Abstract
The present work reports the photo-biomodulatory effect of red (632.8 nm) and near infrared (785 and 830 nm) lasers on burn injury in Swiss albino mice. Animals were induced with a 15-mm full thickness burn injury and irradiated with various fluences (1, 2, 3, 4, and 6 J/cm2) of each laser wavelength under study having a constant fluence rate (8.49 mW/cm2). The size of the injury following treatment was monitored by capturing the wound images at regular time intervals until complete healing. Morphometric assessment indicated that the group treated with 3-J/cm2 fluence of 830 nm had a profound effect on healing as compared to untreated controls and various fluences of other wavelengths under study. Histopathological assessment of wound repair on treatment with an optimum fluence (3 J/cm2) of 830 nm performed on days 2, 6, 12, and 18 post-wounding resulted in enhanced wound repair with migration of fibroblasts, deposition of collagen, and neovascularization as compared to untreated controls. The findings of the present study have clearly demonstrated that a single exposure of 3-J/cm2 fluence at 830-nm enhanced burn wound healing progression in mice, which is equivalent to 5 % povidone iodine treatment (reference standard), applied on a daily basis till complete healing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wound repair is a natural course of action against injury which comprises multifaceted step [1, 2], regulated upon activation of a series of molecules such as cytokines, growth factors, and hormones [3, 4], playing a crucial role in the restoration process in response to injury. Any impairment in the cascade may result in delayed healing. Several systemic and local factors may also contribute to the cause, leading to the development of non-healing wounds [5]. In burns, the nature of injury is complex and is often accompanied by microbial infections further complicating the healing process [6]. Wide range of treatment modalities is being practiced in clinics to treat burn injuries [7, 8]. Despite these advances, the management of burns still remains a concern and, therefore, development of new approaches to reduce physical and psychological stress associated with burns is still needed.
Low-level laser therapy (LLLT) is a non-invasive technique and is regularly being tested over the decades for its possible application in tissue regeneration, as it is known to induce a photo-biological response [9] by altering cellular redox potential in cells. The effect of this favorable response triggered at molecular level [10] is being actively investigated as reported both in vitro [11–13] and in vivo [14, 15] studies. However, one limitation of this method is that the light energy for stimulation in LLLT at threshold can stimulate therapeutic outcome in low fluence but an inhibitory effect at high fluence [15]. It is highly dependent on several complex laser parameters [16], and the choice of these parameters plays a major role in the effectiveness of treatment or otherwise, which could lead to negative therapeutic outcomes as depicted in biphasic dose-response curve observed in LLLT [17].
In an effort to facilitate the choice of laser parameters, the present work was designed to interpret the influence of various fluences of 632.8, 785, and 830 nm having equal power density (8.49 mW/cm2). The study will help in selecting an optimal fluence and appropriate laser wavelength for burn wound healing in mice. The experimental animals were induced with full thickness burns followed by treatment with various fluences of selected wavelengths under study. The optimum fluence and wavelength for best healing was assessed through morphometric analysis. Histopathological analysis was performed at various time points for the optimum fluence to understand the effect of the wavelength in ameliorating healing events in burn induced mice.
Materials and methods
Selection of animals
Animals were handled as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Govt. of India. Experiments conducted on animals were with prior approval from the Institutional Animal Ethics Committee (IAEC), Manipal University, India. Swiss albino mice (total = 221) of either sex aged 6–8 weeks, weighing 25–30 g were selected from an inbred colony of mice, maintained under controlled conditions of temperature (23 ± 2 °C), humidity (50 ± 5 %), and light/dark (12 and 12 h) cycle, access to sterile water and food ad libitum at our institutional animal house facility. All the animals were monitored carefully throughout the experimental period and housed individually in polypropylene cages comprising of sterile paddy husk as quilt.
