Abstract
Third-degree burn wounds are considered severe injuries because they destroy all the skin layers and may affect subcutaneous tissues, fasciae, muscles, and bones. To favor the healing process of the injured tissues, it is very useful to diminish the occurrence of the inflammatory process. The present study was aimed at comparing the effect of different energetic densities of AlGaInP laser on the inflammatory process and in the healing of third-degree burn wounds in Wistar rats. This study was approved by the Ethics Committee, in which 36 adult male rats were selected and suffered the induction of third-degree burn injury. These rats were divided as follows: group 1—control (treated with silver sulfadiazine), group 2—received energy density of 3 J/cm2, and group 3—received energy density of 6 J/cm2. All animals daily received an occlusive bandage with silver sulfadiazine and 8 % papain. The laser therapy was performed alternatively three times a week. The animals were evaluated on the 3rd, 7th, 14th, and 21st days after the initial lesion and euthanized for the macroscopic, histologic, and morphometric analysis. A higher production of collagen was observed at 7 days and a greater re-epithelialization at 21 days in group 3 (6 J/cm2). Furthermore, the latter when compared to the other groups presented macroscopically a better aspect of the scar at 21 days with more granulation tissue and fibrosis. We conclude that the AlGaInP laser used in dosages of 3 and 6 J/cm2 favors the healing of third-degree burn wounds induced in rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Burn wounds are cutaneous lesions caused by the direct or indirect action of heat which are caused by direct flame, contact with boiling water or hot liquids, scalding and the contact with hot surfaces, electric current, and chemical agents [1].
It is estimated that in Brazil, about a million accidents occur with burn wounds per year [2]. Studies about the profile of 138 institutionalized patients with burn wounds revealed that 71 % were males, 67.4 % were burned accidentally, and that the main cause of the lesion was an open flame [3]. Among the risk factors for the accidents with burn wounds in domestic environment were low social, economic, and educational levels of the mother or caretakers, houses too small for the number of residents, and precarious kitchen supplies [4].
The healing of a burn wound is a process composed by a series of interdependent and simultaneous complex stages. These stages are described as: inflammatory, epithelialization, cellular, and fibroplasias [5, 6]. The healing of the burn wound follows the same stages of the inflammatory process as any other injury [5].
In spite of many advances in the caretaking of victims with severe burn wounds, secondary infections persist in which the equilibrium of the microbiota from the skin is modified enabling the establishment and growth of pathogenic bacteria [7, 8]. Therefore, the quicker the healing, the lesser time the tissue remains exposed to microorganisms. One of the factors that prolong the repair process is chronic inflammation. Hence, the inflammatory process caused by the burn wound may be reduced by the use of low-intensity laser as an auxiliary treatment [9]. On the other hand, these lesions may need surgical procedures, and to insure their success, a neovascularization of the site is necessary which may also be favored by the use of low-intensity laser therapy [10].
The use of the laser in burn wound treatment is based on its anti-inflammatory action with consequent analgesia. This was observed in experiments which used lasers with different wavelength, dosages, and application method [11–13]. Studies that reported the use of laser on surgical wound repair concluded that the lesions that were treated with low-intensity laser therapy presented an increase in the neovascularization and in the fibroblastic proliferation, also a higher contraction of the wound and greater speed of the epithelial migration when compared to lesions from the control group [14, 15].
Recent investigations demonstrated the influence of laser therapy in the healing of the skin and other tissues. However, it still presents some controversies due to the great variability and divergence of the irradiation parameters and also the discrepancies on the biological findings [16–18].
In spite of these effects, the action of the laser on tissues is still not fully comprehended, and there are important differences between the application methods which leave doubts on which is the best energetic dosage to be used in the healing adjuvant therapy. Furthermore, experimental studies with histopathological approaches are justifiable as to provide scientific basis to the clinical-therapeutic use of laser on the treatment of burn wounds and to provide parameters in relation to the observed effects, contributing to clarify the results of this therapeutic modality as to promoting the healing of burn wounds. The present study aims to verify the effect of the aluminum gallium indium phosphide (AlGaInP) low-intensity laser with different energetic dosages on the healing of third-degree burn wounds in Wistar rats.
Methodology
Samples and ethic aspects
An experimental and prospective study was performed by using 36 Wistar rats provided by the Animal Facility of the Federal University of Goias (UFG). This study was approved by the Ethics Committee in Human and Animal Research from the Clinics Hospital from UFG—no. 098/2009.
