Abstract
Photomodulation therapy (PBMT) using light-emitting diode (LED) has been proposed as an alternative to conventional osteoporosis therapies. Our aim was to determine the effect of irradiation with a light-emitting diode on receptor activator of NF-κB ligand (RANKL)-mediated differentiation of mouse bone marrow macrophages into osteoclasts and compare it to alendronate treatment. The cells were irradiated with LED at 635±10 nm, 9-cm spot size, 5 mW/cm2, and 18 J for 60 min/day in a CO2 incubator. The differentiation of irradiated and untreated RANKL-stimulated bone marrow macrophages into osteoclasts was evaluated by tartrate-resistant acid phosphatase (TRAP) staining and by molecular methods. These included assessing messenger RNA (mRNA) expression of osteoclastic markers such as TRAP, c-Fos, Atp6v0d2, DC-STAMP, NFATc1, cathepsin K, MMP9 and OSCAR; phosphorylation of various MAPKs, including extracellular signal-regulated kinase ERK1/2, P38, and JNK; NF-κB translocation; and resorption pit formation. Results were compared to those obtained with sodium alendronate. Production of reactive oxygen species was measured by a 2’,7’-dihydrodichlorofluorescein diacetate assay. LED irradiation and alendronate inhibited mRNA expression of osteoclast-related genes, such as TRAP, c-Fos, and NFATc1, and reduced the osteoclast activity of RANKL-stimulated bone marrow macrophages. LED irradiation, but not alendronate, also inhibited the production of reactive oxygen species (ROS); phosphorylation of ERK, P38, and IκB; and NF-κB translocation. These findings suggest that LED irradiation downregulates osteoclastogenesis by ROS production; this effect could lead to reduced bone loss and may offer a new therapeutic tool for managing osteoporosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a common chronic disease that increases the risk of bone fracture [1]. In patients with osteoporosis, there is an imbalance between bone formation and resorption, which favors bone loss and progressive skeletal fragility, thereby increasing the risk of fracture [2]. Under physiological conditions, bone remodeling is maintained by tight regulation of osteoclasts (OCLs) and osteoblasts; excessive activation of OCLs can cause bone loss [3]. OCLs are multinucleated cells that develop from hematopoietic precursors of the monocyte-macrophage lineage, whereas osteoblasts derive from mesenchymal stem cells. The differentiation of OCLs from precursor cells is induced by two key molecules: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB (NF-κB) ligand (RANKL). RANKL-induced activation of RANK on OCL progenitor cells causes upregulation of tumor necrosis factor (TNF) receptor-associated mediators. This leads to activation of osteoclastogenic signaling cascades involving reactive oxygen species (ROS), NF-κB, and mitogen-activated protein kinase (MAPK) [3, 4]. ROS in particular may be responsible for the development of osteoporosis. Some in vitro and animal studies have shown that oxidative stress inhibits bone formation by modulating the differentiation and survival of osteoblasts and OCLs [5–8].
Most drugs currently used to treat osteoporosis suppress OCL-mediated bone resorption. The most common antiresorptive drugs are nitrogen-containing bisphosphonates, which were developed for the treatment of middle- to late-stage osteoporosis in postmenopausal women and men [9]. Bisphosphonates adsorb to bone mineral, are taken up by OCLs, and inhibit farnesyl pyrophosphate synthase. The latter effect blocks the prenylation of key signaling molecules that mediate cytoskeletal organization, which is necessary for vesicular trafficking and activation of mature OCLs [10].
Recently, irradiation with light-emitting diodes (LED) at 635 nm wavelength has been applied to many clinical fields. Some investigators have suggested that photobiomodulation therapy (PBMT) may modulate wound healing, cell proliferation, differentiation, collagen synthesis, as well as osteoblast development, and bone formation [11–14]. PBMT have been employed successfully on superficial wounds, such as in the skin. However, light above 600 nm wavelength can penetrate the epithelial tissue, potentially improving the healing of injured muscle or bone [15]. In a study involving both in vitro and in vivo models, low-level irradiation with light was found to alleviate inflammation by inhibiting prostaglandin E2 production and messenger RNA (mRNA) expression of cyclooxygenases 1 and 2 [16, 17]. The authors suggested that irradiation with weak light at a specific wavelength could promote scavenging of intracellular ROS, mitigate oxidative stress, and alleviate several pathological conditions [18–20].
Although many reports have demonstrated the benefits of laser irradiation on osteoblast differentiation and bone formation, little is known about the effect of light therapy on the development of OCLs or bone resorption [14, 15, 21–23].
