Abstract
Low-energy laser irradiation (Ga–Al–As semiconductor laser, output 50 mW) was applied to rat osteoclast precursor cells for 1, 3, 6, or 10 min at 24-h intervals during the culture period. The number of tartrate-resistant acid phosphatase positive multinucleate cells was increased by approximately 1.3-fold in the 3- and 6-min irradiation groups. Further, osteoclasts appeared on day 2 in the laser irradiation groups but not until day 3 in the control groups. In immunohistochemical staining for receptor activator of NF-κB (RANK), the laser irradiation groups showed significantly greater amounts of staining in comparison with the control group on days 2 and 3. Reverse transcription-polymerase chain reaction results also showed that the expressions of RANK were upregulated. In the pit formation assay, resorption pits were significantly more abundant in the laser irradiation groups than in the controls. The present results suggest that low-energy laser irradiation facilitates differentiation and activation of osteoclasts via RANK expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, various biostimulatory effects of low-energy laser irradiation have been reported, including wound healing [1–3], fibroblast [4, 5], and chondral [6] proliferation, collagen synthesis [7–9], and nerve regeneration [10]. In particular, the acceleration of bone regeneration by laser treatment has been the focus of recent studies [11, 12].
In the field of orthodontics, low-energy laser irradiation can be utilized for several different treatments in the clinical practice of orthodontics such as in the reduction of postadjustment pain [13] or in the treatment of traumatic ulcers promoted by the appliance in the oral mucosa [14]. However, little information is available concerning the effect of low-energy laser irradiation on bone remodeling during orthodontic tooth movement. Tooth movement is related to the response to applied orthodontic forces that cause remodeling of the periodontal tissues, especially alveolar bone. The typical 2- to 3-year treatment period is burdensome for patients; thus, it is very important to accelerate alveolar bone remodeling during treatment to shorten the time required. Our laboratory previously reported the stimulatory effects of low-energy laser irradiation on bone regeneration in the median palatine suture area during rapid maxillary expansion in rats [15]. Further, Ozawa et al. [16] demonstrated that laser irradiation stimulates cellular proliferation and differentiation of osteoblast lineage bone nodule-forming cells, especially in committed precursors, resulting in an increase in the number of differentiated osteoblastic cells as well as in bone formation. In addition, Kawasaki and Shimizu [17] reported that low-energy laser irradiation stimulated the amount of tooth movement and formation of osteoclasts on the pressure side during experimental tooth movement in vivo. Since bone remodeling is a physiological process that involves the resorption of bone by osteoclasts and synthesis of bone matrix by osteoblasts [18, 19], those findings are not surprising. A recent study showed that low-energy laser irradiation accelerated the velocity of orthodontic movement of human teeth [20]. Therefore, low-energy laser irradiation may accelerate bone remodeling and shorten the orthodontic treatment period.
Osteoclasts are specialized members of the monocyte/macrophage family that differentiate from hematopoietic precursors [21]. The expression of tartrate-resistant acid phosphatase (TRAP) is a characteristic of the macrophage/osteoclast lineage and is often used as one of the lineage markers [22]. Activity of osteoclasts in vitro is measured by excavation of pits in bone or dentin slices, and this is the feature that undoubtedly identifies mature, active osteoclasts [23]. Recently, a receptor activator of NF-κB ligand (RANKL) has been identified as an osteoclast differentiation factor [24] and has been shown to bind to a receptor activator of NF-κB (RANK) on the surface of osteoclastic cells. While detailed molecular mechanisms for each of the diverse functions of RANK are expected to be revealed in the near future, there has already been considerable progress in elucidating the RANK signaling pathways in a few specific cell types, especially osteoclasts and osteoclast precursor cells. Further, it is thought that RANK might be involved with osteoclast formation that is accelerated by laser irradiation.
In contrast, very little is known about the effects of laser irradiation on bone resorption, though some reports have demonstrated effects on bone formation. The present study was designed to examine the effects of low-energy laser irradiation on the formation of osteoclasts and RANK expression from rat osteoclast precursor cells in vitro.
