Abstract
The aim of the present study was to determine whether low-level laser therapy (LLLT) in conjunction with aerobic training interferes with oxidative stress, thereby influencing the performance of old rats participating in swimming. Thirty Wistar rats (Norvegicus albinus) (24 aged and six young) were tested. The older animals were randomly divided into aged-control, aged-exercise, aged-LLLT, aged-LLLT/exercise, and young-control. Aerobic capacity (VO2max0.75) was analyzed before and after the training period. The exercise groups were trained for 6 weeks, and the LLLT was applied at 808 nm and 4 J energy. The rats were euthanized, and muscle tissue was collected to analyze the index of lipid peroxidation thiobarbituric acid reactive substances (TBARS), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) activities. VO2 0.75max values in the aged-LLLT/exercise group were significantly higher from those in the baseline older group (p <0.01) and the LLLT and exercise group (p <0.05). The results indicate that the activities of CAT, SOD, and GPx were higher and statistically significant (p <0.05) in the LLLT/exercise group than those in the LLLT and exercise groups. Young animals presented lesser and statistically significant activities of antioxidant enzymes compared to the aged group. The LLLT/exercise group and the LLLT and exercise group could also mitigate the concentration of TBARS (p > 0.05). Laser therapy in conjunction with aerobic training may reduce oxidative stress, as well as increase VO2 0.75max, indicating that an aerobic exercise such as swimming increases speed and improves performance in aged animals treated with LLLT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the aging process, accumulation of mitochondrial DNA mutations, impairment of oxidative phosphorylation, and an imbalance in the expression of antioxidant enzymes results in an overproduction of reactive oxygen species (ROS). This mitochondrial dysfunction-elicited ROS production forms a cycle, which is the basis of mitochondrial free radical theory of aging [1]. Reactive oxygen species may play a role in the neuromuscular degeneration process, with a constant loss of fibers and muscle function [2]. This could be more pronounced in the elderly population in whom a sedentary lifestyle and age-related physiological dysfunctions could impair antioxidant defenses and increase susceptibility to oxidative stress and muscle damage [3].
Exercise represents the physical stress that transiently disrupts homeostasis, and the skeletal muscle is most directly affected by physical activity. Studies have indicated that exercise may induce structural damage to muscle cells and the production of metabolic by-products, such as lactate and ROS [4]. However, research has claimed that physical training increases the activity of the antioxidant enzymes superoxide dismutase (SOD) and glutathione peroxidase (GPx) that help combat free radical formation of oxidative metabolism [5]. Although there is some inconsistency within the literature, it is clear that both aerobic and anaerobic exercises can increase free radical production, which may cause acute oxidative stress. However, the extent of redox homeostasis disturbance induced by an acute bout of exercise depends on many factors, including inter alia, exercise mode, intensity and duration, participant’s state of training, gender, age, and nutritional habits [3].
Low-level laser therapy (LLLT) has also been used to boost repair processes by reducing pro-inflammatory markers, including tumor necrosis factor (TNF)-α and interleukin (IL)1-β, as well as increasing anti-inflammatory cytokines, such as IL-10 [6–8].
More recently, researchers have shown that in addition to the modulatory effects of LLLT on inflammatory processes, LLLT also reduces creatine kinase levels immediately after exercise [9]. LLLT also acts on the markers of oxidative stress, such as protein carbonyls, SOD, and thiobarbituric acid reactive substances (TBARS) [10], in addition to delaying muscle fatigue and improving physical performance [11–15].
Based on anti-inflammatory properties of exercise training and LLLT, we aimed to determine whether LLLT in association with aerobic training interferes with oxidative stress, thus influencing the performance of aged rats subjected to exercise.
Materials and methods
Experimental animals
Thirty Wistar rats (Norvegicus albinus) were used in this study, with groups consisting of 24 aged animals (24 months old; mean body weight, 517.7 ± 27.54 g) and six young animals (12 weeks old; mean body weight, 266, 19:30 0 ± g). The animals were acquired from the animal facility of the Federal University of São Paulo (UNIFESP), where they were housed and kept under controlled light and temperature, and with water and food “ad libitum”. All experimental procedures were carried out in accordance with the standards established by the Brazilian College for Animal Experimentation (COBEA). The animals were handled in compliance with the national guidelines for the humane treatment of laboratory animals, and all experimental procedures were approved by the Research Ethics Committee of the UNIFESP.