The aim of the present study was to analyze the effect of LED irradiation on RANKL-stimulated mouse bone marrow macrophages (BMMs) and compare it to that of bisphosphonate alendronate. Because few research efforts have examined the effect of LED irradiation on the formation and activity of OCLs, this study may facilitate the development of a new treatment for osteoporosis.
Methods
Cell culture
Four-week-old male BALB/c mice were obtained from Damul Science (Daejeon, Korea). We obtained bone marrow cells from the tibiae and femurs by flushing these bones with α-MEM medium (Invitrogen, Carlsbad, CA, USA) as previously described [24]. After red blood cells were removed with ACK buffer (0.01 mM EDTA, 0.011 M KHCO3, 0.155 M NH4Cl, pH 7.3), they were resuspended in complete α-MEM medium containing 10 % (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated 24 h in the presence of 10 ng/mL M-CSF (Cell Guidance Systems Ltd., Cambridge, UK). We collected non-adherent cells and cultured them with 30 ng/mL M-CSF for 3 days to generate bone marrow macrophages (BMMs). To generate pre-OCLs and OCLs, we cultured BMMs (5 × 104/cm2) in complete α-MEM medium containing 30 ng/mL M-CSF and 37.5 or 75 ng/mL RANKL (Cell Guidance Systems, Ltd.) for 72 h (until the cells differentiated into multinucleated mature OCLs).
Light source and irradiation
A LED with a peak wavelength of 635 nm was used to irradiate the BMMs in the 9-cm petri dish with a power density of 5 mW/cm2 at target and dose of energy density 18 J/cm2 in the presence of M-CSF and RANKL for 1 h/day during osteoclast differentiation. A custom-made LED irradiation tool kit was used to assemble the light source in a chamber containing humidified air with 5 % CO2 at 37 °C (Biophoton, Gwangju, Korea). We chose an irradiation spot size that fitted in a 9-cm petri dish. To examine the effect of LED irradiation, we cultured groups of cells with or without LED irradiation for the indicated time.
Cell viability assay
Cell viability was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich, St. Louis, MO, USA) assay. Cells were seeded at 5 × 103 in 200 μL of medium in 96-well plates and cultured for 1 day at 37 °C. After each experiment, the medium was removed and 20 μL MTT (50 μg/mL) was added to the wells. Cells were incubated at 37 °C for 4.5 h to allow the color to develop, and the formazan product was solubilized by addition of 50 μL dimethyl sulfoxide (Calbiochem Bio-Mol, La Jolla, CA, USA). Optical density at 570 nm was measured using a microtiter plate reader (Bio-Rad, Hercules, CA, USA).
Tartrate-resistant acid phosphatase (TRAP) staining
Seventy-two hours after the RANKL treatment with or without LED irradiation or alendronate treatment, cells were washed once with PBS and fixed in 10 % formalin (in PBS) for 5 min. After three washes with distilled water, TRAP solution (Sigma-Aldrich) was added and staining was allowed to proceed for 30–40 min. After the stained cells were examined under a light microscope (Olympus, Tokyo, Japan), images were captured with a digital camera (Olympus). Multinucleated cells were counted manually.
TRAP solution assay
This assay can be used to quantify total TRAP enzymatic activity. We used it to count OCLs. Briefly, cells in 96-well plates were washed once with PBS and lysed in 80 μL cold lysis buffer (90 mM citrate pH 4.8, 0.1 % Triton X-100, and 80 mM sodium tartrate) for 10 min. Following, 80 μL of substrate solution (20 mM p-nitrophenyl phosphate) was added, and the cells were incubated for 30 min at 37 °C. To stop the reaction, 40 μL 0.5 N NaOH was added, and optical density was measured at 405 nm. Results were expressed as a percentage of the control.
Osteoclast activity assay
We assessed OCL activity by quantifying resorption pit formation. For this purpose, BMMs were seeded in plates coated with a thin calcium phosphate film (Corning Inc., Tewksbury, MA, USA). Cells were incubated with M-CSF and RANKL for 7 days until the resulting mature OCLs resorbed the calcium phosphate film and were then dissolved in 5 % sodium hypochlorite. Images of the resorption pits were taken using a light microscope. The ratio of the resorbed area to the total area was calculated by means of using ImageJ software (http://rsbweb.nih.gov/ij/).