Materials and methods
Cell culture
Rat osteoclast precursor cells that had been purified from rat bone marrow cells [25] were purchased from Hokudo Co. (Hokkaido, Japan). The cells were seeded onto 16-well Lab-Tek chamber slides (Nunc, Naperville, IL, USA) at a density of 4×104 cells/100 μl and maintained in commercial medium (α-MEM supplemented with 10 unit/ml of penicillin, 10 μ/ml of gentamicin, and 10% fetal calf serum) with both macrophage colony stimulating factor (M-CSF) and RANKL (both at 10 ng/ml) for 8 days. The medium was changed every 3 days.
Laser irradiation
In this study, we used a Ga–Al–As diode laser (ora-laser 2100, ORALIA Dentalproukte GmbH, Konstanz, Germany), which has a continuous wavelength of 810 nm and a maximum power output of 50 mW (Fig. 1). The laser beam was delivered by an optical fiber 5 mm in diameter that was defocused at the tip using concave lenses and used to irradiate a uniform circular area 7 mm in diameter at the cell-layer level. The power density of the laser beam was measured using a laser power meter, and irradiation was performed from 14 mm above the cell layer. In this manner, 16-well Lab-Tek chamber slides were simultaneously and uniformly irradiated for a period of 8 days. The periods of exposure were 1, 3, 6, or 10 min/day, which corresponded to 9.33, 27.99, 55.98, or 93.30 J/cm2, respectively, per exposure. When not being laser-irradiated, the cells were kept in a 5% CO2 incubator at 37°C. As controls, chamber slides were placed on a clean bench for the corresponding time points without irradiation.
TRAP staining
When the irradiation schedule was completed, the cells were washed twice with phosphate-buffered saline (PBS) and stained with TRAP, using a commercial staining kit (Hokudo Co.).
RANK immunohistochemistry
Immunohistochemical staining was performed as follows: When the irradiation schedule was completed, rat osteoclast precursor cells were washed three times with PBS and were then fixed in 4% (v/v) paraformaldehyde. After washing in PBS, the samples were incubated with anti-RANK polyclonal antibody (Santa Cruz Biotechnology, CA, USA; working dilution, 1:200) for 2 h at 4°C and stained using Histofine simple stain MAX-Po kits (Nichirei Co., Tokyo, Japan), according to the manufacturer’s protocol. The samples were rinsed with PBS and final color reactions were performed using the substrate reagent 3,3-diaminobenzidine tetra-hydrochloride; this was followed by counterstaining with hematoxylin. As immunohistochemical controls, some samples were incubated in the same way with either nonimmune rabbit IgG or 0.01 M PBS alone, instead of the primary antibody.
RT-PCR analysis
Detection of mRNA encoding RANK from rat osteoclast precursor cells was performed using a reverse transcription-polymerase chain reaction (RT-PCR) assay. We extracted the RNA from rat osteoclast precursor cells with the Qiagen RNeasy Mini Kit, following the manufacturer’s protocol. Total RNA was converted to cDNA by ReverTra Ace (Toyobo Co., Japan). PCR amplification was done using KOD Dash (Toyobo Co.) in a thermal cycler (PTC-0200 DNA Engine, MJ Research, Inc., USA). After a hot start, temperature cycling was done as follows: denaturation at 98°C for 10 s, primer annealing at 63°C for 10 s, and extension at 74°C for 15 s for 30 cycles. PCR primers for RANK and β-Actin were purchased from SIGMA Genosys Co. (Hokkaido, Japan). The PCR primers for amplification were designed with reference to the sequences of cDNA reported for RANK [26] and β-Actin [27]. The primers were designed as follows: RANK (5′-TTAAGCCAGTGCTTCACGGG-3′ and 5′-ACGTAGACCACGATGATGTCGC-3′), β-Actin (5′-ATGAGGATCCTCACCGAGCGCGGCTCAGC-3′ and 5′-ACACCACTGTGTTGGCGTACAGGTCTTTGC-3′). PCR fragments were resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
Pit formation assay
The resorptive activity of mature osteoclasts formed in vitro was evaluated by their ability to form resorption pits on dentin slices (diameter, 6 mm; thickness, 0.15 mm) and submicron synthetic calcium phosphate thin films (BD Bio Coat Osteologic Discs; BD Biosciences, New Jersey, USA). Briefly, osteoclast precursor cells were cultured in a commercial medium in wells containing RANKL and M-CSF (both at 10 ng/ml) at 37°C. After being incubated for 8 days, the samples were washed three times with PBS. The slices were placed for 30 min in 1 M NH4OH and cleaned by ultrasonication to remove adherent cells and were then washed and dried. After drying, the dentin slices were mounted onto stubs for scanning electron microscopy and sputter-coated with platinum or onto glass slides for light microscopy. The entire surface of each dentin slice was examined using a scanning electron microscope (S-2700; Hitachi, Tokyo, Japan) or a reflected light microscope with bone resorption quantified using an eyepiece graticule. We performed an experiment using von Kossa staining and, since natural dentin often causes scattered results, calcium phosphate. The rat osteoclast precursor cells were incubated on submicron synthetic calcium phosphate thin films for 10 days. The samples were washed three times with PBS and placed for 30 min in 1 M NH4OH, after which they were cleaned via ultrasonication to remove adherent cells and were then washed and dried. Fresh 5% silver nitrate was then added for 30 min, after which the samples were stained with von Kossa stain and dehydrated to obtain the area covered by calcium phosphate.