Experimental groups
The 24 aged animals were randomly divided into four groups, with six animals per group:
-
1.
Aged-control group with no LLLT irradiation and no exercise training
-
2.
Aged-LLLT group (GLI) treated with LLLT irradiation and no exercise training
-
3.
Aged- Exercise group (GLTI) no treated with irradiation and subjected to exercise training
-
4.
Aged-LLLT/exercise group (GLTI) treated with irradiation and subjected to exercise training
Furthermore, an additional experimental group (young-control group with no LLLT irradiation and no exercise training) included six young animals.
Functional fitness assessment (maximal oxygen uptake, VO2max)
Functional fitness was assessed using a motorized treadmill coupled with a gas analyzer (Panlab; Harvard Bioscience Company, MA, USA) in which VO2 and VCO2 were continuously recorded. Three days prior to testing, the rats were introduced to the treadmill for 15 min (5-min stages) as follows: day one 25 cm/s, 35 min/s, and 35 cm/s; day two 25 cm/s, 45 min/s, and 55 cm/s; and day three 25 cm/s, 55 min/s, and 65 cm/s. To evaluate VO2max, each rat performed a 2-min warm-up at 25 cm/s, with the treadmill speed increased by 9 cm/s every 2 min until physical exhaustion occurred. The VO2max was calculated as an allometric score (mL/kg0.75/min), which is the VO2max/lean body mass ratio and was performed before and after exercise training [16].
Exercise training
The exercise training consisted of swimming in a custom-built fiberglass pool (diameter, 130 cm; and height, 80 cm). The water was heated with a gas heating system from 32 to 34 °C. The training protocol was divided into two phases:
1. Adaptation: On the first day of exercise, the animals swam for 15 min, and the times increased by 15 min on each subsequent day until the sixth day when the animals were able to swim for 90 min.
2. Main training: The training time was maintained at 90 min, 6 times a week for 6 weeks.
Low-level laser application
The DMC Photon Laser III ® (DMC, Sao Carlos, SP, Brazil) system was used for irradiation under the parameters in Table 1. The laser was applied transcutaneously, and the irradiation was performed at three anatomical locations for 40 s/location in the central, proximal, and distal area of the gastrocnemius muscle10. Laser was applied before every training session for both groups (those that underwent exercise training and the group that received LLLT only).
Euthanasia
At the end of the 6-week training period, the animals from each group were identified, weighed, and euthanized by decapitation according to the protocol detailed in the Report of the AVMA Panel on Euthanasia [17].
The gastrocnemius was collected immediately after euthanasia. The incised areas were surgically removed with a 1-cm margin of skin surrounding the lesion to the depth of the fascia. The muscle (two from each animal) was frozen for subsequent analysis, including measurement of the index of lipid peroxidation TBARS, glutathione (GSH), SOD, and catalase (CAT) activities.
Biomarkers of oxidative stress
In order to verify the antioxidant status, enzyme activities of CAT, SOD, and GPx were determined in the gastrocnemius. As an indicator of lipid peroxidation (damage to the muscle membrane), we determined the concentrations of substances that react with TBARS in the same tissue, based on the protocol reported by [18].
Catalase
To determine the level of CAT activity, tissue samples were placed on ice in Eppendorf tubes containing 1 mL of 0.05 N phosphate buffer (composition in g/L KH2PO4, 1.34 and 2H2 NaHPO4, 7, 1), sonicated, and centrifuged at 10,000 rpm for 5 min. The supernatant was separated and stored at −20 °C for subsequent analysis using commercial kits (Cayman Chemical®, Michigan, USA).
Superoxide dismutase and glutathione peroxidase
For determination of GPx and SOD activities, tissue samples were washed once with PBS (pH 7.4) containing heparin (0.16 mg/mL) to remove blood cells. Immediately after the samples were homogenized (on ice) in 1 mL of HEPES buffer (20 mM, pH 7.2) (containing 1 mM of EGTA, 7 mM of mannitol, and 210 mM of sucrose), they were centrifuged for 15 min at 10,000 rpm (4 °C). The supernatant was separated and stored at −20 ° C for subsequent analysis of total SOD (cytoplasmic and mitochondrial) and GPx using commercial kits (Cayman Chemical®, Michigan, USA).