Mouse model for full thickness burn
Dorsal surface of each animal was depilated using electronic mouse clipper (Remington, Spectrum Brands Inc., Germany). Animals were administered with a cocktail of anesthesia (65-mg/kg Ketamine -Aneket, Neon Laboratories, India, and 8-mg/kg Diazepam—Calmpose, Ranbaxy Laboratories, India) intraperitoneally. The depilated area was swabbed with disinfectant solution, and thermal injury was induced by placing a hot aluminum rod (15-mm diameter), immersed in boiling water bath for 2 min and placed on dorsum surface of the mice for 60 s to induce burn. Duration required to achieve full thickness injury was determined by conducting the procedure on animals (n = 5, each) for 30, 60, and 90 s. Histology was performed to analyze the severity of burn and was confirmed by histopathologist, scored in a blinded way.
Experimental design
About 170 animals induced with full thickness burn were randomly divided into 3 major groups (Table 1); Group 1: untreated control, devoid of any treatment, Group 2: 5 % povidone iodine (reference standard) (Wokadine, Wockhardt, Mumbai, India), topical application on a daily basis and Groups 3–5: served as laser treatment groups for 632.8, 785, and 830 nm, irradiated with fluences 1, 2, 3, 4, and 6 J/cm2, respectively, only once immediately after wounding. Laser sources include; Helium-Neon laser—632.8 nm (CVI Melles Griot, US), and two diode lasers—785 and 830 nm (CNI Optoelectronics Tech. Co., Ltd.) The laser beam was coupled with an optical fiber, carried further and coupled to a beam expander for ensuring uniform laser beam, covering inflicted area of the mice as shown in Fig. 1. All the laser irradiation experiments were conducted after 30 min of laser warm-up time to enable stable laser power throughout the experimental procedure. Table 2 illustrates the laser parameters used in the study for irradiating burn inflicted mice.
Morphometric analysis
The animals with burn lesions were monitored, and the images were captured using digital camera with fixed distance. The area of the burnt lesions was calculated using AutoCAD 2011, version 18.1 (Autodesk, Inc., California, USA) software. The wound area at a particular day was expressed in percentage of day one by considering the wound area on day one as 100 % and calculated as follows:
The average healing time of animals from each group was calculated and is represented as mean healing time in days. The experiments were conducted in duplicates (n = 5), and data were represented as mean ± standard error of mean. Statistical analysis was performed using GraphPAD Prism 5 (GraphPad Software, Inc., California, USA) software. Unpaired t test, one-way ANOVA and two-way ANOVA with a post-hoc test was used for comparisons. p < 0.05 was considered significant.
Histopathology
Animals (n = 36) induced with full thickness burns were randomly divided into 3 groups; Group 1: untreated control, devoid of any treatment, Group 2: 5 % povidone iodine (reference standard) (Wokadine, Wockhardt, Mumbai, India), topical application on a daily basis and Group 3: laser treatment group (optimum fluence and wavelength). Three animals from each group were euthanized on 2, 6, 12, and 18 days post-injury, and burn tissues were excised, fixed in Bouin’s solution, processed in series of alcohol, cleared in xylene, and embedded in paraffin as described elsewhere [18]. Sections of thickness 4–5 μm were obtained using microtome and stained with Hematoxylin and Eosin (H and E). Inflammatory levels, neovascularization and deposition of collagen by fibroblast proliferation, and epithelialization were assessed qualitatively by a histopathologist in a blinded fashion.
Results
Standardization of burn injury
Burn injury model for the present study was established by histopathological evaluation. The histological changes observed after 30, 60, and 90 s burn induction is shown in Fig. 2. The full thickness burn achieved with 30 s of burn procedure was limited to epidermis and just extending to dermal region of the skin (Fig. 2b). In case of full thickness burn with 60 s of burn induction, the severity of damage extended till the reticular dermis to subcutaneous tissue, damaging all the skin layers (Fig. 2c). In case of 90-s burn induction (Fig. 2d), the wound extended beyond the subcutis to the muscle layer, as compared to intact architecture of the unwounded skin (Fig. 2a). The severity of the injury was assessed by an experienced histopathologist and confirmed that 60 s of burn procedure was sufficient to induce full thickness burn in Swiss albino mice.
