Abstract
The aim of this study was to evaluate the effects of red laser, infrared, photodynamic therapy, and green light-emitting diode (LED) on the healing process of skin burns through clinical and histopathologic analysis in rats. For this, 100 animals were randomly divided into five groups: G1—untreated control (CTR), G2—red laser (LVER), G3—infrared (LINF), G4—photodynamic therapy (PDT), and G5—green LED. Burn was induced on the dorsum of the rat and the treatment of the experimental groups was red light (10 J/cm2, 10 s, 40 mW, and λ660 nm), infrared (10 J/cm2, 10 s, 40 mW, and λ780 nm), green LED irradiation (60 J/cm2, 10 s, λ520, and 550 nm), and photodynamic therapy (10 J/cm2, 40 mW, and λ660 nm), the latter combined with methylene blue photosensitizer at concentration 0.5 μg/mL. Applications were performed daily until day prior to sacrifice of the animal at 3, 7, 14, and 21 days with intraperitoneal anesthetic overdose. The specimens collected were clinically examined and soon after processed and stained with hematoxylin-eosin and Picrosirius for analysis under light and polarized light microscopy, respectively. Animals treated with LVER, LINF, PDT (p < 0.001), and LED (p < 0.05) stimulated production and maturation of collagen, and increased the consumption of food and water compared to the CTR (p < 0.001). Laser λ660 nm and λ780 nm showed the largest wound reductions in all groups (p = 0.001). In conclusion, red laser, infrared, photodynamic therapy, and green LED favored the healing process of third-degree burns in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A burn is a type of open wound in which the healing process occurs by secondary intention [1], resulting in loss of body fluids, inflammation, swelling, infection, pain, and shock, all these factors can result in death [2–5].
In third-degree burns, the lesion affects the entire skin thickness, involving the epidermis and dermis, and, in some cases, reaches the subcutaneous tissues, muscles, and bones. They may have white or brown appearance, or have leather texture [6–8].
One of the treatments shown in literature to accelerate the healing process of burns is the laser therapy [6, 9–17] and more recently photodynamic therapy (PDT) [13, 18] and light-emitting diodes (LEDs) [19–22]. The first studies were conducted by Mester and colleagues, who observed that laser application is capable of modulating biological processes, in particular, to stimulate tissue regeneration [23].
Low-intensity laser has demonstrated beneficial effects on the healing process of burns, acting in the inflammatory infiltrate, re-epithelialization, collagen formation and maturation in addition to its analgesic and anti-inflammatory action [6, 9–11, 14, 15, 24]. However, there is still a lack of standardization in the choice of the optimum wavelength, power, frequency, and energy density associated with the optical properties of the irradiated tissue [25, 26].
The aim of this study was to evaluate the effects of red laser, infrared, PDT, and green LED on the healing process of skin burns through clinical and histopathologic analysis in rats.
Material and methods
Animals
In this study, 100 male Wistar rats weighting 200 to 250 g from the animal sector of the Department of Medicine, Federal University of Campina Grande, were used and kept in polypropylene cages lined with shaving bedding and submitted to controlled temperature (23 ± 2 °C) and lighting (12 h of light/dark cycle) with food and water ad libitum. The animals were randomly divided into five groups: G1—untreated control (CTR), G2—red laser (LVER), G3—infrared (LINF), G4—photodynamic therapy (PDT), and G5—green LED, each group of 20 animals was divided into subgroups of five animals for each time of 3, 7, 14, and 21 days of sacrifice. This study was approved by the Ethics Committee on Animal Use (CEUA)/CESED (protocol no. 0019/240712).
Food and water consumption
Analytical scales were used to control animal weight and food consumption, in addition to a water bottle for rats in order to calculate the amount of water consumed. The animals were weighed before each experiment and each cage received a daily amount of 200 g of diet and 250 mL of water. The feed intake was calculated as the difference of weight between two consecutive days, while water intake was quantified with a measure cylinder, and the consumption was calculated by the difference between two consecutive days.