Biomarkers of lipid peroxidation
Concentrations of thiobarbituric acid
For the determination of TBARS concentrations, tissue samples were placed on ice in an Eppendorf container containing 1.5 mL of 0.05 N phosphate buffer (composition in 1.34 g/L of KH2PO4, 7 g/L of NaHPO4 and 1 g/L of 2H2O) in POLYTRON® homogenized and centrifuged for 5 min at 10,000 rpm. The supernatant was then separated and stored at −20 ° C for subsequent analysis using commercial kits (Cayman Chemical®, Michigan, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA). The post-training VO2max values and the biomarkers of oxidative stress were compared using a one-way ANOVA with a Tukey post hoc test. Data were expressed as mean ± standard deviation, and differences with p values <0.05 were considered significant.
Results
Functional fitness assessment (maximal oxygen uptake, VO2max)
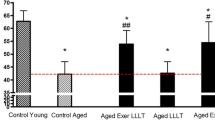
Assessment of cardiopulmonary function was conducted using a standard approach before and after the swim training. In the comparison between the control group and the young-aged control group for values of VO2max 0.75 at baseline, the aged groups that received the intervention (LLLT/exercise, LLLT, or exercise). We also present results of the average speed of the animals at baseline and after the intervention (Fig. 1).
Comparison of the mean and standard deviation on functional fitness assessment (maximal oxygen uptake, allometric VO2 máx0.75), *p < 0.05 and **p < 0.01 and, using Tukey’s test with comparisons against the Young-Control group; # p < 0.05 and ## p < 0.001 using Tukey’s test with comparisons against the Aged-Control group; ɸ p < 0.05 using Tukey’s test comparing the LLLT-exercise group with the Aged-LLLT group and exercise group
Biomarkers for oxidative stress
Activity of catalase
The mean values (μmol/min/mg) of CAT were as follows: young group, 0.75 ± 0.1; aged, 0.32 ± 0.09; aged LLLT/exercise, 1.50 ± 0.18; aged-LLLT, 0.64 ± 0.19; and aged-exercise, 1.1 ± 0.13. CAT levels in the aged rats were significantly different from that in young group (p < 0.05) before training. CAT enzyme levels were not significantly higher in the aged group before training than in the intervention group (LLLT/exercise, LLLT or exercise) (p < 0.05 and p < 0.01). CAT enzyme levels were higher in the groups that received the interventions (LLLT/exercise, LLLT, or exercise) (p < 0.05). These results demonstrate an increase of 78.6 % in CAT activity in the LLLT/exercise group compared to that in the aged-control group and an increase of 70.9 % in the exercise group compared with that in the aged-control group (Fig. 2).
Comparison of the mean and standard deviation the activity of the Catalase enzyme (CAT) in the gastrocnemius muscle of the young and aged animals. a Represents the activity of the enzyme CAT after 6 weeks of aerobic training through swimming, where *p < 0.05 and **p < 0.001, using Tukey’s test with comparisons against the Young-Control group; # p < 0.05 and ## p < 0.001 using Tukey’s test with comparisons against the Aged-Control group; ɸ p < 0.05 using Tukey’s test comparing the LLLT-exercise group with the Aged-LLLT group and exercise group and λ p < 0.05 using Tukey’s test comparing the LLLT group vs exercise group. b Represents the perceptual increased activity of the enzyme relative to the control group Aged
Activity of superoxide dismutase
The mean concentrations of SOD (U/mL) were as follows: young group, 9.8 ± 2.1; aged, 4.1 ± 1.4; aged-LLLT/exercise, 15.3 ± 1.8; aged-LLLT, 9.4 ± 2.0; and aged-exercise, 10.1 ± 1.7. SOD levels in the aged rats was significantly different from that in the young group (p < 0.05) before training and was significantly different between the young group (no training) and the aged LLLT/exercise group (p <0.01). When comparing the aged group before training with the groups that underwent interventions (LLLT/exercise, LLLT, or exercise), all showed significantly higher values (p < 0.05 and p < 0.01). There was an elevation in SOD levels in the groups that received the interventions (LLLT/exercise) LLLT (p < 0.05) and exercise. These results demonstrate an increase of 73.2 % in the activity of SOD in the LLLT/exercise group compared to that in the aged-control group and an increase of 59.4 % in the exercise group compared with that in the aged-control group (Fig. 3).