Three animals were accommodated per cage and received water and autoclaved commercial ration ad libitum, and the changes of bed were performed twice a week. The animals were manipulated in a careful manner and always by the morning by the same researcher which was trained and supervised by a veterinarian doctor. An adaptation of the animals in the proposed environment was performed so as to prioritize their well-being.
Lesion protocol
In day 0, the animals were anesthetized with a solution of 0.01 l/g of ketamine 10 % and xylazine 2 % intraperitoneally. After that, the depilation was performed on the dorsal region of the animal followed by the third-degree thermal lesion by immersion of this region in water at 95 °C for 14 s. For that, the animal was placed inside a polyvinyl chloride (PVC) plastic cylinder with a 2 × 2-cm opening and sealed extremities.
Protocol for the group treatment
The 36 Wistar rats used weighed between 300 and 350 g with third-degree burn wounds divided into three groups of 12 animals each. The first group (G1) was the control group which received only bandages with physiological serum for the cleaning of the wound and the application of an association of silver sulfadiazine ointment and papain 8 % gel. Group two (G2) received a bandage with physiological serum for the cleaning of the wound, followed by the application of laser in the energy density of 3.0 J/cm2 and the application of an association of silver sulfadiazine ointment and papain 8 % gel. Group three (G3) received a bandage with physiological serum for the cleaning of the wound, followed by the application of laser in the energy density of 6.0 J/cm2 and the application of an association of silver sulfadiazine ointment and papain 8 % gel. During the treatment period, all animals received daily occlusive and sterile bandages. For the occlusion, a sterile gauze was used in which a blanket made with longcloth was attached. The analgesia was performed throughout the experiment with tramadol hydrochloride, 30 mg/kg.
Each one of these groups was divided into subgroups containing three animals each for observations at 0, 3, 7, 14, and 21 days after the wound induction (DAI). The animals were euthanized with a lethal injection of sodium thiopental 100 mg/kg at 3, 7, 14, and 21 days after the wound inductions for the follow-up of the macroscopic, morphometric, and microscopic parameters.
Laser application
The AlGaInP—Diode continuum laser (KLD Biosistemas®) was used with the following specifications: wavelength of 660 nm, power density of 35 mW, medium output power of 20 mW, beam spot of 0.035 cm2, and selection of the continuum mode. The application of the laser occurred three times a week in a half-light environment in perpendicular position in a punctual mode over the burned area obeying the time of laser application provided by the device in each point. The radiation was applied in four points within the area. For the laser application, a supporting device was built so the laser beam remained in a 90° position over the lesion. The animals were previously contained in a plastic tube, in ventral decubitus, with extension of their limbs.
Macroscopic evaluation
On the established days for analysis, the lesions were photographed, and phases of the healing process were macroscopically examined, in other words, inflammation, proliferation, and maturation. The following parameters were analyzed: color of the lesion, differentiation of the areas of hyperemia, stasis, necrosis, erythema, edema, presence of crusts, neovascularization, hemorrhage, re-epithelialization, and time for the lesion closure. All these aspects were analyzed in a subjective way by the same researcher without knowing which group was being observed.
Morphometric evaluation
For the analysis of the contraction of the wound, all lesions were photographed with a digital camera attached to a tripod at 20 cm distance from the lesion, and afterwards, the images were analyzed with the Image J (NIH) version 1.3.1 software.
In the image, the area of the wound was circled using the above software by a researcher without knowing which group was being analyzed. The values were calculated by the following formula:
Where CL = contraction level, T 0 = day of the lesion induction, and T days of euthanize = days 3, 7, 12, and 21 after the lesion induction.
Microscopic evaluation
The injured tissue was removed through a biopsy procedure, processed, and blocked into paraffin and then sectioned into 4-μm sections and stained with hematoxylin and eosin (H&E). The samples of the cutaneous lesions were removed as to include the borders of the adjacent skin and all the healing tissues in its depth.
The general pathologic processes were analyzed on the crust, dermis, hypodermis, deep tissue, and lesion border (Table 1). The analysis of the lesions was qualitative as follows: absent (0), discrete 1–25 % of the slide (1), moderate 26–50 % of the slide (2), and accentuated above 51 % of the slide (3).
Mesoscopic analysis
Through mesoscopy, the size of the open wound was measured by using the Image J software.
Statistical analysis
The statistical analysis was performed using the Sigma Stat 2.3 software. Descriptive statistics were applied to determine the mean and standard deviation and to evaluate the differences between the groups analyzed. The variables were tested for normal distribution and homogeneous variance. When they presented normal distribution, analysis of variance was used, and when the distribution was non-normal or the variance was nonhomogenous, nonparametric tests were used (Kruskal–Wallis). The differences noted were considered significant when p < 0.05.