Statistical methods
Values were calculated and are presented as the means ± standard deviation (SD). Student’s t test was used for analyzing the difference of the two groups. Differences were considered significant when p<0.05.
Results
Effects of laser irradiation on osteoclast formation
To detect TRAP-positive mononuclear and multinuclear osteoclasts, we performed TRAP staining following 8 days of laser irradiation. The number of TRAP-positive cells was significantly increased by laser irradiation for 1, 3, and 6 min/day (1.2-, 1.3-, and 1.2-fold, respectively) as compared with the respective controls, whereas there was no significant stimulation seen with irradiation for 10 min/day (Fig. 2a,b).
Effects of laser irradiation on osteoclast formation (TRAP staining). Osteoclast precursor cells (4×104 cells/100 μl) were cultured for 8 days with various doses of laser irradiation. a Representative TRAP staining field: (a) control; (b) laser irradiation, 1 min/day; (c) laser irradiation, 3 min/day; (d) laser irradiation, 6 min/day; (e) laser irradiation, 10 min/day. b TRAP-positive multinucleated cells containing three or more nuclei were counted. Data are expressed as the means ± SD of 15 wells (*p<0.05, **p<0.01)
Time dependency of laser stimulation in TRAP-positive cells
As Fig. 2 shows, the greatest increase in the number of TRAP-positive cells occurred with laser irradiation for 3 min/day. In view of this result, following the changes of time dependency in TRAP-positive cells, immunohistochemistry and RT-PCR analysis of RANK were performed for 3 min/day. The time dependency of laser stimulation was also investigated by treating cultures at various stages during the course of osteoclast-like cell development. Figure 3 already shows TRAP-positive cells on day 2 in the laser irradiation groups but such cells did not appear until day 3 in the control groups.
Time course of laser-mediated stimulation of TRAP-positive cells. Osteoclast precursor cells (4×104 cells/100 μl) were cultured with or without laser irradiation (3 min/day). TRAP-positive cells (arrowheads) were detected on day 2 in the laser group but not until day 3 in the control group. a Laser irradiation, day 2. b Laser irradiation, day 3. c Control, day 2. d Control, day 3
Immunohistochemistry results of the effects of laser irradiation on RANK
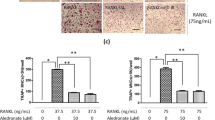
Receptor activator of NF-κB is the sole signaling receptor for RANKL in the process of inducing the development and activation of osteoclasts [28]. To investigate the effects of laser irradiation on the development and activation of osteoclasts, we examined RANK expression in the control and laser groups. In immunohistochemistry staining for RANK, the laser irradiation groups showed stronger positive staining than the control group on days 2, 3, 4, 6, and 8, while the staining for both groups was not detected on day 1.
Effects of laser irradiation on RANK mRNA by RT-PCR analysis
To elucidate the molecular mechanisms of alteration of immunohistochemical staining for RANK by laser irradiation, we investigated RANK mRNA expression in both the control and laser-irradiated cells via RT-PCR analysis. As immunohistochemical staining for RANK was increased by 2 and 3 days of irradiation for 3 min/day, gene expression was also investigated in the same conditions.