Comparison of the mean and standard deviation the activity of the Superoxide Dismutase (SOD) in the gastrocnemius muscle of the Young and Aged animals. a Represents the activity of the SOD after 6 weeks of aerobic training through swimming, where *p < 0.05 and, using Tukey’s test with comparisons against the Young-Control group; # p < 0.05 and ## p < 0.001 using Tukey’s test with comparisons against the Aged-Control group; ɸ p < 0.05 using Tukey’s test comparing the LLLT-exercise group with the Aged-LLLT group and exercise group. b Represents the perceptual increased activity of the enzyme relative to the control group Aged
Activity of glutathione peroxidase
The mean concentrations of GPx (nmol/min/100 mg) were as follows: young group, 12.0 ± 1.9; aged, 6.2 ± 1.6; aged-LLLT/exercise, 17.3 ± 1.4; aged-LLLT, 10.4 ± 2.1; and aged-exercise, 13.1 ± 1.2. GPx levels in the aged rats were significantly different from that in the young group (p < 0.05) before training and between the young group (no training) and the aged LLLT/exercise group (p < 0.01). When comparing the aged group before training with the groups that received the intervention (LLLT/exercise, LLLT, or exercise), all showed significantly higher values (p < 0.05 and p < 0.01). There was an elevation in GPx associated with the interventions (LLLT/exercise, LLLT (p <0.05), or exercise. These results demonstrate an increase of 64.1 % in GPx in the LLLT/exercise group compared with that in the aged-control group and an increase of 52.6 % in the exercise group compared with that in the aged-control group (Fig. 4).
Comparison of the mean and standard deviation the activity of the Glutathione Peroxidase (GPx) in the gastrocnemius muscle of the young and aged animals. a Represents the activity of the GPx after 6 weeks of aerobic training through swimming, where *p < 0.05 and, using Tukey’s test with comparisons against the Young-Control group; # p < 0.05 and ## p < 0.001 using Tukey’s test with comparisons against the Aged-Control group; ɸ p < 0.05 using Tukey’s test comparing the LLLT-exercise group with the Aged-LLLT group and exercise group
Biomarkers of lipid peroxidation
Concentrations of thiobarbituric acid
The mean concentrations of TBARS (nmolMDA/mg) were as follows: young group, 10.0 ± 1.1; aged, 18.2 ± 2.4; aged-LLLT/exercise, 7.3 ± 1.8; aged-LLLT, 9.3 ± 1.8); and aged-xercise, 11.1 ± 1.6. The TBARS levels in the aged rats were significantly different from that in the young group (p < 0.01) before training and between the young group (no training) and the aged-LLLT/exercise group (p < 0.01). When comparing the aged group before training with the groups that received the intervention (LLLT/exercise, LLLT, or exercise), all showed minor significant differences (p < 0.05 and p < 0.01). Furthermore, the association between LLLT and exercise resulted in minor significant differences in TBARS compared with that in the aged-LLLT group and the aged-exercise group (p < 0.05) (Fig. 5).
Comparison of the mean and standard deviation the concentrations the thiobarbituric acid (TBARS) in the gastrocnemius muscle of the young and aged animals. Concentrations of the TBARS after 6 weeks of aerobic training through swimming, where **p < 0.01 and, using Tukey’s test with comparisons against the Young-Control group; # p < 0.05 and ## p < 0.001 using Tukey’s test with comparisons against the Aged-Control group; ɸ p < 0.05 using Tukey’s test comparing the LLLT-exercise group with the Aged-LLLT group and exercise group
Discussion
Aging is connected to oxidative stress, and the literature indicates that aerobic physical activity can improve cardio-respiratory function in the elderly and is important in the control of chronic diseases such as high blood pressure and diabetes mellitus. However, there is controversy in the literature regarding the role of physical exercise in oxidative stress. According to several studies, LLLT reduces oxidative stress markers and improves the defenses of antioxidants in several clinical and experimental circumstances. Furthermore, recent studies have shown that LLLT may improve the physical performance of top athletes and can alleviate muscle fatigue. In the current study, we hypothesized that LLLT in conjunction with aerobic training may be an effective approach to reduce oxidative stress markers in skeletal muscle, and that LLLT plus swim training could improve the functional performance of older rats. Although physical activity of moderate-to-high intensity can generate oxidative stress at different magnitudes [19], the literature does not provide a consensus. In some cases, it has been shown to increase oxidative stress in aerobic and anaerobic exercises, while other studies have shown that TBARS levels remain unchanged in response to both types of exercises [20].