Results
The induction of the burn wound using a PVC tube and water at 95 °C with 14 s of exposure favored a greater uniformity of the lesion. The histological analysis demonstrated necrosis and elimination of the structure of the epidermis, papillary dermis, reticular dermis, and hypodermis which confirmed the third-degree burn wound.
The use of papain 8 % associated to silver sulfadiazine and to the occlusive bandage showed no formation of crust during the entire period of treatment which also maintained the humidity of the lesion. The lesions did not present macroscopic characteristics of infection.
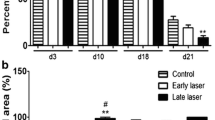
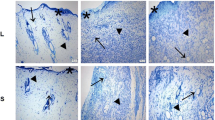
In relation to the macroscopic alterations, at 3 DAI, it was possible to observe a secondary edema in all groups. In addition, the G2 (3 J/cm2) presented greater hyperemia on the border of the lesions when compared to other groups (Fig. 1). At 7 DAI, G2 presented a smaller area of necrosis than G3 (6 J/cm2) and G1 (control) (Figs. 1 and 3). At 14 DAI, G2 presented bigger granulation tissue and fibrosis than the other groups which also presented necrosis in the center of the lesion (Figs. 2 and 3). At 21 DAI, there was greater re-epithelialization and fibrosis in G3 when compared to the other groups (Figs. 2 and 4).
Macroscopic analysis of the aspect of the wound after 14 and 21 days of treatment. a Accentuated necrosis (arrowhead) in the center of the lesion and moderate fibrosis (arrow). b Accentuated fibrosis (arrow) and granulation tissue (segmented arrow). c Accentuated fibrosis (arrow) and necrosis (arrowhead). d–f Presence of granulation tissue (segmented arrow)
Photomicroscopy of the skin of a rat with third-degree burn wound at 7 days with production of collagen. CT group (control): Deep tissue with precocious collagens (arrowhead), hyperemia, neovascularization (arrow), and accentuated inflammatory infiltration (H&E, scale = 20 μm). Group 3 J/cm2: Thicker collagen (arrowhead), with more organized fibers (H&E, scale = 20 μm). Group 6 J/cm2: Thicker and more organized collagen (arrowhead) (H&E, scale = 20 μm)
Photomicroscopy of the skin of a rat with third-degree burn wound at 21 days with production of collagen. CT group (control): open wound (arrow), re-epithelialization of the borders (dotted arrow), hyperemia and fur (arrow head) in the re-epithelialization area (dotted arrow), (H&E, scale = 100 μm). Group 3 J/cm2: hyperemia, hyperplasia of epidermis (dotted arrow), and open wound (H&E, scale = 100 μm). Group6 J/cm2: accentuated re-epithelialization (dotted arrow), organized collagen in the dermis below the re-epithelialization area and below the open wound (arrow), presence of inflammatory infiltration (H&E, scale = 100 μm)
At 3 DAI, there was an increase on the size of the wound in G1 which was associated with a secondary edema. On the other hand, the treated groups presented a tendency to reduce the wound size. On the other experimental days, there was no difference in the contraction of the wounds (Fig. 5).
In the microscopic analysis, it was possible to observe that at 7 DAI the intensity of collagen was significantly higher (p < 0.05) in G2 and G3 when compared to G1 (Fig. 4). It was possible to observe a significant re-epithelialization of the border of the lesion at 21 DAI in G3 when compared to G1 (Fig. 5). These data were confirmed through mesoscopic analysis which measured the size of the open wound which was significantly smaller in G3, with a mean of 16 mm, when compared to G2, mean of 152 mm, and G1, mean of 132 mm (Fig. 5). Simultaneous to this re-epithelialization, it was possible to observe type I collagen and in the other areas of the wound, type III collagen. All other analyzed parameters did not present significant differences between the treated and control groups.
Discussion
The AlGaInP laser was chosen for this study because it comprises the effects related to the energy density and also has a mean of output potency of 20 mW which is much superior to the HeNe or even the AsGa lasers. This enables a smaller time of exposure as potency and time are proportionally inverted values. Another advantage is due to the fact that the generator material (semiconductors) is in diode form which facilitates the device design as well as its use as there is no need for optic fibers in relation to the HeNe laser [19].