Burn
The animals were anesthetized with an anesthetic combination of 100 mg/kg of 10 % ketamine (Cetamin ®, Syntec, São Paulo, SP, Brazil) and 5 mg/kg of 2 % Xilazyna (Dopaser ®, Syntec, São Paulo, SP, Brazil) applied to the intraperitoneal region; then, the back of the animal was shaved with a disposable razor blade associated with water and mild soap. Burn was induced on the dorsum of all animals with a 1.0 × 1.0 × 1.0 cm3 iron, which was heated with the aid of a torch until it was red hot. The heating was due to the blue flame of a torch in direct contact with the iron for 40 s. When reaching this state, the instrumental was immediately pressed against the back of the animal, remaining for 20 s, burning the skin.
Pilot study
Two animals were randomly selected in order to define burn type and standardization. Both animals were anesthetized and shaved, and the metal instrument was heated in torch for 40 s, differing in skin burn time, 20 and 30 s, respectively. After microscopic analysis of the slides stained with hematoxylin and eosin, the time of 20 s was chosen, in which the complete destruction of the epidermis and dermis, characteristic of third-degree burn, was observed.
Light irradiation
In the irradiation LVER (InGaAIP/λ660 nm), LINF (AsGaAl/λ780 nm), and PDT groups (InGaAIP/λ660 nm), low-intensity laser was used (MMO TWIN FLEX Evolution®, São Carlos, Brazil) with dose, power, time, and total energy of 10 J/cm2, 40 mW, 10 s, and 0.4 J, respectively, and LED irradiation (KONDORTECH, São Paulo, Brazil) with 60 J/cm2, 60 mW, 10 s, total energy of 0.6 J, and λ520 to 550 nm/InGaN. Irradiation was applied immediately after burn in a timely manner in the four points coinciding with the wound angles, depositing an amount of 10 J/cm2, totaling 40 J/cm2 per session in LVER, LINF, and PDT groups, while LED was applied at 60 J/cm2, totaling 240 J/cm2 at each point. The applications were performed daily, except on the day of sacrifice of the animal, where no irradiation was performed. In the PDT group, drops of methylene blue photosensitizer were used daily for 5 min before irradiation of light λ660 nm; the methylene blue concentration was 0.5 μg/mL, as concentrations above 1 μg/mL are toxic to the skin [27].
Euthanasia of animals and wound retraction index
After the experimental period for each subgroup (3, 7, 14, and 21 days), the animals were submitted to new peritoneal anesthesia in amount three times higher than that used in the first time: 300 mg/kg of 10 % ketamine (Cetamin ®, Syntec, São Paulo, SP, Brazil) and 15 mg/kg of 2 % Xilazyna (Dopaser ®, Syntec, São Paulo, SP, Brazil). After euthanasia, the wound retraction index (WRI) was calculated with the aid of digital calipers according to the formula: WRI (%) = initial area − area of sacrifice day ÷ initial area × 100. The initial area was 1 cm2 and the area of sacrifice day was calculated by the square of the radius multiplied by pi (r 2π).
Histological processing
After death confirmation evidenced by cessation of vital signs and corneal opacification, the specimen was removed. Using a scalpel, the wound was excised with a safety margin of 0.5 cm and packaged in plastic container containing 10 % formaldehyde. After the fixation period, the specimen was processed according to routine hematoxylin-eosin (HE) and Picrosirius methods.
Morphological aspects of inflammatory cells and collagen fibers
In the HE morphological analysis, inflammatory cells were described in treatment groups within the same sacrifice times. Histological sections stained with Picrosirius and analyzed under polarized light were used for descriptive and quantitative analysis of collagen deposition. Collagen fibers were classified according to their birefringence pattern (greenish/yellow-greenish or orange), morphological appearance (wavy or stretched, thick or thin, short or long), and disposition (reticular, parallel, or interlaced).