Morphometric assessment of burn injury
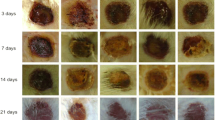
The progression of wound healing was monitored by capturing digital images (Fig. 3) and wound contraction rate for each group has been illustrated in Table 3. As anticipated, a significantly enhanced wound contraction on day 3 (p < 0.05), day 9 (p < 0.05), day 15 (p < 0.05), day 21 (p < 0.001), and day 27 (p < 0.01) was observed in reference standards as compared to untreated controls. The group treated with 2 J/cm2 fluence of 632.8 nm showed statistically significant difference on day 27 (p < 0.05) as compared to untreated controls, whereas no significant difference was observed for the group treated with various fluences of 785 nm. On the other hand, animals receiving 3-J/cm2 fluence of 830 nm have also shown profound wound contraction as observed on day 21 (p < 0.01) and day 27 (p < 0.001), compared to untreated controls (Table 3). An additional bar graph representing the % of wound closure on day 27 for all the wavelengths under study and respective fluences, depicting best and poor fluence is shown in Fig. 4.
In order to understand the overall contraction and the time taken by the animals to heal, the average healing time was calculated for all the experimental groups (Fig. 5). The average healing time was found to be 35.4 ± 0.4 days for untreated controls and 29.6 ± 1.0 days for 5 % povidone iodine treatment group. The average healing time for 632.8-nm treatment group was observed to be 34.2 ± 0.8, 29.8 ± 0.9, 31.2 ± 0.6, 34.0 ± 1.2, and 34.2 ± 1.0 days post-wounding for 1, 2, 3, 4, and 6 J/cm2 fluence, respectively (Fig. 5a). Similarly, for 785-nm treatment group, the average healing time was found to be 32.8 ± 1.3, 31.6 ± 0.5, 31.8 ± 0.8, 34.2 ± 1.0, and 34.2 ± 0.9 days (Fig. 5b). Likewise, 830-nm treatment group exhibited average healing time of 32 ± 1.0, 31.2 ± 1.0, 29.2 ± 0.8, 29.8 ± 0.5, and 31.6 ± 1.1 days for 1, 2, 3, 4, and 6 J/cm2, respectively, as shown in Fig. 5c. As predicted, the reference standard revealed a significant (p < 0.001) mean healing time as compared to untreated controls. Statistical analysis was also performed for test groups and observed a significant difference in 632.8 nm exposed with 2 (p < 0.001) and 3 J/cm2 (p < 0.05) as compared to untreated controls. Although 785 nm exhibited a slight response to healing, which is better than untreated control, the difference observed was statistically insignificant as shown in Fig. 5b. However, 830 nm exhibited a better response with significant difference when exposed with 2 (p < 0.05), 3 (p < 0.001), 4 (p < 0.001), and 6 J/cm2 (p < 0.05), respectively, as compared to untreated controls.
Histopathological analysis
The H and E-stained sections of untreated, positive control and test (830 nm 3 J/cm2) groups were qualitatively assessed by an experienced pathologist in a blinded manner. Figure 6 represents the outcomes of histopathological observations. Group treated with 3 J/cm2 fluence of 830 nm displayed mild inflammation in between the subcutaneous region with marked edema and tissue necrosis on day 2 post-irradiation. While untreated controls were mostly necrotic and edematous with persistence of intense inflammation, presence of neutrophils and lymphocytes extensively at subcutaneous region. At day 6 post-wound irradiation, with the presence of mild inflammation with occasional neutrophils, moderate amount of plump fibroblasts was found, deposition of collagen, minimal granulation tissue formation at wound edges and mild to moderate level of neovascularization was observed at subcutaneous region. In contrast, minimal neovascularization and fibroblast proliferation with dense inflammation persisted in untreated controls. By day 12 post-wound irradiation (830 nm 3 J/cm2), regenerating epithelium was observed with better neovascularization, moderate amount of granulation tissue with denser packing of collagen was observed as compared to untreated controls, where the presence of plasma cells at mild to moderate levels was observed. Mild to moderate levels of macrophages were observed at days 2, 6, and 12 post-wounding in almost all the groups under study. At day 18, epithelialization at wound margin with abundant neovascularization and denser connective tissue with random arrangement of collagen fibers was observed in group 830 nm exposed to 3 J/cm2. In contrast, in untreated controls, the connective tissue was still loose and moderate level of neovascularization was observed. Scanty neutrophils and plasma cells persisted at low levels in an edematous tissue.