Isolation of total RNA and quantitative real-time PCR (RT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. At the indicated time points, RNA (1 μg) was reverse-transcribed using oligo-dT primers (10 μg) and dNTPs (10 mM). The mixture was incubated at 65 °C for 5 min, and complementary DNA (cDNA) was synthesized by incubation at 42 °C for 50 min with first-strand buffer (50 mM Tris-HCl pH 8.3, 75 mM KCl, and 3 mM MgCl2), 100 mM dithiothreitol (DTT), an RNase inhibitor, and Superscript II reverse transcriptase (Invitrogen). The cDNA was amplified using the following primer sets: TRAP, 5’-TACCTGTGTGGACATGACC-3’ (forward) and 5’-CAGATCCATAGTGAAACCGC-3′ (reverse); c-Fos, 5′-GGTGAAGACCGTGTCAGGAG-3′ (forward) and 5′-TATTCCGTTCCCTTCGGATT-3′ (reverse); Atp6v0d2, 5′-GAAGCTGTCAACATTGCAGA-3′ (forward) and 5′-TCACCGTGATCCTTGCAGAAT-3′ (reverse); DC-STAMP, 5′-TGGAAGTTCACTTGAAACTACGTG-3′ (forward) and 5′-CTCGGTTTCCCGTCAGCCTCTCTC-3′ (reverse); NFATc1, 5′-GAGTACACCTTCCAGCACCTT-3′ (forward) and 5′-TATGATGTCGGGGAAAGAGA-3′ (reverse); cathepsin K, 5′-TGTATAACGCCACGGCAAA-3′ (forward) and 5′-GGTTCACATTATCACGGTCACA-3′ (reverse); MMP9, 5′-TCCAGTACCAAGACAAAG-3′ (forward) and 5′-TTGCACTGCACGGTTGAA-3′ (reverse); OSCAR, 5′-CTGCTGGTAACGGATCAGCTCCCCAGA-3′ (forward) and 5′-CCAAGGAGCCAGAACCTTCGAAACT-3′ (reverse); and GAPDH, 5′-TCAAGAAGGTGGTGAAGCAG-3′ (forward) and 5′-AGTGGGAGTTGCTGTTGAAGT-3′ (reverse). RT-PCR was conducted on an Exicycler 96 Real-Time Quantitative Thermal Block (Bioneer Co., Daejeon, Korea) in a 20-μL reaction mixture containing 10 μL SYBR Green Premix (Bioneer Co.), 10 pM forward primer, 10 pM reverse primer, and 1 μg cDNA. The cycling conditions comprised of an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 1 min, annealing at 60 °C for 30 s, and extension at 72 °C for 1 min. Fluorescence resulting from the incorporation of the SYBR Green dye into double-stranded DNA during PCR was quantified by the threshold cycle (Ct) method. Relative levels of expression of each gene were normalized to GAPDH.
Preparation of nuclear extracts
BMMs were rinsed in PBS and then collected into Eppendorf tubes. Cytosolic protein extraction buffer (0.5 mL; 10 mM HEPES pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, and 0.05 % NP-40) was added, and the cells were centrifuged at 805×g for 10 min at 4 °C. The supernatant contained mostly cytoplasmic constituents. To obtain a pellet of nuclear material, 0.4 mL of nuclear protein extraction buffer (5 mM HEPES pH 7.9, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, 26 % glycerol (v/v), and 300 mM NaCl) was added, the tubes were inverted several times (for mixing), and they were placed on a small rotator shaker for 15 min. Finally, the mixture was centrifuged at 12,000×g for 3 min. The supernatant containing the proteins from the nuclear extract was carefully transferred to a new tube. The nuclear and cytosolic extracts were stored in aliquots at −80 °C until western blot analysis. The protein content of each sample was determined using the BCA protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA).
Western blot analysis
BMM lysates were prepared in 200-μL cold lysis buffer (50 mM Tris-HCl pH 7.5, 1 % NP-40, 150 mM NaCl, 0.02 % sodium azide, 150 μg/mL phenylmethanesulfonyl fluoride, 2 μg/mL aprotinin, 20 μg/ml leupeptin, and 1 μg/mL pepstatin A). Proteins from the cell lysates were separated on a 10–12 % sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene difluoride membrane (GE Healthcare, Little Chalfont, UK). Membranes were blocked in TBST (2.42 g/L Tris base adjusted with HCl to pH 7.6, 8 g/L NaCl, and 0.5 % Tween 20) containing 5 % skimmed milk for 30 min, and rinsed briefly in TBST. Membranes were incubated overnight at 4 °C in 5 % nonfat milk in TBST using antibodies against P65-NF-κB (1:1000; Cell Signaling Technology, Beverly, MA, USA), histone H1 (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), actin (1:2000; Santa Cruz Biotechnology), phospho-Jun N terminal kinase (JNK) (1:1000; Cell Signaling Technology), JNK (1:1000; Cell Signaling Technology), phospho-P38 (1:1000; Cell Signaling Technology), P38 (1:1000; Cell Signaling Technology), phospho-ERK1/2 (1:1000; Cell Signaling Technology), ERK1/2 (1:1000; Cell Signaling Technology), phospho-IκB (1:1000; Cell Signaling Technology), IκB (1:1000; Cell Signaling Technology), or GAPDH (1:2000; Santa Cruz Biotechnology). After rinsing in TBST, membranes were incubated for 1 h with anti-rabbit and anti-mouse horseradish peroxidase-conjugated secondary antibodies (1:2000; Santa Cruz Biotechnology). Finally, membranes were washed in TBST, and protein immunoreactivity was detected using the enhanced chemiluminescence (Santa Cruz Biotechnology) detection kit.