Quantitative analysis of the collagenization area
The area occupied by collagen deposition in the wound was quantified by optical density of the image analysis system in eight microscopic fields for each slide with ×100 amplification. The system consists of a CCD Sony DXC -101 video camera applied to an Olympus CX31 microscope, from which the images were sent to a monitor (Sony Trinitron). Through a scanning system (Olympus C-7070 WideZoom), images were loaded into a computer (Pentium 133 MHz) and processed using the ImageTool software, which provided the interpretation and values corresponding to the measurement of collagen fibers through quantifying their original measures, transforming the measure of the scanned image (pixel) into micrometered measure using a 50-μm bar. The thresholds for collagen fibers were established for each blade after increasing the contrast up to a point at which the fibers were easily identified as birefringent (collagen). The area occupied by the fibers was determined by thresholds of the different densities of collagen colors and highlighted by a selection tool.
Statistical analysis
Statistical analysis used the Shapiro-Wilk test to verify data normality, with significance level of 5 % for the differences observed when p < 0.05. The statistical significance of the collagenization area and quantitative analysis of water consumed were evaluated by analysis of variance (ANOVA) with Tukey and Bonferroni post tests, respectively, except for water consumption at 21 days, feed consumption, and WRI calculation at 3, 7, and 21 days, which used the Kruskal-Wallis test. The WRI in 14 days was evaluated by ANOVA with Tukey post test.
Results
Wound retraction index
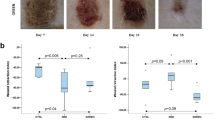
In 3 days, the WRI of LVER (median, −53 %), LINF (−53 %), and PDT (−76 %) groups was higher than in the CTR group (−154 %) (p = 0.012). In 7 days, LVER (median, −53 %), LINF (median −53 %), and PDT groups (−76 %) showed greater wound retraction (p = 0.004) compared to CTR group (−124 %). In 14 days, the WRI of LINF (mean 0.4 ± 22.9 %), LVER (mean 7.8 ± 14.1 %), LED (−9.6 ± 15.4 %), and PDT groups (1.0 ± 14.3 %) were higher (p = 0.002, p = 0.0001, p = 0.013, and p = 0.002, respectively) than the CTR group (−49 ± 18.2 %). In 21 days, the LINF group (median 87 %) showed the largest wound retraction (p = 0.001) when compared to LVER (median 72 %), PDT (72 %), CTR (5 %), and LED groups (5 %), and LVER (72 %) and PDT groups (72 %) had higher WRI (p = 0.001) than CTR (5 %) and LED groups (5 %) (Figs. 1 and 2).
Wound retraction index of each treatment group during sacrifice times. Significant difference among LVER, LINF, and PDT groups and CTR (*p = 0.006). Significant difference between LVER, LINF, and PDT groups (**p = 0.0004). Significant difference among LVER, LINF, PDT, and LED groups and CTR (***p = 0.002, ***p = 0.001, ***p = 0.002, and ***p = 0.013, respectively). Significant difference between LINF and LVER, PDT, LED, and CTR (****p = 0.001) and between PDT, LED, and CTR (****p = 0.001)
Food and water consumption
In 3 days of treatment, there was no difference in food and water consumption between groups. At 7 days, the LINF (mean, 43.71 ± 2.21 mL) and PDT groups (42.28 ± 3.19 mL) ingested larger amount of water (p = 0.001) compared to LED (18.85 ± 4.63 mL) and CTR groups (36.71 ± 1.25 mL); the LVER group (mean 39.28 ± 0.75 mL) ingested larger amount of water (p = 0.013) compared to the CTR group (mean 36.71 ± 1.25 mL). Food intake did not differ between groups. In 14 days, LVER (median 23 g), LINF (24.5 g), PDT (23 g), and LED groups (21.5 g) consumed higher amount of food (p = 0.02) compared to CTR group (median 18 g). Animals treated with LINF (mean, 43.71 ± 1.89 mL) and PDT (mean, 42 ± 2.48 mL) had higher water consumption (p < 0.001) compared to LED (38.64 ± 2.13 mL) and CTR groups (mean, 37 ± 1.17 mL). The LINF group (mean, 43.71 ± 1.89 mL) ingested larger amount of water (p < 0.001) than the LVER group (mean, 40.28 ± 1.38 mL). The LVER group (mean, 40.28 ± 1.38 mL) ingested larger amount of water compared to CTR (1.17 ± 37 mL) and LED groups (38.64 ± 2.13 mL), p < 0.001 and p = 0.032, respectively. Furthermore, the LED group consumed larger amount of water compared to CTR group (p = 0.032). After 21 days, the LVER (median 25 g) LINF (26 g), and PDT groups (25 g) consumed larger amount of food (p = 0.001) compared to CTR group (mean 20 g), while LVER (median 40 mL) LINF (43 mL), PDT (41 mL), and LED groups (39 mL) ingested larger amount of water (p < 0.001) compared to the CTR group (median 38 mL).