In the present study, the application of the punctual mode with the laser pen attached in a holder and the animal contained in a plastic tube made possible the standardization of the dosage in all the extension of the wound. The application in the scanning mode which is adopted by other authors [20] presents a difficult methodology in an experimental model because the velocity of the laser pen dislocation must be constant, as well as the distance between the laser pen and the wound should be maintained constant and should not be greater than 1 cm which could compromise the standardization of the dosage.
In this experiment, the evolution of the healing process in the third-degree burn wound in groups which suffered irradiation and control ones occurred accordingly to the phases of the reparative process. The tissue necrosis caused by the burn wound induction caused lesion until the hypodermis which, according to the literature [21], results in alteration of the metabolism and release of substances that trigger an inflammatory response. There was a significant improvement of these characteristics in G3 which is in accordance to the findings reported by Pereira [12] which also used similar methodology.
In this study, there were no clinical signs of infection or crust formation which is different from what is reported by another study [22] which verified the presence of bacterial colonies and hyperkeratosis, a focal and discrete area in epidermis which consisted of a crust of orthokeratotic cells.
Probably the use of a topic occlusive bandage with silver sulfadiazine prevented the bacterial colonization in this study. The presence of crust was not observed in this study which shows the efficacy of the chemical debridement with 8 % papain and the benefits of the occlusive bandage in burn wounds. According to the literature, the presence of crusts on the wounds prevents totally or partially the penetration of the laser radiation [23].
In this study, it was possible to observe a greater quantity of collagen at 7 DAI in G3 when compared to G2 and G1, and at the same experimental day, a smaller area of necrosis in G2. Other authors using the same wavelength also obtained better results in the early phases of the healing process [24, 25].
The third-degree burn wounds treated with AlGaInP laser in the punctual mode in a dosage of 6 J/cm2 presented better macroscopic aspect and greater re-epithelialization than the other groups at 21 DAI; however, the total closure of the wound was not observed. These data are in accordance to Gál et al. [26] which used low-level laser therapy composed by InGaAlP laser (670 nm) on the healing process in wounds experimentally induced in Sprague–Dawley rats during the first week and observed an anti-inflammatory effect.
Other studies [9] described the complete healing of the wound at 14 days probably due to the fact that cutaneous wounds have different microenvironments, causal agents, and variations in the low-intensity laser therapy as this author induced the lesions with steel iced in nitrogen. Mello et al. [22] also adopting an experimental model with HeNe laser in the dosage of 4 J/cm2 in the scanning mode obtained a total healing with 14 days of treatment. We believe that this divergence with our results is due to the laser’s wavelength used and daily applications while we performed laser application only three times a week.
At 21 DAI, the macroscopic characteristics observed were of the proliferative phase in which there is a formation of the granulation tissue evidenced mainly in G3. The presence of fibroblasts, collagen, and blood vessels is essential to the tissue regeneration [19].
Conclusion
The application of the AlGaInP laser in the dosage of 3 J/cm2 was more effective in the initial phases of the healing process, while the dosage of 6 J/cm2 was better on the late stages of the healing process because with the first there was a smaller area of necrosis at 7 days, and the latter favored a collagen production at 7 days and greater re-epithelialization at 21 days. As the healing products are used differently according to the phases of the healing process and the wound conditions, the present study indicates that the laser should also be used differently.
References
Vale ECS (2005) Primeiro atendimento em queimaduras: a abordagem do dermatologista. An Bras Dermatol 80:9–19
Lima Júnior EM, Novaes FN, Piccolo NS, Serra MC (2008) Tratado de queimaduras no paciente agudo, 2nd edn. Atheneu, São Paulo
Montes SF, Barbosa MH, Sousa Neto AL (2011) Aspectos clínicos e epidemiológicos de pacientes queimados internados em um Hospital de Ensino. Rev Esc Enferm USP 45:369–373