Immunofluorescence assay
To determine the intracellular localization of the NF-κB P65 subunit, we seeded cells on four-well chamber slides. After either irradiation or alendronate treatment, cells were fixed in cold 3.8 % paraformaldehyde, permeabilized by incubation with 0.2 % Triton X-100 in cold PBS for 30 min, and blocked in 5 % BSA in PBST (0.2 % Tween 20 in PBS) at room temperature for 30 min. A rabbit anti-NF-κB P65 subunit antibody (1:200; Santa Cruz Biotechnology) diluted in PBST containing 5 % BSA was used as the primary antibody and was incubated with the cells for 2 h at room temperature. After thorough washes with PBST, cells were incubated with a FITC-labeled goat anti-rabbit IgG antibody (10 μg/mL, diluted in PBST containing 5 % BSA) for 1 h at room temperature in the dark, and were then washed with PBST for 10 min. Thereafter, cells were stained with 5 μg/mL 4’,6-diamidino-2-phenylindole (DAPI) for 30 min at 37 °C in the dark and then thoroughly washed with PBS. Cells were examined under a confocal microscope (Carl Zeiss, Oberkochen, Germany) with a 60× oil objective using excitation and emission wavelengths of 490 and 540 nm, respectively, for FITC and 360 and 450 nm, respectively, for DAPI. Images were captured as serial sections in the XY plane.
Detection of ROS
ROS levels were measured using a 2’,7’-dihydrochlorofluorescein diacetate (DCF-DA; Sigma) assay. DCF-DA enters cells passively, where it is enzymatically deacetylated by esterases to non-fluorescent 2’,7’-dihydrodichlorofluorescein (DCF-H). Oxidants, such as O2 −, convert DCF-H to highly fluorescent DCF. Intracellular ROS were assessed immediately after irradiation of RANKL-stimulated BMMs. Cells grown on four-well chamber slides were incubated with 10 μM DCF-DA for 20 min, followed by washes with PBS containing 10 nM glucose. DCF fluorescence intensity was recorded using a confocal microscope (Olympus) with excitation and emission at 488 and 525 nm, respectively. To measure intracellular ROS levels, we washed the cells with PBS containing 10 nM glucose and incubated them with 10 μM DCF-DA for 20 min. Cells were detached with a trypsin-EDTA solution, and ROS were measured by flow cytometry (Beckman Coulter, Fullerton, CA, USA) using 485 nm excitation and 530 nm emission filters.
Statistical analysis
All experiments were performed in triplicate. Data are expressed as mean ± SD and statistical significance was determined using the SPSS software for Windows (version 12.0; SPSS, Chicago, IL, USA). Statistical significance was set to p < 0.05.
Results
LED irradiation and alendronate treatment inhibit RANKL-induced differentiation of BMMs into OCLs
To ensure that the therapeutic concentration of alendronate had no effect on cell viability during OCL differentiation, and for comparison to LED irradiation, MTT assay were used. BMMs were cultured in the presence of various concentrations of alendronate (0, 5, 10, 50, 100, 500, or 1 000 μM) for 3 days (Fig. 1a). Concentrations up to 50 μM had no significant effect on cell viability. Therefore, 50 μM was chosen for all subsequent experiments with alendronate. Next, the MTT assay was used to exclude the possibility that inhibition of differentiation was due to reduced viability and/or proliferation of OCL precursor cells. Results revealed that LED irradiation had no cytotoxic effect on BMMs differentiation into OCLs (Fig. 1b).