Inflammatory infiltrate
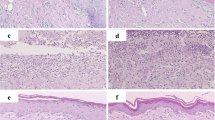
In the morphological analysis in 3 days, groups revealed inflammatory infiltrate composed of neutrophils that permeated the muscle fibers in the wound margins. In the central portion of the burn, the inflammation was predominantly lymphocytic and limited to the hypodermic loose fibrous connective tissue, and a prominent vascular dilatation (arterioles and venules), sometimes associated with leukocyte margination (Fig. 3a).
Histological sections of CTR (a), LINF (b), LVER (c), and LED (d) groups at 3, 7, 14, and 21 days, respectively (HE, ×100). Photomicrographs a and b show in detail the inflammatory infiltrate composed of neutrophils that permeated the muscle fibers at the wound margin, and in c and d, the predominance of lymphocytes, macrophages, and neutrophils (HE, ×400)
In 7 days, the groups exhibited predominantly neutrophilic inflammatory infiltrate located both in the margins and at the central portion of the wound. In the latter region, the inflammatory infiltrate was limited to hypodermic loose fibrous connective tissue (Fig. 3b). As identified in the 3-day period, significant dilation of arterioles and venules was observed, with occasional leukocyte margination.
After 14 days, groups showed inflammatory infiltrate composed of lymphocytes, macrophages, and neutrophils, the latter predominated on the surface areas of the wound. Both on the margin as in the central areas of the wound, it was possible to detect the presence of exuberant granulation reaction with newly formed blood vessels and young fibroblasts arranged amid an extracellular matrix composed of slender and elongated collagen bundles (Fig. 3c). Burns were covered by serohematic crust of variable thickness and exhibited re-epithelialization in early stages.
In 21 days, the groups showed inflammatory infiltrate composed of neutrophils, lymphocytes, and macrophages, with predominance of the mononuclear component. The entire length of the wound showed exuberant granulation reaction with newly formed blood vessels, and young fibroblasts arranged amid an extracellular matrix composed of collagen bundles, sometimes short, sometimes elongated, with varied thicknesses (Fig. 3d). Compared with the 14-day period, collagen fibers were thicker and more densely arranged. Burns were covered by serohematic crust of variable thickness and exhibited re-epithelialization in early stages.
Collagen quantification using polarized light
At 3 and 7 days, there was no collagen formation in the treatment groups. In 14 days, the LINF group exhibited a larger area of collagen formation compared to CTR and LED (p < 0.001) and to PDT and LVER (p < 0.05), while LVER and PDT groups showed greater area of collagen formation (p < 0.05) in relation to CTR (Fig. 4a). At 21 days, the LINF group showed larger area of collagen formation in relation to CTR, LVER, LINF, and PDT groups (p < 0.001), and the LVER group showed area of collagen formation much larger (p < 0.001) compared to CTR and LED (Fig. 4b).