Vendrusculo TM, Balieiro CRB, Echevarría-Guanilo ME, Farina Junior JA, Rossi LA (2010) Queimaduras em ambiente doméstico: características e circunstâncias do acidente. Rev Lat Am Enfermagem 18:444–451
Blanes L (2011) Tratamento de feridas. Cirurgia vascular: guia ilustrado. São Paulo [on line]. http://www.bapbaptista.com. Accessed 01 Nov 2011
Fernandes LRA (2005) Fisiologia da cicatrização–feridas e curativos. [on line]. http://www.unimes.br/aulas/MEDICINA/Aulas2005/1ano/Procedimentos_basicos_em_medicina/feridas_e_curativos.html. Accessed 01 Nov 2010
Azevedo AMZ, Machado MJ, Chassot GC (2002) Assistência de enfermagem nos pacientes queimados com curativo aberto na unidade de internação. Mom Perspec Saúde 15:1–7
Santucci SG, Gobara S, Santos CR, Fontana C, Levin AS (2003) Infections in a burn intensive care unit: experience of 7 years. J Hosp Infect 53:6–13
Bourguignon-Filho AM, Feitosa ACR, Beltrão GC, Pagnoncelli RM (2005) Utilização do laser de baixa intensidade no processo de cicatrização tecidual. Rev Port Estomatol Cir Maxilofac 46:37–43
Veçoso MC (1993) Laser em fisioterapia. Lovise, São Paulo
Bayat M, Vasheghani M, Razavi N (2008) Effects of low-level laser therapy on mast cell number and degranulation in third-degree burns of rats. JRRD 45:931–938
Pereira RM (2005) Efeitos dos lasers de baixa potência em três diferentes comprimentos de onda no processo de cicatrização de queimaduras de 3° grau. Thesis. Universidade Vale do Paraíba
Schlager K, Oehler K, Huebner M, Schmuth L, Spoetl L (2000) Healing of burns after treatment with 670-nanometer low-power laser light. J Dermatol Surg Austria 105:1635–1639
Santuzzi CH, Buss HF, Pedrosa DF, Freire MOV, Nogueira BV, Goncalves WLS (2011) Uso combinado da laserterapia de baixa potência e da inibição da ciclooxigenase-2 na reepitelização de ferida incisional em pele de camundongos: um estudo pré-clínico. An Bras Dermatol 86:278–283
Júnior AMR, Oliveira RG, Farias RE, Andrade LCF, Aarestrup FM (2006) Modulação da proliferação fibroblástica e da resposta inflamatória pela terapia a laser de baixa intensidade no processo de reparo tecidual. An Bras Dermatol 81:150–156
Reddy GK (2004) Photobiological basis and clinical role of low-intensity lasers in biology and medicine. J Clin Laser Med Surg 22:141–150
Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M (2005) Low-level laser therapy for wound healing: mechanism and efficacy. Dermatol Surg 31:334–340
Maiya GA, Kumar P, Rao L (2005) Effects of low intensity helium-neon (He-Ne) laser irradiation on diabetic wound healing dynamics. Photomed Laser Surg 3:187–190
Guirro ECO, Guirro RRJ (2004) Fisioterapia dermato funcional: fundamentos, recursos, patologias, 3rd edn. Manole, São Paulo
Lange F, Kroth A, Steffani JA, Lorencetti N (2003) Influência da laserterapia no processo cicatricial de queimaduras de terceiro grau. Fisioter Bras 4:335–340
Gomes DR, Serra MC, Pellon MA (1995) Queimaduras. Revinter Ltda, Rio de Janeiro
Mello PB, Sampedro RMF, Piccinini AM (2007) Efeitos do laser HeNe e do modo de aplicação no processo de cicatrização de queimaduras em ratos. Fisioterapia Pesquisa 14:6–13
Low J, Reed A (2001) Eletroterapia explicada princípios e prática. Manole, São Paulo
Oliveira PC, Meireles GCS, Santos NR, Carvalho CM, Souza APC, Santos JN, Pinheiro ALB (2008) The use of light photobiomodulation on the treatment of second-degree burns: a histological study of a rodent model. Photomed Laser Surg 26:289–299
Meirelles GCS (2005) Análise comparativa do efeito dos lasers GaAlAs de 660 nm e 780 nm na cicatrização de úlceras por queimadura em dorso de ratos diabéticos e não-diabéticos: estudo histológico. Thesis. Universidade Federal da Bahia
Gál P, Vidinsky’ B, Toporcer T, Mokrý M, Mozes S, Longauer F, Sabo J (2006) Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg 24:480–488
Acknowledgments
The authors would like to thank Sharon Lois Vinaud for the English review. The authors would also like to thank the Burning Wounds Hospital of Goiania (Hospital de Queimaduras de Goiania) and Eduardo Di Oliveira Pires from the Núcleo Integrado de Reabilitação e Educação for providing the AlGaInP laser for this study, and Dr. Nelson Sarto Piccolo from the Nelson Piccolo Institute and Farmácia Artesanal for providing the papain.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Moraes, J.M., Eterno de Oliveira Mendonça, D., Moura, V.B.L. et al. Anti-inflammatory effect of low-intensity laser on the healing of third-degree burn wounds in rats. Lasers Med Sci 28, 1169–1176 (2013). https://doi.org/10.1007/s10103-012-1213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1213-1