LED irradiation and alendronate treatment inhibit RANKL-induced differentiation into osteoclasts (OCLs). a Cell viability was determined determined by MTT assay at various concentrations of alendronate (0, 5, 10, 50, 100, 500, and 1000 μM). b Cultured for 3 days in the presence of M-CSF and RANKL (0, 37.5, or 75 ng/mL) with (Ir) or without (Con) LED irradiation, and cell viability was determined. c LED irradiation (LED Ir) and alendronate treatment decreased the number of TRAP-positive multinucleated cells. TRAP-positive cells were photographed under a light microscope (×100 magnification). The scale bar is 20 μm. d TRAP-positive cells were counted; *p < 0.05 compared to the control; **p < 0.05 compared to RANKL-stimulated cells. e TRAP activity in BMMs was measured as absorbance at 450 nm by means of an enzyme-linked immunosorbent assay (ELISA) reader
Next, the effect of LED irradiation and alendronate treatment on osteoclastogenesis was compared. To this end, primary BMMs stimulated with RANKL (37.5 or 75 ng/mL) were first exposed to either LED irradiation or 50 μM alendronate, followed by TRAP staining. RANKL-stimulated BMMs differentiated into mature TRAP-positive multinucleated OCLs in a dose-dependent manner; however, their numbers decreased significantly upon LED irradiation or alendronate treatment (Fig. 1c, d). TRAP activity was also significantly reduced by LED irradiation or alendronate treatment (Fig. 1e).
LED irradiation inhibits the activity of mature OCLs in vitro
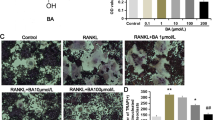
To determine whether LED irradiation and alendronate treatment affected OCL activity, the resorption area was measured. RANKL treatment (37.5 or 75 ng/mL) increased the resorption area, however irradiation with LED light or addition of alendronate led to decrease the resorption pit, significantly (Fig. 2a, b). This suggested that both LED irradiation and alendronate treatment inhibited OCL differentiation and decreased the resorption activity of mature OCLs.
Effect of LED irradiation on bone resorption in RANKL-induced BMMs. a Photomicrographs of the bone resorption activity of osteoclasts (×100 magnification). Space bar, 20 μm. b Pit areas were quantified by using ImageJ software; *p < 0.05 compared to the control; **p < 0.05 compared to RANKL-stimulated cells. Ir irradiation, Al alendronate
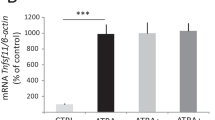
LED irradiation is more effective than alendronate treatment in suppressing mRNA expression of TRAP, c-Fos, ATP6V0d2, DC-STAMP, NFATc1, cathepsin K, MMP9, and OSCAR in RANKL-stimulated BMMs
To further explore the changes in RANKL-mediated osteoclastogenesis induced by LED irradiation or alendronate treatment, the expressions of osteoclastogenesis-related genes were investigated by RT-PCR (Fig. 3). LED irradiation and alendronate treatment significantly reduced mRNA expression of TRAP and MMP9 in RANKL-stimulated BMMs during cell culture. Instead, mRNA expression of c-Fos, ATP6V0d2, DC-STAMP, and NFATc1 in RANKL-treated cells was downregulated more effectively by LED irradiation than alendronate treatment during cell culture period. Finally, cathepsin K mRNA expression in RANKL-treated cells was not affected by either alendronate or LED irradiation.
LED irradiation attenuates activation of the NF-κB pathway in RANKL-stimulated BMMs
To study the activation of NF-κB, which drives RANKL-induced osteoclastogenesis, phosphorylation of IκB and translocation of NF-κB were assayed by western blotting and immunofluorescence. Compared with alendronate treatment, LED irradiation significantly suppressed RANKL-induced phosphorylation and degradation of IκB (Fig. 4a) and inhibited translocation of P65 to the nucleus, as confirmed separately by western blot (Fig. 4b) and immunofluorescence (Fig. 4c).
LED irradiation suppresses activation of the NF-κB pathway in RANKL-stimulated BMMs. a Phosphorylation of IκB was analyzed by western blotting in RANKL-stimulated BMMs in the presence or absence of either LED irradiation or alendronate at the indicated time points. The membranes were stripped, and antibodies against non-phosphorylated IκB were used as a control. LED Ir irradiation. b Nuclear translocation of the NF-κB P65 subunit was analyzed by western blotting of the cytosolic and nuclear fractions. Histone H1 from the nuclear fraction and β-actin from the cytosol were used as protein loading controls. The results of densitometric analysis of P65 in the cytosolic and nuclear fractions are presented as the mean ratio ± SD from three separate experiments. c Immunofluorescent images under a confocal microscope after staining for NF-κB P65 subunit (green) and nuclei (blue). The magnification is ×100. The scale bar is 20 μm
LED irradiation suppresses MAPK phosphorylation in RANKL-stimulated BMMs
To determine the role of signal transduction and the mechanistic effect of LED irradiation on RANKL-mediated differentiation into OCLs, MAPK activities were examined. To this end, we measured phosphorylation of JNK (Fig. 5a), ERK (Fig. 5b), and P38 (Fig. 5c) after 10 min of exposure to RANKL (37.5 ng/mL) in the presence or absence of either LED irradiation or alendronate treatment. LED irradiation significantly inhibited RANKL-mediated phosphorylation of ERK1/2 compared with alendronate treatment. A considerable decrease in P38 phosphorylation was also observed in LED-irradiated cells. In contrast, JNK phosphorylation was not affected by either treatment. These results suggest that the effect of LED irradiation on the MAPK signaling pathway was mediated mainly by inhibition of ERK and P38 phosphorylation.