Collagenization areas formed at 14 days (a) and 21 days (b) by CTR, LVER, LINF, PDT, and LED groups. Significant difference with CTR (*p < 0.05). Significant difference between CTR and LED groups (**p < 0.001). Significant difference between PDT and LVER (***p < 0.05). Significant difference with CTR (#p < 0.05). Significant difference between LED and CTR (****p < 0.001). Significant difference with CTR, LVER, PDT, and LED (αp < 0.001). Significant difference with CTR (*#p < 0.001). Significant difference with CTR (**#p < 0.01)
Morphological description of collagen using polarized light
In 14 days, collagen fibers in CTR (Fig. 5a), LVER (Fig. 5b), LINF (Fig. 5c), PDT (Fig. 5d), and LED (Fig. 5e) groups showed, according to their birefringence pattern, predominance of type I collagen in LVER, LINF, PDT, and LED groups (n = 3 rats/60 %), while the CTR group showed type I collagen in 20 % of animals (n = 1 rat). Regarding morphological appearance and disposition, there were no variations in descriptions. All animals showed wavy, thin, short, and reticular collagen fibers.
Panels f to j of Fig. 5 show collagen formation in 21 days in CTR, LVER, LINF, PDT, and LED groups, respectively. At this time, LVER, LINF, PDT, and LED groups showed, in relation to their birefringence pattern, predominance of type I collagen in all animals, and the CTR group showed type I collagen in 60 % of animals (n = 3 rats). The morphological appearance and disposition of collagen fibers in LVER and PDT groups were drawn, thick, long, and reticular in three animals (60 %); in LINF and LED groups, 20 % of animals showed this feature and the CTR group showed in all rats, wavy, thin, short, and reticular fibers (100 %).
Discussion
Despite divergences among researchers [25, 26, 28, 29], laser therapy has been proposed as one of the treatment modalities for soft tissue injuries, mainly due to its biostimulant properties that can accelerate the healing process of burns [6, 14, 15]. In the present study, LVER, LINF, PDT, and LED groups consumed greater amount of water and food in relation to CTR group. This result is possibly due to the analgesic effect, since all animals were of the same sex, race, and range weight, from the same location and kept under the same temperature and lighting conditions. Immunohistochemical studies claim that laser therapy inhibits the expression of the enzyme cyclooxygenase-2 [29], preventing the conversion of arachidonic acid into prostaglandin [30].
The mechanism of action of LVER and PDT is through the mitochondria, which can promote tissue biomodulation by increasing the respiratory chain and adenosine triphosphate synthesis, favoring the healing process through cellular proliferation, production of nucleic acids, collagen synthesis, and reducing inflammation [13, 31]. On the other hand, the use of LINF and LED also showed similar results, being the process in operation at the level of cell membrane [31, 32].
In the WRI analysis, negative values were observed mainly in 3 and 7 days possibly due to interstitial edema, which increases the macroscopic dimensions of the wound. In the other times, the increased wound retraction is probably the result of myofibroblastic differentiation. Ribeiro et al. [14] reported that LVER and LINF stimulate and increase of myofibroblasts in the burn, suggesting the transformation of fibroblasts into myofibroblasts in the early stages.
LINF has the highest skin penetration power compared to the other groups [33]. This feature may have influenced in the more rapid replacement of collagen fibers from type III to type I and significant collagen formation in 14 and 21 days compared to CTR. Studies have shown the benefits of LVER [6, 9–11, 14, 15], LED [18–20], LINF [12, 24, 33–35], and PDT [13] in the healing of burns. In agreement with these findings, it has been reported that laser therapy is able to regulate the release of cytokines responsible for fibroblast proliferation and collagen synthesis, such as fibroblast growth factor alpha (FGF-α) and transforming growth factor beta (TGF-β), respectively [36, 37].