Microscopic images of histopathological changes on 2, 6, 12, and 18 day post-wounding in untreated control (a–d), 5 % Povidone iodine (e–h) and 830 nm 3 J/cm2 (i–l). H & E-stained sections of untreated control showed persistence of dense inflammation on day 2 and 6 at subcutaneous region with slight neovascularization and fibroblast proliferation. However, 830 nm 3 J/cm2 displayed mild inflammation, mild to moderate level of neovascularization and fibroblast proliferation. By day 12 and 18, the group treated with 830 nm 3 J/cm2 exhibited better epithelialization at wound margin and abundant neovascularization with fibroblast proliferation and deposition of denser connective tissue, compared to untreated control persisting with scanty inflammatory cells, loose connective tissue, and moderate level of neovascularization. (HE, ×400)
Discussion
The use of in vitro models in the field of burns evaluating the progression of healing has limitations in assessing the complexity behind the disease. Nevertheless, in vivo rodent models like mouse and rat have been found suitable for studying burns injury and evaluating the progression of healing [19]. Further, mouse are easy to handle and cost effective as well making the monitoring process post-burn injury simple due to its reduced healing time [20]. Therefore, in the present study, mouse has been used for evaluating the treatment outcomes in burn wound healing. Standardization of burn wound model seeking reproducibility in the study outcomes is another important aspect when studying factors related to burn injury. To induce burns on animals, the present study adapted heating metal method [21] and standardized the time duration for creating full thickness burns at our facility through histopathological assessment of the corresponding changes as compared to untreated controls (Fig. 2). The histopathological analysis revealed that 60 s of burn procedure was suitable for inducing full thickness burn in Swiss albino mice.
The biphasic dose-response generated in LLLT is frequently related with the “Arndt Schultz law,” which states that “weak stimuli increases physiologic activity, moderate stimuli inhibit activity and very strong stimuli abolish activity.” In context to LLLT, the stimulus could be irradiation time or irradiance (laser beam intensity) [17]. The therapeutic effect of laser therapy is highly dependent on the factors like laser beam intensity, laser exposure, and the fluence delivered. However, a recently published article [22] has clearly stated the importance of key illumination parameters in improving the repeatability and reliability of study outcomes. Based on these, the laser parameters described in Table 2 such as beam area, power, irradiance, fluence, and exposure time were kept constant, except for the wavelength, the influence of which was evaluated in the present study. Hence, the observed photo-biomodulatory effects in the present study following LLLT could be contributed by the laser wavelengths under study. Nevertheless, the wavelengths to be evaluated were selected based on the oxidized forms of the copper centers present in cytochrome c oxidase (CCO), which are responsible for the photo-biomodulatory effect and are sensitive to red and near infrared lights (620–820 nm) [23], as reported by Karu [24]. There are several studies in the literature of using 632.8-nm irradiation on burn wound healing reporting efficacious outcomes [25, 26]. However, studies using 785-nm irradiation on burn wound healing showing progressive outcomes are very few [27]. Recently, there are fewer reports of using 830-nm irradiation as well on burn wound healing [28, 29]. The use of three laser wavelengths (632.8, 785, and 830 nm) in the present study intended to understand the influence of laser fluences for all the wavelengths in burn wound healing and to make a comparative evaluation for selecting an optimal fluence of a wavelength showing accelerated tissue regeneration. Our intension was also to evaluate whether the observed photo-biomodulatory effects was due to the laser fluence specific to a particular wavelength or to the laser fluence alone.
