diameter beam from the erbium glass laser into 175 microbeams, whereas the XD optic separates only into 49 microbeams per shot [15]. For this reason, stronger superficial effects were expected to be induced by the XF optic. This is consistent with the observation that less disruption of the dermal–epidermal junction and deeper penetration occurred after treatment with the XD optic compared to the XF optic [14, 15]. We assume that epidermal microinjuries also occur with dermal laser remodelling.
Inflammation and its cellular mediators are involved in the pathogenesis of hypertrophic scars. It was shown that IL6, IL8 and monocyte chemotactic protein-1 (MCP-1) were significantly increased in fibroblasts of hypertrophic scars compared to normal fibroblasts [22]. Furthermore, scarless embryonal healing tends to be characterised by minimal inflammatory reaction mediated by reduced IL6 and IL8 expression [23].
In our study, the expression of IL6 and IL8, as well as the chemokines CXCL1, CXCL2, CXCL5, CXCL6 and CXCL10 were downregulated following laser treatment. The chemokines are well-known to have chemotactic and activating functions on neutrophils, mainly during acute inflammatory responses. We propose that these erbium glass laser-induced anti-inflammatory effects might positively influence scar remodelling and treatment of hypertrophic or keloid scars.
Interestingly, non-ablative fractional photothermolysis treatment of photodamaged human skin using a 1550-nm erbium doped fibre laser, which induced dermal and stronger epidermal damage, resulted in an initial upregulation of interleukin-1β and tumour necrosis factor-α [24]. This initial inflammatory response was also induced by ablative laser systems in vivo [25]. A cause for these molecular differences in inflammatory response could be the stronger epidermal damage induced by these (ablative) laser systems and the in vivo presence of multiple cells including inflammatory cells such as macrophages and Langerhans cells which interact in vivo on intercellular levels in a cross-linked matter.
MMPs, which are involved in extracellular matrix remodelling, degrade and remove damaged structural extracellular matrix proteins, such as collagen. This was originally thought to be their primary function [26, 27]. However, recent evidence suggests that MMPs also influence other wound healing responses, such as inflammation and re-epithelialisation. MMPs are also involved in the remodelling of abnormal scars [28]. It was shown that tissue inhibitors of metalloproteinase (TIMPs) were downregulated in hypertrophic scars [29]. An increased ratio of MMPs to TIMPs expression could have positive effects on senescence of fibroblasts in hypertrophic scars hereby inhibiting hypertrophic scar formation [30].
In this study, we found that MMP9 mRNA and protein expression were increased by erbium glass laser treatment. Consistent with these results, it was previously reported that mechanical compression induces MMP9 release and activation in hypertrophic scars [31]. This upregulation could be an effector mechanism responsible for hypertrophy regression.
Non-ablative fractional photothermolysis treatment [24] and ablative laser systems [25] cause similar molecular upregulation of MMP gene expression in human in vivo wound healing studies or in human skin explant models. Besides these underlying molecular alterations, changes in gene regulation of heat shock proteins, hyaluronic acid synthetases and hyaluronidases have been postulated to play significant roles in skin remodelling after ablative or non-ablative laser treatment [25].
Other studies assessed mature burn scars treated with a fractional CO2 laser for clinical parameters, histological architecture and the biochemical mechanisms responsible for clinical improvement [32, 33]. Besides the clinical benefit, they found significant improvement in collagen architecture and alterations in the procollagen expression following laser treatment [32, 33]. Furthermore, MMP1 expression was significantly upregulated 48 h after laser treatment [32].
Previous results indicate that MMP3 is responsible for fibroblast contraction and initiating wound contraction [34]. Furthermore, a recent study revealed that laser irradiation regulates MMP expression. In monolayers of the keratinocyte cell line HaCaT, the expression of MMP1, MMP2 and MMP12 decreased at different time points [35]. Consistent with these results, we found that MMP3 mRNA and protein expression were reduced after erbium glass laser treatment.
We assume that erbium glass laser treatment exerts its effects on extracellular matrix remodelling via a fine-tuned regulation of MMP expression.
A limitation of this in vitro skin model is that it can certainly not meet all the requirements of in vivo conditions. Human skin physiology is a very complex fine-tuned system regulated on many different levels. Various internal and external variables influence wound healing and remodelling processes in human skin in vivo.
This human 3D skin model contains only keratinocytes and fibroblasts. However, it represents a simplified model allowing reproducible and comparable data in vitro [5, 6]. The observed gene regulatory effects are specifically attributed to keratinocytes and fibroblasts. The model allows performing analysis of gene regulation on both the RNA and the protein level, as well as histological analysis. It can be utilised to gain data at the same time point in one model, but also at different time points (time course), which is hardly possible in clinical in vivo studies in humans due to ethical reasons. In addition, the organotypic skin model employed in these studies allows performing experiments in various replicates and complete research projects can be conducted with cells from one single donor.