The VO2max values presented in the current study point to an improvement in aerobic performance with LLLT associated with exercise (i.e., swimming) in aged rats. This group (LLLT/exercise) had a higher VO2max 0.75 than the other aged and young groups that received either only training or LLLT. During exercise, many physiological systems dynamically interact with each other [21]; among these are the cardiopulmonary interactions and the muscle’s ability to utilize oxygen [22]. The VO2max is accepted and regarded as the best measure to determine cardiovascular stability and the ability to perform physical exercise [23]. Although VO2max has been shown to decline with age in some studies (at a rate of approximately 10 % per decade in sedentary participants), other investigators have reported that neither stroke volume nor maximal cardiac output decreases with age. Furthermore, some research has also indicated that there is no evidence of reduced oxygen extraction in older versus younger individuals [24].
Our results corroborate the above authors [24, 25] considering that the comparison of VO2max0.75 between the young and elderly control groups were higher than in the young groups. However, our results demonstrated a significant increase in VO2max in the LLLT and exercise groups, as the group held only exercise training for swimming.
A clinical study by [10] evaluated the effects of LLLT on exercise performance (VO2max, time to exhaustion, aerobic threshold, and anaerobic threshold), oxidative stress, the levels of antioxidant enzymes (SOD and CAT), and muscle status in humans (creatine kinase and lactate dehydrogenase). In a double-blind placebo-controlled randomized crossover trial with 22 untrained male volunteers subjected to irradiation by LLLT, the authors analyzed exercise performance (VO2max, time to exhaustion, aerobic threshold, and anaerobic threshold), levels of oxidative damage to lipids and proteins, the activities of the antioxidant enzymes SOD and CAT, and markers of creatine kinase muscle damage and lactate dehydrogenase. They concluded that the use of LLLT before a progressive-intensity running exercise increased exercise performance and decreased exercise-induced oxidative stress and muscle damage. This suggests that the modulation of the redox system by LLLT could be related to the delay in skeletal muscle fatigue observed after the use of LLLT.
Reactive oxygen species, such as superoxide anions (O2·−) and hydroxyl radicals (OH·−), cause the oxidation of membrane phospholipids, proteins, and DNA, and such modifications have been implicated in a variety of pathological conditions. Under physiological conditions, the toxic effects of an increase in free radicals can be prevented by antioxidant enzymes, such as SOD, GPx, and CAT, and by non-enzymatic antioxidants. However, when there is an excess production of free radicals, oxidative stress has deleterious effects on the structural and functional integrity of cells and tissues [26]. The results of the current study for the antioxidant activity of LLLT/exercise, LLLT, and exercise only showed an increase in activity of CAT and SOD. These results were further confirmed by the analysis of GPx.
In the present study, we observed that the aged group presented higher mean TBARS concentrations than the exercise group, LLLT group, and LLLT/exercise group that indicates a reduction of lipid peroxidation in the membrane of the muscle fiber in the intervention group. The LLLT/exercise condition also resulted in an increase in the activity of antioxidant enzymes (CAT, SOD, and GPx), in addition to a decrease in the concentration of TBARS in the gastrocnemius muscle of the aged rats.
CAT found in the peroxisomes plays a specific role in metabolizing hydrogen peroxide, a highly toxic substance to the cell. SOD has three isoforms in the human body: SOD1 in the cytoplasm, SOD2 in the mitochondria, and SOD3 in the extracellular fluid. This enzyme catalyzes the dismutation (oxidation and reduction redox reaction) of the superoxide in oxygen and hydrogen peroxide. GPx, found in the mitochondria, reduces hydroperoxides to alcohols and hydrogen peroxide to water [18].
Ferraresi et al. [27], reported that phototherapy (LLLT and light-emitting diode therapy) has been used to combat ROS and reactive nitrogen species that are produced during physical exercise. Therefore, this reduces mitochondrial function that contributes to the reduction of muscle fatigue subsequently improving muscle performance [25]. conducted a study with the aim of evaluating the influence of LLLT on the parameters of oxidative stress and DNA damage in skeletal muscle and plasma of rats with heart failure. They concluded that laser therapy appears to reduce SOD activity and DCFH oxidation levels, changing the oxidative balance in the skeletal muscle of HF rats. Otherwise, high doses of LLLT seem to increase DNA damage.