As Fig. 5 shows, because the visualized PCR products corresponding to β-Actin were the same in both samples, it was acceptable to have the amount of PCR product reflect each mRNA level. The bands for RANK mRNA of rat osteoclast precursor cells were visible at 30 cycles, with the bands of the laser-stimulated osteoclast precursor cells appearing more intense than those of the corresponding controls (Fig. 5).
Pit formation assay
Resorption of mineralized tissue was investigated by measuring the capacity of in vitro formed osteoclasts to resorb dentin and submicron synthetic calcium phosphate on thin films. When osteoclast precursor cells were cultured for 8 days on dentin slices with or without laser irradiation, resorption lacunae appeared on the surfaces of the slices. However, resorption pits were more abundant in the laser irradiation groups than in the control group.
Laser irradiation and control group cells were incubated on submicron thin films of synthetic calcium phosphate for 10 days. In pit formation assays using calcium phosphate, the resorption pits were more abundant in the 1-, 3-, and 6-min laser irradiation groups as compared with the control group.
The resorption pit area in a single well was determined by an image processing system (Win Roof, Mitani Co., Japan). Significant differences were observed between the 1-, 3-, and 6-min irradiation groups and the control. However, the 10-min irradiation group did not show any differences from the control in the resorption pit area. Further, resorption lacunae were significantly stimulated by laser irradiation of 1, 3, and 6 min/day: 1.2, 1.3, and 1.4 times, respectively, those of the control (p<0.01), whereas no significant stimulation was seen with irradiation of 10 min/day (Fig. 7b).
Discussion
There are only a few experimental studies regarding the biostimulatory mechanisms of laser irradiation on fibroblasts and blood polymorphonuclear leukocytes in vitro [7, 29]. Therefore, it is considered important to clarify the mechanisms of the effects of laser irradiation in molecular and cell-based studies. Our apparatus previously demonstrated effective performance in a study on bone metabolism [15–17]. Based on those results, we used a Ga–Al–Gs diode laser in the present study to examine the effects of irradiation on osteoclast formation.
The present findings suggest that low-energy laser irradiation for 8 days significantly stimulated the number of TRAP-positive cells, as their numbers were increased by factors of 1.2, 1.3, and 1.2 with laser irradiation of 1, 3, and 6 min/day, respectively, in comparison with the control cells that received no laser irradiation. In contrast, no significant increase was seen with 10 min/day (Fig. 2b). In an in vivo study, Kawasaki and Shimizu [17] reported that the number of multinuclear osteoclasts in the irradiation group increased 1.6-fold compared with the nonirradiation group and suggested that laser irradiation may stimulate the fusion of mononuclear macrophages to mature osteoclasts. In addition, TRAP-positive cells were found on day 2 in the irradiation groups but not until day 3 in the control groups (Fig. 3), suggesting that laser irradiation may stimulate not only fusion but also differentiation in the early stages of cell growth.
Osteoclasts are multinucleated cells responsible for bone resorption in modeling and remodeling. Mature osteoclasts differentiate from progenitors of the hematopoietic/macrophage lineage through cell-to-cell interactions with osteoblasts. The discovery of RANKL, a member of the tumor necrosis factor (TNF) superfamily originally identified as a T-cell-derived immunomodulatory cytokine [24, 30], has helped to elucidate the mechanisms of osteoclast differentiation and function that are regulated by osteoblasts [28, 31]. It is expressed on the surfaces of stromal/osteoblastic cells in response to various bone-resorptive bone cytokines and hormones and binds to its cognate receptor, RANK [24, 32], which is expressed on the cell surfaces of osteoclasts and their progenitors to induce osteoclast differentiation and enhance osteoclast cell survival. Further, critical roles of RANK signaling pathways in osteoclastic bone resorption have been demonstrated by in vitro studies of gene manipulation. It has also been shown in vitro that RANKL and M-CSF can substitute for supporting cells of the stromal/osteoblastic lineage to induce osteoclast differentiation from spleen- or bone-marrow-derived progenitors [33]. Thus, RANKL/RANK signaling and M-CSF are both necessary for osteoclast differentiation. In the present study, we examined the effects of laser irradiation on the expression of RANK in osteoclast precursor cells cultured with RANKL and M-CSF. Figure 4 shows that RANK expression in the laser irradiation group was stronger than that in the nonirradiation group on days 2 and 3. Furthermore, the RT-PCR results also showed that the expressions of RANK were upregulated on days 2 and 3 (Fig. 5). Therefore, osteoclast differentiation following laser irradiation may be induced via RANK expression.