The cells used for the 3D skin model are human primary skin cells. Human primary cells show inter-individual genetic differences due to donor-specific variances. Therefore, an experiment to experiment variability due to the inherent variation in genetics of the donors has to be considered; however, each experiment was performed with primary human keratinocytes and dermal fibroblasts from only one skin donor each.
In conclusion, this human 3D model system may be useful in the future for monitoring ex vivo effects of various laser systems on keratinocyte/fibroblast physiology, skin morphology and gene expression. Due to the fully developed dermal structure, collagen skin models offer good experimental possibilities for testing non-ablative laser systems, particularly on the dermal layers. It allows reliable data and can be applied to comparative studies that analyse the effect of different laser systems, hereby avoiding animal experiments or clinical trials in humans.
References
Neis MM, Wendel A, Wiederholt T, Marquardt Y, Joussen S, Baron JM, Merk HF (2010) Expression and induction of cytochrome p450 isoenzymes in human skin equivalents. Skin Pharmacol Physiol 23(1):29–39
Cornelissen C, Marquardt Y, Czaja K, Wenzel J, Frank J, Luscher-Firzlaff J, Luscher B, Baron JM (2012) IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J Allergy Clin Immunol 129(2):426–433, 433 e421-428
Astashkina A, Grainger DW (2014) Critical analysis of 3-D organoid in vitro cell culture models for high-throughput drug candidate toxicity assessments. Adv Drug Deliv Rev 69–70:1–18
Mathes SH, Ruffner H, Graf-Hausner U (2014) The use of skin models in drug development. Adv Drug Deliv Rev 69–70:81–102
European Centre for the Validation of Alternative Methods (ECVAM). https://eurl-ecvam.jrc.ec.europa.eu/validation-regulatory-acceptance/topical-toxicity/skin-irritation. Accessed 10/28/2015
Marquardt Y, Amann PM, Heise R, Czaja K, Steiner T, Merk HF, Skazik-Voogt C, Baron JM (2015) Characterization of a novel standardized human three-dimensional skin wound healing model using non-sequential fractional ultrapulsed CO2 laser treatments. Lasers Surg Med 47(3):257–265
Sardana K, Manjhi M, Garg VK, Sagar V (2014) Which type of atrophic acne scar (ice-pick, boxcar, or rolling) responds to nonablative fractional laser therapy? Dermatol Surg 40(3):288–300
Cho SB, Lee SJ, Cho S, Oh SH, Chung WS, Kang JM, Kim YK, Kim DH (2010) Non-ablative 1550-nm erbium-glass and ablative 10 600-nm carbon dioxide fractional lasers for acne scars: a randomized split-face study with blinded response evaluation. J Eur Acad Dermatol Venereol 24(8):921–925
Taudorf EH, Danielsen PL, Paulsen IF, Togsverd-Bo K, Dierickx C, Paasch U, Haedersdal M (2015) Non-ablative fractional laser provides long-term improvement of mature burn scars—a randomized controlled trial with histological assessment. Lasers Surg Med 47(2):141–147
Guimaraes PA, Haddad A, Sabino Neto M, Lage FC, Ferreira LM (2013) Striae distensae after breast augmentation: treatment using the nonablative fractionated 1550-nm erbium glass laser. Plast Reconstr Surg 131(3):636–642
Puri N (2013) A study on fractional erbium glass laser therapy versus chemical peeling for the treatment of melasma in female patients. J Cutan Aesthet Surg 6(3):148–151
Lee GY, Lee SJ, Kim WS (2011) The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol 25(12):1450–1454
Moneib H, Tawfik AA, Youssef SS, Fawzy MM (2014) Randomized split-face controlled study to evaluate 1550-nm fractionated erbium glass laser for treatment of acne vulgaris—an image analysis evaluation. Dermatol Surg 40(11):1191–1200
Childs J, Altshuler G (2011) XD Microlens™ compression optic for deep-dermis non-ablative fractional treatment. http://www.palomarmedical.com/uploaddocs/xdmicrolenscompressionoptic.pdf. Accessed 04/03/2015
Doherty S, Brooke S, Childs J, Tabatadze D, Erofeev A, Smirnov M, Altshuler G (2011) XF Microlens™ Optic and XD Microlens™ compression optic for non-ablative fractional skin treatment with the Palomar Icon™ System. http://www.palomarmedical.com/uploaddocs/xd_xfpaper2011.pdf. Accessed 04/03/2015