In a study aimed at analyzing the effects of LLLT on oxidative stress and fibrosis in an experimental model of an Achilles tendon injury [26], described that LLLT has been reported to increase SOD levels. The increase in this antioxidant enzyme, by reducing oxidative stress, could alleviate the injury to the soft tissue and prevent development of fibrosis. Furthermore [28], reported that lipid peroxidation can be assessed by TBARS, a biochemical index of oxidative damage by free radicals. The authors conducted a study to evaluate the effects of the low-intensity infrared laser on plasma protein content and oxidative stress in blood and concluded that LLLT increases plasma protein content and causes oxidative stress, lipid peroxidation, and increases myeloperoxidase activity, which could depend on the fluency and frequency, in blood samples.
Although the effects of LLLT on ROS production are still controversial, it has been suggested that LLLT could enhance ROS production of human neutrophils by activating the superoxide converting system. LLLT irradiation enables a more rapid activation of this system; however, laser irradiation has been demonstrated to reduce oxidative stress in different situations such as isolated human neutrophils, during abdominal surgery, and in vitro in liposome membranes [26].
Conclusion and summary
In the current study, there was an improvement in the antioxidant defense system in the gastrocnemius of aged animals that received irradiation with LLLT and aerobic training compared to that in the other groups (exercise, LLLT, and aged-control groups). These results indicate that regular physical exercise plus LLLT could be an important strategy to reduce the supra physiological production of ROS, as its levels increase antioxidant enzyme activity and reduce lipid peroxidation in the gastrocnemius. It is possible that this influenced, at least partially, the increase VO2max0.75 of the animals tested.
References
Wang CH, Wu SB, Wu YT, Wei YH (2013) Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med (Maywood) 238:450–460
Marzetti E, Calvani R, Cesari M et al (2013) Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int J Biochem Cell Biol 45:2288–2301
Bouzid MA, Hammouda O, Matran R, Robin S, Fabre C (2014) Changes in oxidative stress markers and biological markers of muscle injury with aging at rest and in response to an exhaustive exercise. PLoS One 9, e90420
Lima FD, Stamm DN, Della-Pace ID et al (2013) Swimming training induces liver mitochondrial adaptations to oxidative stress in rats submitted to repeated exhaustive swimming bouts. PLoS One 8, e55668
Powers SK, Ji LL, Leeuwenburgh C (1999) Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 31:987–997
Souza NH, Marcondes PT, Albertini R, Mesquita-Ferrari RA, Fernandes KP, Aimbire F. Low-level laser therapy suppresses the oxidative stress-induced glucocorticoids resistance in U937 cells: Relevance to cytokine secretion and histone deacetylase in alveolar macrophages. J Photochem Photobiol B 2013;(26)130C:327-336.
Alves AC, Vieira R, Leal-Junior E et al (2013) Effect of low-level laser therapy on the expression of inflammatory mediators and on neutrophils and macrophages in acute joint inflammation. Arthritis Res Ther 15:R116
Laraia EM, Silva IS, Pereira DM et al (2012) Effect of low-level laser therapy (660 nm) on acute inflammation induced by tenotomy of Achilles tendon in rats. Photochem Photobiol 88:1546–1550
Sussai DA, Carvalho Pde T, Dourado DM, Belchior AC, dos Reis FA, Pereira DM (2010) Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci 25:115–120
De Marchi T, Leal Junior EC, Bortoli C, Tomazoni SS, Lopes-Martins RA, Salvador M (2012) Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci 27:231–236
Antonialli FC, De Marchi T, Tomazoni SS, et al (2014) Phototherapy in skeletal muscle performance and recovery after exercise: effect of combination of super-pulsed laser and light-emitting diodes. Lasers Med Sci 29: 1967–1976
Leal Junior EC, Lopes-Martins RA, Frigo L et al (2010) Effects of low-level laser therapy (LLLT) in the development of exercise-induced skeletal muscle fatigue and changes in biochemical markers related to postexercise recovery. J Orthop Sports Phys Ther 40:524–532