Immunohistochemistry findings of the effects of laser irradiation on RANK. Osteoclast precursor cells (4×104 cells/100 μl) were cultured with or without laser irradiation (3 min/day). Immunohistochemical staining for RANK (arrowheads) revealed stronger staining in the laser irradiation groups than in the control group on days 2, 3, 4, 6, and 8
To examine whether laser irradiation induces osteoclast activation, we cultured osteoclast precursor cells on dentin slices and submicron synthetic calcium phosphate thin films with or without laser irradiation. We found that resorption pits were significantly stimulated by factors of 1.2, 1.3, and 1.4 as compared with the controls, following laser irradiation of 1, 3, and 6 min/day, respectively (Figs. 6 and 7a,b). Therefore, laser irradiation may induce osteoclast activation as well as differentiation. In contrast, TRAP staining and pit formation were not significantly stimulated by irradiation of 10 min/day. Ueda and Shimizu [34] demonstrated that there is an optimal mode of laser irradiation for bone formation. Based on the present results, we considered that low-frequency laser irradiation, especially for 3 min/day, may be optimal for osteoclast formation. Interestingly, Dortbudak et al. [35] reported that bone resorption at bony implant sites were not affected by low-energy laser irradiation. These contrasting results may have been due to differences between the different types of laser used and/or different types of experimental model. Further studies are necessary to confirm this phenomenon.
Scanning electron micrograph images of resorption pits on dentin slice surfaces. Osteoclast precursor cells were cultured for 8 days on dentin slices with or without laser irradiation. a Control. b Laser irradiation, 1 min/day. c Laser irradiation, 3 min/day. d Laser irradiation, 6 min/day. e Laser irradiation, 10 min/day
Effects of laser irradiation on resorption pits. A pit formation assay was conducted using submicron synthetic calcium phosphate thin films and von Kossa staining. a Osteoclast precursor cells were cultured for 10 days on submicron synthetic calcium phosphate thin films with or without laser irradiation: (a) Control; (b) laser irradiation, 1 min/day; (c) laser irradiation, 3 min/day; (d) laser irradiation, 6 min/day; (e) laser irradiation, 10 min/day. b Determination of resorption pit area following von Kossa staining and dehydration. Data are expressed as the means ± SD of six wells (*p<0.01)
As for the effects of laser irradiation on proliferation and differentiation in osteoblasts, Ozawa et al. [16] reported that laser irradiation in the early stages of osteoblast-like cells isolated from fetal rat calvariae significantly stimulated cellular proliferation, ALP activity, and osteocalcin gene expression thereafter. Furthermore, laser irradiation in the earlier stages of cultures significantly stimulated the proliferation of osteoblasts, resulting to a greater number (1.7-fold) and a larger area (3.4-fold) of bone nodules that developed in the culture dish on day 21. These results suggest that laser irradiation may stimulate proliferation and differentiation, resulting to an increase in the number of more differentiated osteoblastic cells and an increase in bone formation. Furthermore, Barushka et al. [36] reported that low-energy laser (He–Ne) irradiation after injury affected the population of osteoblasts and osteoclasts in the injured site. On the basis of the findings of the present study, it is possible that low-energy laser irradiation may accelerate the process of bone remodeling by stimulating osteoblast and osteoclast differentiation.