It was observed that the daily dose of 40 J/cm2 applied at 21 days in LVER and LINF groups was effective in stimulating fibroblasts in collagen formation and wound retraction, contradicting studies that claim that doses above 38 J/cm2 have inhibitory effects [13]. Probably, this difference in the efficiency of the light–tissue interaction is due to the lack of standardization in the choice of wavelength, power, frequency, and energy density associated to the optical properties of the irradiated tissue [25, 38].
A significant amount of collagen formed in 21 days by the LVER and LINF groups can be confused with the formation of hypertrophic scars [39]. However, during the morphological analysis, the formation of the final healing process phase (maturation or remodeling) was not observed [40, 41]. Thus, it was observed in this study that the collagen synthesis formed no scars and is the result from the acceleration of the healing process. The partial re-epithelialization observed in this study is justified by authors who showed that LVER [10, 15] and LINF [15] irradiation in third-degree burns for 21 days favored the healing process. However, there was no complete re-epithelialization, probably due to the short monitoring time in the repair process for this type of injury.
There are few studies on the role of PDT on the healing process of burns, especially when red light is used on methylene blue diluted to nontoxic concentrations (0.5 μg/mL) [27]. Thus, the significant results found in the synthesis, formation, collagen maturation, and food and water consumption in relation to CTR may result from the combination of methylene blue, which binds to the target cell, with the light at wavelength of 660 nm. In the presence of medium oxygen, photosensitizer is activated and may react with neighboring molecules, leading to the production of singlet oxygen, which reacts with cellular components, either bacteria or cancer cells, causing cell damage [42]. This provides certain advantages to PDT such as noninvasive nature, easy repeatability, and antimicrobial action [43], as the presence of bacteria in the wound can delay the healing process [44].
Significant collagen formation was observed at 21 days using green LED compared to CTR, and Taga et al. [45] reported that LED can stimulate fibroblasts to produce collagen, increasing the mRNA expression of type-I procollagen. Thus, further studies should be carried out to better understand the action of green light on the healing process of third-degree burns, as there are only few studies in literature.
In conclusion, LVER, LINF, PDT, and green LED showed beneficial effects in accelerating the healing process of third-degree burns in rats. These groups stimulated fibroblasts in the production and maturation of collagen, the analgesic effects probably related to increased water and feed consumption compared to the CTR group, and higher wound retraction at 21 days in animals treated with LVER and LINF compared to the other groups.
References
Foulkes R, Davidson L, Gateley C (2011) Full thickness burn to a latissimus dorsi flap donor site due to a heat pad—there is still a need to improve patient information. BMJ Case Rep. doi:10.1136/bcr.05.2011.4224
Sharma BR (2007) Infection in patients with severe burns: causes and prevention thereof. Infect Dis Clin N Am 21:745–759
Vale ECS (2005) Initial management of burns: approach by dermatologists. An Bras Dermatol 80:9–19
Barrilo DJ, Pausen SM (2003) Management of burns to the hand. Wounds 15:4–9
Wassermann D (2002) Évaluation et premiers soins d’une brûlure thermique. Rev Prat 52:2228–2233
Fiório FB, Albertini R, Leal-Junior EC, de Carvalho PT (2014) Effect of low level laser therapy on types I and III collagen and inflammatory cells in rats with induced third-degree burns. Lasers Med Sci 29:313–319