Wound closure can be referred as the boundaries of the injury travelling towards the center and can be assessed through parameters like percentage of wound contraction and average healing time [15, 30]. The groups irradiated with laser exhibited a better wound contraction rate as compared to untreated controls. During the progression of healing, an initial expansion of the wound [31] was observed at initial days in all the groups, which subsequently reduced to the original size and eventually contracted. We observed that 830-nm treatment group was more potent as observed in morphometric analysis when compared to other two wavelengths. While 632.8 nm showed a partial effect in the healing of burn injury, 785 nm showed a limited effect in the study. Although, the healing time for 632.8 nm (2 J/cm2) was almost similar to that of 830 nm (3 and 4 J/cm2), the independent t test (data not shown) revealed 830-nm treatment significantly reduced the healing time (p < 0.01) as compared to 632.8 nm (mean ± SEM, 30.7 ± 0.4 days vs. 32.6 ± 0.4 days) treatments. Furthermore, two-way ANOVA was also performed (data not shown) to determine the interaction between the wavelength and fluence for the healing time, but no such interaction in the present study was observed (p > 0.05). The morphometric observation revealed that the group treated with 3-J/cm2 fluence of 830 nm exhibited best response with an optimum effect when compared to other groups and statistically significant when compared to untreated controls. In order to further evaluate the efficacy of 3-J/cm2 fluence of 830 nm showing optimum tissue regeneration, histopathological analysis was also performed. Wound healing can be investigated at several levels of the healing progression with histopathology by utilizing stains specific to cellular and tissue components of the skin. As reported in literature [31, 32], the use of H and E staining has the ability to qualitatively assess numerous pathological parameters and the same was used in the present study to assess the efficacy of 3-J/cm2 fluence of 830 nm on burn wound healing. The histological observations revealed that the group treated with 3-J/cm2 fluence of 830 nm exhibited favorable outcomes compared to untreated controls. This led to a more beneficial response in controlling the levels of inflammation and thereby regulating proliferation, neovascularization and remodeling during the healing. These findings were in accordance with the earlier studies using low doses of near-infrared wavelengths [28, 32, 33]. Further, it is a recognized fact that proliferation phase is characterized by formation of new blood vessels, granulation tissue formation, and re-epithelialization in histological analysis as observed in the present study on day 12 and 18 post-wounding with 3-J/cm2 fluence of 830 nm. A similar observation was also reported on cutaneous wound healing by Rezende and co-workers with 1.3-J/cm2 fluence of 830-nm treatment [32]. This may be due to the type of wound (cutaneous) examined as well as the laser fluence rate (53 mW/cm2) used in their study as compared to the present study on full thickness burns irradiated with fluence rate of 8.49 mW/cm2. Several studies have also reported the use of near-infrared wavelengths at low doses to stimulate enhanced deposition of collagen and tensile strength of collagen fibers [32, 34]. In the present study, treatment with 3-J/cm2 fluence of 830 nm showed a similar observation with a modest amount of granulation tissue and denser packing of connective tissues in the wound bed.
Healing of injury requires continuous supply of energy in the form of ATP to repair damaged tissues and provide all the necessary nutrients to restore the healthy tissues. Mitochondria which is a source of ATP is particularly sensitive to monochromatic lights due to the presence of transmembrane protein CCO involved in the respiratory chain. Karu et al. have shown the existence of a wide range of absorption spectrum of CCO ranging from visible to near-infrared region of the electromagnetic spectrum [24, 35]. The response observed in the present study at 830-nm treatment may be due to the oxidized activity of CCO [34] and its ability to penetrate deeper into the tissues. In contrast, 632.8 nm is readily absorbed by various components in the skin and tissues, limiting the penetration depth to less than 3–5 mm [36]. On the other hand, 785 nm had limited effect, perhaps due to the reduced form of CCO at wavelengths 750–770 nm with limited biochemical activity [10, 34].
Povidone iodine is known for its wide spectrum of microbicidal activity against bacteria, protozoa, fungi, and virus and has been accepted to treat burn injuries in clinical settings [37–39]. The free iodine is slowly discharged from the povidone iodine complex and aids in anti-microbial activity by lipid iodination, cytoplasmic and membrane oxidation. Povidone iodine has also shown excellent healing properties on both open [40, 41] and burn wounds [42, 43], acting as an effective barrier against microbial infection and promotes wound healing [44]. Based on these evidences, povidone iodine (5 %) was used as a reference standard to evaluate the efficacy of all the laser treatment groups under study. In the present study, the group treated with optimum laser fluence and wavelength was performed only once after the injury, while 5 % povidone iodine was applied on daily basis as prescribed by the clinician, till complete healing. Surprisingly, 3-J/cm2 single exposure of 830-nm treatment group exhibited a similar effect as that of 5 % povidone iodine treatment in enhancing burn wound healing. This observation was noticed both in biophysical and histopathological evaluations of 3-J/cm2 fluence of 830-nm treatment confirming the healing potential of the laser wavelength and fluence. The advantage of using LLLT as a treatment modality is its non-invasive nature and has a huge potential of photo-biomodulatory effects when applied at an optimum dose of a suitable wavelength. While in topical application there is a chance of disturbing the wound and most importantly the possible side effects of Povidone iodine, i.e., excessive iodine resorption in clinical consequences may lead to systemic effects [45]. However, investigating the physical interaction between the photons and CCO [46] in mitochondria could help in solving the mystery of LLLT mechanism and could relate to the results observed in the present study of 3-J/cm2 treatment with 830 nm in enhancing burn wound healing in mice.