RANKL has been identified to be an osteoclast differentiation factor [24] that binds to RANK on the cell surface of osteclastic cells. On the other hand, osteoprotegerin (OPG) is a novel secreted member of the TNF receptor superfamily that works as a decoy receptor of the RANKL/RANK signaling system, thereby inhibiting osteoclastogenesis [32, 37]. Thus, OPG, RANKL, and RANK build up a critical system that controls bone resorption by regulating the number and activity of osteoclasts [28]. In relation to the OPG/RANKL/RANK system and orthodontic tooth movement, since RANKL expression was observed in compressed periodontal ligament (PDL) cells, it is possible that osteoclastogenesis is promoted on the compression side during orthodontic tooth movement [26]. Orthodontic force induced RANKL and RANK expression in osteoclasts of periodontal tissue [38]. Oshiro et al. [39] reported that in OPG-deficient mice, there was greater induction of TRAP-positive multinucleated cells in the PDL on the compressed side and in the adjacent alveolar bone after experimental application of orthodontic force than in their wild-type OPG (+/+) littermates. Kanzaki et al. [40] reported that OPG gene transfer to periodontal tissue inhibited RANKL-mediated osteoclastogenesis and inhibited experimental tooth movement. These studies suggest that during orthodontic tooth movement, the OPG/RANKL/RANK system in the periodontal tissues is an important determinant regulating balanced alveolar bone resorption. As low-energy laser irradiation accelerated the orthodontic movement velocity, it may also have affected the expression of RANKL and OPG in osteoblasts and PDL cells, as well as of RANK, during orthodontic tooth movement. Studies in animal models are in progress to clarify whether this function indeed exists.
In conclusion, low-energy laser irradiation of 1, 3, and 6 min/day significantly stimulated osteoclast formation in vitro. Further, RANK expression in osteoclast precursor cells was detected at an early stage (day 2) in the laser-irradiated groups. These results suggest that laser irradiation may induce differentiation and activation of osteoclasts via the expression of RANK.
References
Kana JS, Hutschenreiter G, Haina D, Waidelich W (1981) Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg 116:293–296
Mester E, Mester AF, Mester A (1985) The biomedical effects of laser application. Lasers Surg Med 5:31–39
Mester E, Nagylucskay S, Tisza S, Mester A (1978) Stimulation of wound healing by means of laser rays. Part III—investigation of the effect on immune competent cells. Acta Chir Acad Sci Hung 19:163–170
Oudry M, Franquin JC, Pourreau-Schreider N, Martin PM (1988) Effect of a helium–neon laser on cellular growth: an in vitro study of human gingival fibroblasts. J Biol Buccale 16:129–135
van Breugel HH, Bar PR (1992) Power density and exposure time of He–Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528–537
Schultz RJ, Krishnamurthy S, Thelmo W, Rodriguez JE, Harvey G (1985) Effects of varying intensities of laser energy on articular cartilage: a preliminary study. Lasers Surg Med 5:577–588
Abergel RP, Meeker CA, Lam TS, Dwyer RM, Lesavoy MA, Uitto J (1984) Control of connective tissue metabolism by lasers: recent developments and future prospects. J Am Acad Dermatol 11:1142–1150
Balboni GC, Brandi ML, Zonefrati R, Repice F (1986) Effects of He–Ne/I. R. laser irradiation on two lines of normal human fibroblasts in vitro. Arch Ital Anat Embriol 91:179–188
Bosatra M, Jucci A, Olliaro P, Quacci D, Sacchi S (1984) In vitro fibroblast and dermis fibroblast activation by laser irradiation at low energy. An electron microscopic study. Dermatologica 168:157–162
Anders JJ, Borke RC, Woolery SK, Van de Merwe WP (1993) Low power laser irradiation alters the rate of regeneration of the rat facial nerve. Lasers Surg Med 13:72–82
Tang XM, Chai BP (1986) Effect of CO2 laser irradiation on experimental fracture healing: a transmission electron microscopic study. Lasers Surg Med 6:346–352
Trelles MA, Mayayo E (1987) Bone fracture consolidates faster with low-power laser. Lasers Surg Med 7:36–45
Lim HM, Lew KK, Tay DK (1995) A clinical investigation of the efficacy of low level laser therapy in reducing orthodontic postadjustment pain. Am J Orthod Dentofac Orthop 108:614–622
Rodrigues MTJ, Ribeiro MS, Groth EB, Zezell DM (2002) Evaluation of effects of laser therapy (λ=830 nm) on oral ulceration induced by fixed orthodontic appliances. Lasers Surg Med 30(Suppl 14):15