Effect of LED irradiation on the phosphorylation of ERK, P38, and JNK in bone marrow macrophages (BMMs) by RANKL (37.5 ng/mL). Phosphorylation of a JNK, b ERK, and c P38 was analyzed by western blotting in RANKL-stimulated BMMs in the presence or absence of either LED irradiation or alendronate at the indicated time points. The membranes were stripped, and antibodies against non-phosphorylated proteins were used as a control. Results were obtained by densitometry and are presented as mean ± SD in the graphs. LED Ir, IR irradiation, Al alendronate
LED irradiation suppresses ROS production in RANKL-stimulated BMMs
To evaluate the capacity of LED irradiation to interfere with RANKL-stimulated production of ROS in BMMs, intracellular ROS were assayed by DCF-DA method. A marked increase in DCF fluorescence, indicative of intracellular ROS production, was observed in the RANKL-treated group with or without alendronate. However, in LED-irradiated cells, DCF fluorescence was significantly reduced (Fig. 6a, b).
LED irradiation suppresses production of reactive oxygen species (ROS) in RANKL-stimulated BMMs. a BMMs stimulated with different concentrations of RANKL were stained with DCF-DA and observed by confocal microscopy; green color indicates intracellular formation of ROS. The magnification is ×200; the scale bar is 20 μm. b DCF fluorescence intensity is compared with that of the control. The data are expressed as mean ± SD; *p < 0.05 compared to control, **p < 0.05 compared to stimulation with RANKL. c The distribution of DCF fluorescence is presented as flow cytometry histograms (x-axis: log of the fluorescence intensity from 100 to 103; y-axis: cell number from 0 to 100). Each graph is representative of three separate experiments; all experiments yielded similar results
Also, the number of DCF-DA positive cells in the 37.5 ng/mL RANKL-treated group decreased significantly upon LED irradiation, from 24.67 to 2.76 %, whereas alendronate treatment caused only a slight decrease, to 9.13 %, as indicated by flow cytometry (Fig. 6c). Judging by these results, LED irradiation effectively attenuated the intracellular ROS in RANKL-stimulated BMMs.
Discussion
Recently, LLLT and LEDT attracted considerable attention because of their antiresorptive properties and enhanced bone formation. LED irradiation at 635 nm has been reported to disrupt the actin ring and dramatically inhibit OCL differentiation [25]. In comparison, very little is known about the mechanism and the signaling cascades affected by light treatment in relation to osteoclast activities, although prior research indicates an effect of LED (or laser irradiation) on OCL differentiation and bone resorption [25, 26].
Alendronate binds to bone minerals and is then taken up by OCLs at the initiation of resorption. Inside the OCLs, alendronate interferes with the formation of geranylgeranyl pyrophosphate (GGPP) by inhibiting an enzyme(s) in the cholesterol biosynthesis pathway, most likely via removal of prenylation from the GTP-binding proteins that control cytoskeletal function, vesicular trafficking, and apoptosis [27]. Accordingly, the present study shows that LED irradiation is a promising treatment of osteoporosis because LED light affects RANKL-induced differentiation into OCLs; this mechanism of action is quite similar with that of other anti-osteoporosis treatments such as alendronate. In our study, LED irradiation inhibited OCL activity and bone resorption in RANKL-stimulated BMMs as effectively as did alendronate treatment.