Leal Junior EC, Lopes-Martins RA, Dalan F et al (2008) Effect of 655-nm low-level laser therapy on exercise-induced skeletal muscle fatigue in humans. Photomed Laser Surg 26:419–424
Leal-Junior EC, Vanin AA, Miranda EF et al (2015) Effect of phototherapy (low-level laser therapy and light-emitting diode therapy) on exercise performance and markers of exercise recovery: a systematic review with meta-analysis. Lasers Med Sci 30:925–939
Santos LA, Marcos RL, Tomazoni SS, et al (2014) Effects of pre-irradiation of low-level laser therapy with different doses and wavelengths in skeletal muscle performance, fatigue, and skeletal muscle damage induced by tetanic contractions in rats. Lasers Med Sci 29:1617–1626
Amadio EM, Serra AJ, Guaraldo SA, et al (2015) The action of pre-exercise low-level laser therapy (LLLT) on the expression of IL-6 and TNF-α proteins and on the functional fitness of elderly rats subjected to aerobic training. Lasers Med Sci 30:1127–1134
AVMA Panel on Euthanasia. American Veterinary Medical Association (2001) 2000 Report of the AVMA Panel on Euthanasia. J Am Vet Med Assoc 218:669–696. Erratum in: J Am Vet Med Assoc 218:1884
Vieira Junior Roberto Carlos, Silva Carolina Mendes Santos, Araújo Michel Barbosa de, Garcia Alesandro, Voltarelli Vanessa Azevedo, Reis Filho Adilson Domingos. Aerobic swimming training increases the activity of antioxidant enzymes and the glycogen content in the skeletal muscle of rats. Rev Bras Med Esporte 2014;19:204–208.
Palazzetti S, Richard MJ, Favier A, Margaritis I (2003) Overloaded training increases exercise-induced oxidative stress and damage. Can J Appl Physiol 28:588–604
Alessio HM, Hagerman AE, Fulkerson BK, Ambrose J, Rice RE, Wiley RL (2000) Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Med Sci Sports Exerc 32:1576–1581
Richardson RS, Tagore K, Haseler LJ, Jordan M, Wagner PD (1998) Increased VO2 max with right-shifted Hb-O2 dissociation curve at a constant O2 delivery in gog muscle in situ. J Appl Physiol 84:995–1002
Moffatt SE, Bearden RJ (2000) VO2 Kinetics and the O2 deficit in heavy exercise. J Appl Physiol 88:407–1412
Fletcher GF, Balady GJ, Amsterdan EA et al (2001) Exercise standards for testing and training: A statement for healthcare professionals from the American Heart Association. Circulation 104:1694–1740
Ogawa T, Spina RJ, Martin WH et al (1992) Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation 86:494–503
Biasibetti M, Rojas DB, Hentschke VS et al (2014) The influence of low-level laser therapy on parameters of oxidative stress and DNA damage on muscle and plasma in rats with heart failure. Lasers Med Sci 29:1895–906
Fillipin LI, Mauriz JL, Vedovelli K et al (2005) Low-level laser therapy (LLLT) prevents oxidative stress and reduces fibrosis in rat traumatized Achilles tendon. Lasers Surg Med 37:293–300
Ferraresi C, Hamblin MR, Parizotto NA (2012) Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med 1:267–286
da Fonseca Ade S, Presta GA, Geller M, de Paoli F, Valença SS (2012) Low-intensity infrared laser increases plasma proteins and induces oxidative stress in vitro. Lasers Med Sci 27:211–217
Acknowledgments
Professor Paulo de Tarso Camillo de Carvalho thanks the Brazilian Research Council-CNPq for the Research Productivity Scholarship (Process no.307665/2012-7).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No competing financial interests exist. Professor Ernesto Cesar Pinto Leal-Junior receives research support from Multi Radiance Medical (Solon, OH-USA), a laser device manufacturer. Multi Radiance Medical had no role in the planning of this study, and the laser device used was not theirs. They had no influence on study design, data collection and analysis, decision to publish, or preparation of the manuscript. The remaining authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Guaraldo, S.A., Serra, A.J., Amadio, E.M. et al. The effect of low-level laser therapy on oxidative stress and functional fitness in aged rats subjected to swimming: an aerobic exercise. Lasers Med Sci 31, 833–840 (2016). https://doi.org/10.1007/s10103-016-1882-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-1882-2