Conclusion
In summary, low-power lasers at wavelength 632.8, 785, and 830 nm have shown beneficial effects in burn wound healing, compared to untreated controls. Further, 3-J/cm2 fluence of 830 nm exhibited the best response among the other wavelengths and fluences under study in enhancing wound repair in full thickness burn injury in mice. The treatment promoted proliferation, neovascularization, deposition of collagen, denser packing of connective tissue, and epithelialization at faster rate to contract the wound, compared to untreated controls. Surprisingly, the response observed in the single exposure of 3-J/cm2 treatment of 830 nm was equivalent to the reference standard applied on daily basis. Upon additional evaluation by different groups, this could be an alternate source of therapy in near future for treating full thickness burn injuries.
References
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Frontiers in bioscience : a journal and virtual library 9:283–289
Schreml S, Szeimies RM, Prantl L, Landthaler M, Babilas P (2010) Wound healing in the 21st century. J Am Acad Dermatol 63(5):866–881. doi:10.1016/j.jaad.2009.10.048
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229. doi:10.1177/0022034509359125
Monaco JL, Lawrence WT (2003) Acute wound healing an overview. Clin Plast Surg 30(1):1–12
Velnar T, Bailey T, Smrkolj V (2009) The wound healing process: an overview of the cellular and molecular mechanisms. The Journal of international medical research 37(5):1528–1542
Kao CC, Garner WL (2000) Acute Burns. Plast Reconstr Surg 101(7):2482–2493
Atiyeh BS, Hayek SN, Gunn SW (2005) New technologies for burn wound closure and healing—review of the literature. Burns : journal of the International Society for Burn Injuries 31(8):944–956. doi:10.1016/j.burns.2005.08.023
Fan WPL, Rashid M, Enoch S (2010) Current advances in modern wound healing. Wounds UK 6(3):22–36
Huang YY, Chen AC, Carroll JD, Hamblin MR (2009) Biphasic dose response in low level light therapy. Dose-response : a publication of International Hormesis Society 7(4):358–383. doi:10.2203/dose-response.09-027.Hamblin
Avci P, Gupta A, Sadasivam M, Vecchio D, Pam Z, Pam N, Hamblin MR (2013) Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Seminars in cutaneous medicine and surgery 32(1):41–52
Fushimi T, Inui S, Nakajima T, Ogasawara M, Hosokawa K, Itami S (2012) Green light emitting diodes accelerate wound healing: characterization of the effect and its molecular basis in vitro and in vivo. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 20(2):226–235. doi:10.1111/j.1524-475X.2012.00771.x
Peplow PV, Chung TY, Ryan B, Baxter GD (2011) Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed Laser Surg 29(5):285–304. doi:10.1089/pho.2010.2846
Zhang Y, Song S, Fong CC, Tsang CH, Yang Z, Yang M (2003) cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. The Journal of investigative dermatology 120(5):849–857. doi:10.1046/j.1523-1747.2003.12133.x
Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR (2007) Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 39(9):706–715. doi:10.1002/lsm.20549
Prabhu V, Rao SB, Rao NB, Aithal KB, Kumar P, Mahato KK (2010) Development and evaluation of fiber optic probe-based helium-neon low-level laser therapy system for tissue regeneration—an in vivo experimental study. Photochem Photobiol 86(6):1364–1372. doi:10.1111/j.1751-1097.2010.00791.x