Singer AJ, Brebbia J, Soroff HH (2007) Management of local burn wounds in the ED. Am J Emerg Med 25:666–671
Johnson RM, Richard R (2003) Partial-thickness burn: identification and management. Adv Skin Wound Care 16:178–187
Núñez SC, França CM, Silva DF, Nogueira GE, Prates RA, Ribeiro MS (2013) The influence of red laser irradiation timeline on burn healing in rats. Lasers Med Sci 28:633–641
de Moraes JM, de Oliveira E, Mendonça D, Moura VB, Oliveira MA, Afonso CL, Vinaud MC, Bachion MM, de Souza LR Jr (2013) Anti-inflammatory effect of low-intensity laser on the healing of third-degree burn wounds in rats. Lasers Med Sci 28:1169–1176
Renno AC, Iwama AM, Shima P, Fernandes KR, Carvalho JG, De Oliveira P, Ribeiro DA (2011) Effect of low-level laser therapy (660 nm) on the healing of second-degree skin burns in rats. J Cosmet Laser Ther 13:237–242
Khoshvaghti A, Zibamanzarmofrad M, Bayat M (2011) Effect of low-level treatment with an 80-Hz pulsed infrared diode laser on mast-cell numbers and degranulation in a rat model of third-degree burn. Photomed Laser Surg 29:597–604
Garcia VG, de Lima MA, Okamoto T, Milanezi LA, Júnior EC, Fernandes LA, de Almeida JM, Theodoro LH (2010) Effect of photodynamic therapy on the healing of cutaneous third-degree-burn: histological study in rats. Lasers Med Sci 25:221–228
Ribeiro MA, Albuquerque RL Jr, Ramalho LM, Pinheiro AL, Bonjardim LR, Da Cunha SS (2009) Immunohistochemical assessment of myofibroblasts and lymphoid cells during wound healing in rats subjected to laser photobiomodulation at 660 nm. Photomed Laser Surg 27:49–55
Meireles GC, Santos JN, Chagas PO, Moura AP, Pinheiro AL (2008) A comparative study of the effects of laser photobiomodulation on the healing of third-degree burns: a histological study in rats. Photomed Laser Surg 26:159–166
de Araújo CE, Ribeiro MS, Favaro R, Zezell DM, Zorn TM (2007) Ultrastructural and autoradiographical analysis show a faster skin repair in He-Ne laser-treated wounds. J Photochem Photobiol B 86:87–96
Hawkins D, Abrahamse H (2006) Effect of multiple exposures of low-level laser therapy on the cellular responses of wounded human skin fibroblasts. Photomed Laser Surg 24:705–714
Sperandio FF, Simões A, Aranha AC, Corrêa L, Orsini Machado de Sousa SC (2010) Photodynamic therapy mediated by methylene blue dye in wound healing. Photomed Laser Surg 28:581–587
Lee GY, Kim WS (2012) The systemic effect of 830-nm LED phototherapy on the wound healing of burn injuries: a controlled study in mouse and rat models. J Cosmet Laser Ther 14:107–110
Fiório FB, Silveira L Jr, Munin E, de Lima CJ, Fernandes KP, Mesquita-Ferrari RA, de Carvalho PT, Lopes-Martins RA, Aimbire F, de Carvalho RA (2011) Effect of incoherent LED radiation on third-degree burning wounds in rats. J Cosmet Laser Ther 13:315–322
Oliveira PC, Pinheiro AL, de Castro IC, Reis JA Jr, Noia MP, Gurgel C, Teixeira Cangussú MC, Pedreira Ramalho LM (2011) Evaluation of the effects of polarized light (λ400–200 nm) on the healing of third-degree burns in induced diabetic and nondiabetic rats. Photomed Laser Surg 29:619–625
Adamskaya N, Dungel P, Mittermayr R, Hartinger J, Feichtinger G, Wassermann K, Redl H, van Griensven M (2011) Light therapy by blue LED improves wound healing in an excision model in rats. Injury 42:917–921
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Dantas MD, Cavalcante DR, Araújo FE, Barretto SR, Aciole GT, Pinheiro AL, Ribeiro MA, Lima-Verde IB, Melo CM, Cardoso JC, Albuquerque Júnior RL (2011) Improvement of dermal burn healing by combining sodium alginate/chitosan-based films and low level laser therapy. J Photochem Photobiol B 105:51–59