What is the difference of LED irradiation with alendronate on osteoclastogenesis? To address this question, RANKL-induced signaling cascades were investigated on osteoclastogenesis. LED irradiation effectively reduced the phosphorylation of IκB and NF-κB P65 translocation in compared with alendronate. Moreover, it appears that LED irradiation the downstream signaling of RANK, ERK, and P38 MAPK signaling pathways including the ROS production. Especially, 635-nm irradiation leads to a decrease in intracellular ROS, resulting in a dramatic recovery of oxidative stress. By investigating the possible involvement of the ROS scavenging properties and subsequent regulation of relevant signaling pathways [8], our study showed that LED irradiation significantly inhibited ERK and P38 MAPK signaling pathways as well as I-kB phosphorylation and NF-κB P65 translocation. Ha et al. announced that RANKL stimulated ROS production, activation of Akt, NF-κB, and ERK in osteoclasts, implying that RANKL-induced ROS production is required for upstream components of these signaling pathways [8]. It is common knowledge that ROS drive OCLs to enhance bone resorption [3]. Recent evidence from clinical studies showed that ROS and/or antioxidant systems may be involved in the pathogenesis of bone loss [28]. Intracellular ROS—that are produced during mitochondrial electron transport or by enzymes such as NADPH oxidase and COXs—perform crucial functions in diverse cellular events, including differentiation, proliferation, and apoptosis [29]. ROS have been reported to enhance the development of OCL and bone resorption [30]. RANKL upregulates intracellular ROS, but antioxidants N-acetyl-cysteine and glutathione inhibit both formation of TRAP-positive multinucleated cells and RANKL-induced activation of Akt, NF-κB, and ERK, which are key signaling pathways during differentiation into OCLs. In the present study, LED irradiation was shown to have antioxidant effects, probably due to its free-radical-scavenging properties and subsequent regulation of relevant signaling pathways. This means that intracellular ROS could be scavenged as a result of light irradiation, leading to a reduction in oxidative stress, and consequent alleviation of oxidative stress-induced osteoclastogenesis; these effects are quite different from the signaling effects of alendronate.
It is known that osteoclastogenesis consists of roughly four stages: commitment, differentiation, fusion, and bone resorption [3]. At the commitment stage, myeloid stem cells originating from hematopoietic stem cells are committed to the OCL fate under the influence of signaling molecules such as MITF and M-CSF. At the differentiation stage, the committed cells turn into mononucleated OCLs under the influence of RANK/RANKL signaling and transcription factors TRAF6 and NFATc1. Then, the mononucleated OCLs activate intercellular fusion as RANKL induces the expression of fusion-mediating molecules, such as DC-STAMP and ATP6V0d2 [31]. In the present study, LED irradiation significantly downregulates c-Fos, ATP6V0d2, DC-STAMP, and NFATc1, whereas alendronate treatment markedly downregulates the expression of MMP9 and OSCAR. Therefore, compared with alendronate treatment, LED irradiation appears to affect the differentiation and fusion stages of osteoclastogenesis.
Taken together, our results show that LED irradiation inhibits RANKL-mediated differentiation into OCLs by suppressing expression of c-Fos, NFATc1, and nuclear translocation of NF-κB. Additionally, compared to alendronate, LED irradiation significantly decreases ROS production and phosphorylation of ERK and P38 in RANKL-stimulated BMMs (Fig. 7). These findings suggest that LED irradiation has a useful inhibitory effect on bone loss via prevention of OCL formation, thus offering a potential alternative for the treatment of osteoporosis.
Schematic overview of putative pathways governing osteoclastogenesis and intracellular production of ROS; possible effects of LED irradiation at 635 nm. In RANKL-induced osteoclastogenesis, increased ERK phosphorylation and ROS production lead to phosphorylation of P38 MAPK and IκB, followed by NF-κB translocation to the nucleus, and finally enhanced osteoclastogenesis. We propose that, LED irradiation attenuates the production of intracellular ROS and NF-κB translocation, culminating in diminished osteoclastogenesis. Alendronate also suppresses osteoclastogenesis (via a different mechanism)
References
Goltzman D (2002) Discoveries, drugs and skeletal disorders. Nat Rev Drug Discov 1:784–796
Sims NA, Martin TJ (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKEy Rep 3:481
Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature 423:337–342
Teitelbaum SL, Ross FP (2003) Genetic regulation of osteoclast development and function. Nat Rev Genet 4:638–649
Isomura H, Fujie K, Shibata K, Inoue N, Iizuka T, Takebe G, Takahashi K, Nishihira J, Izumi H, Sakamoto W (2004) Bone metabolism and oxidative stress in postmenopausal rats with iron overload. Toxicology 197:93–100