Gupta A, Dai T, Hamblin MR (2014) Effect of red and near-infrared wavelengths on low-level laser (light) therapy-induced healing of partial-thickness dermal abrasion in mice. Lasers Med Sci 29(1):257–265. doi:10.1007/s10103-013-1319-0
Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533. doi:10.1007/s10439-011-0454-7
Prabhu V, Rao SB, Chandra S, Kumar P, Rao L, Guddattu V, Satyamoorthy K, Mahato KK (2012) Spectroscopic and histological evaluation of wound healing progression following Low Level Laser Therapy (LLLT). J Biophotonics 5(2):168–184. doi:10.1002/jbio.201100089
Erdle BJ, Brouxhon S, Kaplan M, Vanbuskirk J, Pentland AP (2008) Effects of continuous-wave (670-nm) red light on wound healing. Dermatologic surgery: official publication for American Society for Dermatologic Surgery [et al] 34(3):320–325. doi:10.1111/j.1524-4725.2007.34065.x
Abdullahi A, Amini-Nik S, Jeschke MG (2014) Animal models in burn research. Cellular and molecular life sciences : CMLS 71(17):3241–3255. doi:10.1007/s00018-014-1612-5
Avniel S, Arik Z, Maly A, Sagie A, Basst HB, Yahana MD, Weiss ID, Pal B, Wald O, Ad-El D, Fujii N, Arenzana-Seisdedos F, Jung S, Galun E, Gur E, Peled A (2006) Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. The Journal of investigative dermatology 126(2):468–476. doi:10.1038/sj.jid.5700069
Hadis MA, Zainal SA, Holder MJ, Carroll JD, Cooper PR, Milward MR, Palin WM (2016) The dark art of light measurement: accurate radiometry for low-level light therapy. Lasers Med Sci 31(4):789–809. doi:10.1007/s10103-016-1914-y
Prindeze NJ, Moffatt LT, Shupp JW (2012) Mechanisms of action for light therapy: a review of molecular interactions. Experimental biology and medicine 237(11):1241–1248. doi:10.1258/ebm.2012.012180
Karu T (1999) Primary and secondary mechanisms of action of visible to near-IR radiation on cells. J Photochem Photobiol B Biol 49(1):1–17. doi:10.1016/S1011-1344(98)00219-X
Bayat M, Vasheghani MM, Razavi N (2006) Effect of low-level helium-neon laser therapy on the healing of third-degree burns in rats. J Photochem Photobiol B Biol 83(2):87–93. doi:10.1016/j.jphotobiol.2005.12.009
Bayat M, Vasheghani MM, Razavi N, Taheri S, Rakhshan M (2005) Effect of low-level laser therapy on the healing of second-degree burns in rats: a histological and microbiological study. J Photochem Photobiol B Biol 78(2):171–177. doi:10.1016/j.jphotobiol.2004.08.012
Mun S, Cheon M, Kim SH, Choi N, Kim S, Yoo Y, Lim S (2013) The effect of laser diode irradiation on wound healing of rat skin. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology 15(6):318–325. doi:10.3109/14764172.2013.807116
Chiarotto GB, Neves LM, Esquisatto MA, Do Amaral ME, Dos Santos GM, Mendonca FA (2014) Effects of laser irradiation (670-nm InGaP and 830-nm GaAlAs) on burn of second-degree in rats. Lasers Med Sci 29(5):1685–1693. doi:10.1007/s10103-014-1573-9
Lee GY, Kim WS (2012) The systemic effect of 830-nm LED phototherapy on the wound healing of burn injuries: A controlled study in mouse and rat models. Journal of cosmetic and laser therapy : official publication of the European Society for Laser Dermatology 14(2):107–110. doi:10.3109/14764172.2011.649762
Hegde VN, Prabhu V, Rao SB, Chandra S, Kumar P, Satyamoorthy K, Mahato KK (2011) Effect of laser dose and treatment schedule on excision wound healing in diabetic mice. Photochem Photobiol 87(6):1433–1441. doi:10.1111/j.1751-1097.2011.00991.x
de Moraes JM, Eterno de Oliveira Mendonca D, Moura VB, Oliveira MA, Afonso CL, Vinaud MC, Bachion MM, de Souza Lino R Jr (2013) Anti-inflammatory effect of low-intensity laser on the healing of third-degree burn wounds in rats. Lasers Med Sci 28(4):1169–1176. doi:10.1007/s10103-012-1213-1