Al-Watban FA, Delgado GD (2005) Burn healing with a diode laser: 670 nm at different doses as compared to a placebo group. Photomed Laser Surg 23:245–250
Bayat M, Vasheghani MM, Razavi N, Taheri S, Rakhshan M (2005) Effect of low-level laser therapy on the healing of second-degree burns in rats: a histological and microbiological study. J Photochem Photobiol B 78:171–177
Barbosa AF, Soares LG, Aciole JM, Aciole GT, Pitta IR, Galdino SL, Pinheiro AL (2011) Evaluation of photodynamic antimicrobial therapy (PACT) against trypomastigotes of Trypanosoma cruzi: in vitro study. AIP Conf Proc 1364:55–59
Schlager A, Oehler K, Huebner KU, Schmuth M, Spoetl L (2000) Healing of burns after treatment with 670-nanometer low-power laser light. Plast Reconstr Surg 105:1635–1639
Rocha CLJV, Rocha Júnior AM, Aarestrup BJV, Aarestrup FM (2012) Inhibition of cyclooxygenase 2 expression in NOD mice cutaneous wound by low-level laser therapy. J Vasc Bras 11:175–181
Lohinai Z, Stachlewitz R, Székely AD, Fehér E, Dézsi L, Szabó C (2001) Evidence for the expression of cyclooxygenase-2 enzyme in periodontitis. Life Sci 70:279–290
Tunèr J, Hode L (2002) Laser therapy: clinical practice and scientific background. Grangeberg: Prima Books AB, Chap 1—Some basic laser physics 1–44
Ribeiro MS, Da Silva DF, De Araújo CE, De Oliveira SF, Pelegrini CM, Zorn TM, Zezell DM (2004) Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study. J Clin Laser Med Surg 22:59–66
Ezzati A, Bayat M, Khoshvaghti A (2010) Low-level laser therapy with a pulsed infrared laser accelerates second-degree burn healing in rat: a clinical and microbiologic study. Photomed Laser Surg 28:603–611
Ezzati A, Bayat M, Taheri S, Mohsenifar Z (2009) Low-level laser therapy with pulsed infrared laser accelerates third-degree burn healing process in rats. J Rehabil Res Dev 46:543–554
Vasheghani MM, Bayat M, Dadpay M, Habibie M, Rezaei F (2009) Low-level laser therapy using 80-Hz pulsed infrared diode laser accelerates third-degree burn healing in rat. Photomed Laser Surg 27:959–964
Pereira MC, de Pinho CB, Medrado AR, Andrade ZA, Reis SR (2010) Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B 98:188–192
Pereira AN, Eduardo CP, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Medrado AR, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
van der Veer WM, Bloemen MC, Ulrich MM, Molema G, van Zuijlen PP, Middelkoop E, Niessen FB (2009) Potential cellular and molecular causes of hypertrophic scar formation. Burns 35:15–29
Balbino CA, Pereira LM, Curi R (2005) Mechanisms involved in wound healing: a revision. Braz J Pharm Sci 41:27–51
Mandelbaum SH, Di Santis EP, Mandelbaum MH (2003) Cicatrization: current concepts and auxiliary resources—part I. An Bras Dermatol 78:393–410
Dai T, Tegos GP, Lu Z, Huang L, Zhiyentayev T, Franklin MJ, Baer DG, Hamblin MR (2009) Photodynamic therapy for Acinetobacter baumannii burn infections in mice. Antimicrob Agents Chemother 53:3929–3934
Huang Z (2005) A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat 4:283–293
Parsons C, McCoy CP, Gorman SP, Jones DS, Bell SE, Brady C, McGlinchey SM (2009) Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials 30:597–602
Tada K, Ikeda K, Tomita K (2009) Effect of polarized light emitting diode irradiation on wound healing. J Trauma 67:1073–1079
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Vasconcelos Catão, M.H.C., Nonaka, C.F.W., de Albuquerque, R.L.C. et al. Effects of red laser, infrared, photodynamic therapy, and green LED on the healing process of third-degree burns: clinical and histological study in rats. Lasers Med Sci 30, 421–428 (2015). https://doi.org/10.1007/s10103-014-1687-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-014-1687-0