Heise R, Skazik C, Marquardt Y, Czaja K, Sebastian K, Kurschat P, Gan L, Denecke B, Ekanayake-Bohlig S, Wilhelm KP, Merk HF, Baron JM (2012) Dexpanthenol modulates gene expression in skin wound healing in vivo. Skin Pharmacol Physiol 25(5):241–248
Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol 115(6):1148–1151
Xu LY, Kilmer SL, Ross EV, Avram MM (2015) Bacterial infections following non-ablative fractional laser treatment: a case series and discussion. Lasers Surg Med 47(2):128–132
Wang CC, Huang CL, Lee SC, Sue YM, Leu FJ (2013) Treatment of cosmetic tattoos with nonablative fractional laser in an animal model: a novel method with histopathologic evidence. Lasers Surg Med 45(2):116–122
Farkas JP, Richardson JA, Hoopman J, Brown SA, Kenkel JM (2009) Micro-island damage with a nonablative 1540-nm Er: glass fractional laser device in human skin. J Cosmet Dermatol 8(2):119–126
Danso MO, van Drongelen V, Mulder A, van Esch J, Scott H, van Smeden J, El Ghalbzouri A, Bouwstra JA (2014) TNF-alpha and Th2 cytokines induce atopic dermatitis-like features on epidermal differentiation proteins and stratum corneum lipids in human skin equivalents. J Invest Dermatol 134(7):1941–1950
Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, Tredget EE (2011) Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J Cell Physiol 226(5):1265–1273
Mofikoya BO, Adeyemo WL, Ugburo AO (2012) An overview of biological basis of pathologic scarring. Niger Postgrad Med J 19(1):40–45
Orringer JS, Rittie L, Baker D, Voorhees JJ, Fisher G (2010) Molecular mechanisms of nonablative fractionated laser resurfacing. Br J Dermatol 163(4):757–768
Helbig D, Paasch U (2011) Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Skin Res Technol 17(1):119–128
Shah JM, Omar E, Pai DR, Sood S (2012) Cellular events and biomarkers of wound healing. Indian J Plast Surg 45(2):220–228
Gill SE, Parks WC (2008) Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 40(6–7):1334–1347
Fujiwara M, Muragaki Y, Ooshima A (2005) Keloid-derived fibroblasts show increased secretion of factors involved in collagen turnover and depend on matrix metalloproteinase for migration. Br J Dermatol 153(2):295–300
Tsou R, Cole JK, Nathens AB, Isik FF, Heimbach DM, Engrav LH, Gibran NS (2000) Analysis of hypertrophic and normal scar gene expression with cDNA microarrays. J Burn Care Rehabil 21(6):541–550
Wang Q, Dong Y, Geng S, Su H, Ge W, Zhen C (2014) Photodynamic therapy inhibits the formation of hypertrophic scars in rabbit ears by regulating metalloproteinases and tissue inhibitor of metalloproteinase-1. Clin Exp Dermatol 39(2):196–201
Reno F, Grazianetti P, Stella M, Magliacani G, Pezzuto C, Cannas M (2002) Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Arch Dermatol 138(4):475–478
Qu L, Liu A, Zhou L, He C, Grossman PH, Moy RL, Mi QS, Ozog D (2012) Clinical and molecular effects on mature burn scars after treatment with a fractional CO(2) laser. Lasers Surg Med 44(7):517–524
Ozog DM, Liu A, Chaffins ML, Ormsby AH, Fincher EF, Chipps LK, Mi QS, Grossman PH, Pui JC, Moy RL (2013) Evaluation of clinical results, histological architecture, and collagen expression following treatment of mature burn scars with a fractional carbon dioxide laser. JAMA Dermatol 149(1):50–57
Bullard KM, Mudgett J, Scheuenstuhl H, Hunt TK, Banda MJ (1999) Stromelysin-1-deficient fibroblasts display impaired contraction in vitro. J Surg Res 84(1):31–34
Melerzanov A, Lavrov A, Sakania L, Korsunskaya I, Petersen E, Sobelev V (2014) Effects of laser radiation on MMP gene expression in keratinocytes. PRIME J 4(3):39–44
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Ruth Heise and Jens M. Baron contributed equally to this work.
Rights and permissions
About this article
Cite this article
Amann, P.M., Marquardt, Y., Steiner, T. et al. Effects of non-ablative fractional erbium glass laser treatment on gene regulation in human three-dimensional skin models. Lasers Med Sci 31, 397–404 (2016). https://doi.org/10.1007/s10103-015-1863-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1863-x