Rezende SB, Ribeiro MS, Nunez SC, Garcia VG, Maldonado EP (2007) Effects of a single near-infrared laser treatment on cutaneous wound healing: biometrical and histological study in rats. J Photochem Photobiol B Biol 87(3):145–153. doi:10.1016/j.jphotobiol.2007.02.005
Gupta A, Keshri GK, Yadav A, Gola S, Chauhan S, Salhan AK, Bala Singh S (2015) Superpulsed (Ga-As, 904 nm) low-level laser therapy (LLLT) attenuates inflammatory response and enhances healing of burn wounds. J Biophotonics 8(6):489–501. doi:10.1002/jbio.201400058
Karu TI, Pyatibrat LV, Kolyakov SF, Afanasyeva NI (2005) Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B Biol 81(2):98–106. doi:10.1016/j.jphotobiol.2005.07.002
Karu TI, Kolyakov SF (2005) Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 23(4):355–361. doi:10.1089/pho.2005.23.355
Peng Q, Juzeniene A, Chen J, Svaasand LO, Warloe T, Giercksky K-E, Moan J (2008) Lasers in medicine. Rep Prog Phys 71(5):056701
de Kock M (1985) Topical burn therapy comparing povidone-iodine ointment or cream plus aserbine, and povidone-iodine cream. The Journal of hospital infection 6(Suppl A):127–132
Vermeulen H, Westerbos SJ, Ubbink DT (2010) Benefit and harm of iodine in wound care: a systematic review. The Journal of hospital infection 76(3):191–199. doi:10.1016/j.jhin.2010.04.026
Goldenheim PD (1993) An appraisal of povidone-iodine and wound healing. Postgrad Med J 69(Suppl 3):S97–105
Campbell N, Campbell D (2013) Evaluation of a non-adherent, povidone-iodine dressing in a case series of chronic wounds. J Wound Care 22(8):401–402. doi:10.12968/jowc.2013.22.8.401, 404-406
Daroczy J (2006) Quality control in chronic wound management: the role of local povidone-iodine (Betadine) therapy. Dermatology 212(Suppl 1):82–87. doi:10.1159/000089204
Norman D (2003) The use of povidone-iodine in superficial partial-thickness burns. Br J Nurs 12(6 Suppl):S30–36. doi:10.12968/bjon.2003.12.Sup1.11250
Yuksel EB, Yildirim AM, Bal A, Kuloglu T (2014) The effect of different topical agents (silver sulfadiazine, povidone-iodine, and sodium chloride 0.9%) on burn injuries in rats. Plastic surgery international 2014:907082. doi:10.1155/2014/907082
Dai T, Huang YY, Sharma SK, Hashmi JT, Kurup DB, Hamblin MR (2010) Topical antimicrobials for burn wound infections. Recent patents on anti-infective drug discovery 5(2):124–151
Steen M (1993) Review of the use of povidone-iodine (PVP-I) in the treatment of burns. Postgrad Med J 69(Suppl 3):S84–92
Lane N (2006) Cell biology: power games. Nature 443(7114):901–903. doi:10.1038/443901a
Acknowledgements
The authors would like to thank DAE-BRNS, Govt. of India for funding (Grant no. 2012/34/47). Authors also thank Prof. K. Satyamoorthy, Director, School of Life Sciences (SLS) for his support and Manipal University for providing necessary facilities at School of Life Sciences, Manipal University to conduct the study. We extend our gratitude to Dr. Vasudevan T. G, Associate Professor, SLS for his support in scientifically editing the manuscript. Our thanks are also due to Mr. Sudhakar Kotian and Mr. Dheeraj for their help in animal handling and care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rathnakar, B., Rao, B.S.S., Prabhu, V. et al. Photo-biomodulatory response of low-power laser irradiation on burn tissue repair in mice. Lasers Med Sci 31, 1741–1750 (2016). https://doi.org/10.1007/s10103-016-2044-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2044-2