Saito S, Shimizu N (1997) Stimulatory effects of low-power laser irradiation on bone regeneration in midpalatal suture during expansion in the rat. Am J Orthod Dentofac Orthop 111:525–532
Ozawa Y, Shimizu N, Kariya G, Abiko Y (1998) Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 22:347–354
Kawasaki K, Shimizu N (2000) Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med 26:282–291
Rodan GA (1996) Coupling of bone resorption and formation during bone remodeling. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis. Academic, New York, pp 289–299
Lemaire V, Tobin FL, Greller LD, Cho CR, Suva LJ (2004) Modeling the interactions between osteoblast and osteoclast activities in bone remodeling. J Theor Biol 229:293–309
Cruz DR, Kohara EK, Ribeiro MS, Wetter NU (2004) Effects of low-intensity laser therapy on the orthodontic movement velocity of human teeth: a preliminary study. Lasers Surg Med 35:117–120
Roodman GD (1996) Advances in bone biology: the osteoclast. Endocr Rev 17:308–332
Roodman GD, Ibbotson KJ, MacDonald BR, Kuehl TJ, Mundy GR (1985) 1,25-Dihydroxyvitamin D3 causes formation of multinucleated cells with several osteoclast characteristics in cultures of primate marrow. Proc Natl Acad Sci U S A 82:8213–8217
Chambers TJ, Revell PA, Fuller K, Athanasou NA (1984) Resorption of bone by isolated rabbit osteoclasts. J Cell Sci 66:383–399
Anderson DM, Maraskovsky E, Billingsley WL, Dougall WC, Tometsko ME, Roux ER, Teepe MC, DuBose RF, Cosman D, Galibert L (1997) A homologue of the TNF receptor and its ligand enhance T-cell growth and dendritic-cell function. Nature 390:175–179
Takeshita S, Kaji K, Kudo A (2000) Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J Bone Miner Res 15:1477–1488
Myers DE, Collier FM, Minkin C, Wang H, Holloway WR, Malakellis M, Nicholson GC (1999) Expression of functional RANK on mature rat and human osteoclasts. FEBS Lett 463:295–300
Kanzaki H, Chiba M, Shimizu Y, Mitani H (2002) Priodontal ligament cells under mechanical stress induce osteoclastogenesis by receptor activator of nuclear factor kappaB ligand up-regulation via prostaglandin E2 synthesis. J Bone Miner Res 17:210–220
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ (1999) Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 20:345–357
Klebanov GI, Kreinina MV, Poltanov EA, Khristoforova TV, Vladimirov YA (2001) Mechanism of therapeutic effect of low-intensity infrared laser radiation. Bull Exp Biol Med 131:239–241
Wong BR, Rho J, Arron J, Robinson E, Orlinick J, Chao M, Kalachikov S, Cayani E, Bartlett FS 3rd, Frankel WN, Lee SY, Choi Y (1997) TRANCE is a novel ligand of the tumor necrosis factor receptor family that activates c-Jun N-terminal kinase in T cells. J Biol Chem 272:25190–25194
Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ (1999) Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A 96:3540–3545
Udagawa N, Takahashi N, Jimi E, Matsuzaki K, Tsurukai T, Itoh K, Nakagawa N, Yasuda H, Goto M, Tsuda E, Higashio K, Gillespie MT, Martin TJ, Suda T (1999) Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone 25:517–523
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21:271–277
Dortbudak O, Haas R, Mailath-Pokorny G (2002) Effect of low-power laser irradiation on bony implant sites. Clin Oral Implants Res 13:288–292
Barushka O, Yaakobi T, Oron U (1995) Effect of low-energy laser (He–Ne) irradiation on the process of bone repair in the rat tibia. Bone 16:47–55
Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Ogasawara T, Yoshimine Y, Kiyoshima T, Kobayashi I, Matsuo K, Akamine A, Sakai H (2004) In situ expression of RANKL, RANK, osteoprotegerin and cytokines in osteoclasts of rat periodontal tissue. J Periodontal Res 39:42–49
Oshiro T, Shiotani A, Shibasaki Y, Sasaki T (2002) Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat Rec 266:218–225
Kanzaki H, Chiba M, Takahashi I, Haruyama N, Nishimura M, Mitani H (2004) Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J Dent Res 83:920–925
Acknowledgements
This research was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (C:14571969, C:14571970) and a 2003 Suzuki Research Grant from Nihon University School of Dentistry at Matsudo (03-1018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aihara, N., Yamaguchi, M. & Kasai, K. Low-energy irradiation stimulates formation of osteoclast-like cells via RANK expression in vitro. Lasers Med Sci 21, 24–33 (2006). https://doi.org/10.1007/s10103-005-0368-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-005-0368-4