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314:197–207
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639
Ha H, Kwak HB, Lee SW, Jin HM, Kim HM, Kim HH, Lee ZH (2004) Reactive oxygen species mediate RANK signaling in osteoclasts. Exp Cell Res 301:119–127
Baron R, Ferrari S, Russell RG (2011) Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 48:677–692
Zhuo Y, Gauthier JY, Black WC, Percival MD, Duong LT (2014) Inhibition of bone resorption by the cathepsin K inhibitor odanacatib is fully reversible. Bone 67:269–280
Ueda Y, Shimizu N (2003) Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells. J Clin Laser Med Surg 21:271–277
Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G (2004) Low-level laser therapy facilitates superficial wound healing in humans: a triple-blind, sham-controlled study. J Athl Train 39:223–229
Hussein AJ, Alfars AA, Falih MA, Hassan AN (2011) Effects of a low level laser on the acceleration of wound healing in rabbits. N Am J Med Sci 3:193–197
Amid R, Kadkhodazadeh M, Ahsaie MG, Hakakzadeh A (2014) Effect of low level laser therapy on proliferation and differentiation of the cells contributing in bone regeneration. J Lasers Med Sci 5:163–170
Grassi FR, Ciccolella F, D’Apolito G, Papa F, Iuso A, Salzo AE, Trentadue R, Nardi GM, Scivetti M, De Matteo M, Silvestris F, Ballini A, Inchingolo F, Dipalma G, Scacco S, Tete S (2011) Effect of low-level laser irradiation on osteoblast proliferation and bone formation. J Biol Regul Homeost Agents 25:603–614
Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, Park J, Kim O, Choi H (2007) The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med 39:614–621
Bjordal JM, Couppe C, Chow RT, Tuner J, Ljunggren EA (2003) A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother 49:107–116
Lim WB, Kim JS, Ko YJ, Kwon H, Kim SW, Min HK, Kim O, Choi HR, Kim OJ (2011) Effects of 635nm light-emitting diode irradiation on angiogenesis in CoCl(2) -exposed HUVECs. Lasers Surg Med 43:344–352
Lubart R, Lavi R, Friedmann H, Rochkind S (2006) Photochemistry and photobiology of light absorption by living cells. Photomed Laser Surg 24:179–185
Kanzaki H, Shinohara F, Kajiya M, Kodama T (2013) The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem 288:23009–23020
Pretel H, Lizarelli RF, Ramalho LT (2007) Effect of low-level laser therapy on bone repair: histological study in rats. Lasers Surg Med 39:788–796
Kazem Shakouri S, Soleimanpour J, Salekzamani Y, Oskuie MR (2010) Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci 25:73–77
Pinheiro AL, Limeira Junior Fde A, Gerbi ME, Ramalho LM, Marzola C, Ponzi EA (2003) Effect of low level laser therapy on the repair of bone defects grafted with inorganic bovine bone. Braz Dent J 14:177–181
Bartell SM, Kim HN, Ambrogini E, Han L, Iyer S, Serra Ucer S, Rabinovitch P, Jilka RL, Weinstein RS, Zhao H, O’Brien CA, Manolagas SC, Almeida M (2014) FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat Commun 5:3773
Lim HJ, Bang MS, Jung HM, Shin JI, Chun GS, Oh CH (2014) A 635-nm light-emitting diode (LED) therapy inhibits bone resorptive osteoclast formation by regulating the actin cytoskeleton. Lasers Med Sci 29:659–670
Bouvet-Gerbettaz S, Merigo E, Rocca JP, Carle GF, Rochet N (2009) Effects of low-level laser therapy on proliferation and differentiation of murine bone marrow cells into osteoblasts and osteoclasts. Lasers Surg Med 41:291–297
Fisher JE, Rogers MJ, Halasy JM, Luckman SP, Hughes DE, Masarachia PJ, Wesolowski G, Russell RG, Rodan GA, Reszka AA (1999) Alendronate mechanism of action: geranylgeraniol, an intermediate in the mevalonate pathway, prevents inhibition of osteoclast formation, bone resorption, and kinase activation in vitro. Proc Natl Acad Sci U S A 96:133–138
Rao LG, Mackinnon ES, Josse RG, Murray TM, Strauss A, Rao AV (2007) Lycopene consumption decreases oxidative stress and bone resorption markers in postmenopausal women. Osteoporos Int 18:109–115
Jiang F, Zhang Y, Dusting GJ (2011) NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63:218–242
Ishizuka H, Garcia-Palacios V, Lu G, Subler MA, Zhang H, Boykin CS, Choi SJ, Zhao L, Patrene K, Galson DL, Blair HC, Hadi TM, Windle JJ, Kurihara N, Roodman GD (2011) ADAM8 enhances osteoclast precursor fusion and osteoclast formation in vitro and in vivo. J Bone Miner Res 26:169–181
Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T (2002) Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell 3:889–901
Acknowledgments
This study was supported by research funds from Chosun University (2014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experimental procedures involving animals were compliant with institutional and governmental requirements and were approved by the Institutional Animal Care and Use Committee (CIACUC2014-A0023) of Chosun University, Gwangju, Korea.
Additional information
Hong Moon Sohn and Youngjong Ko contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sohn, H.M., Ko, Y., Park, M. et al. Comparison of the alendronate and irradiation with a light-emitting diode (LED) on murine osteoclastogenesis. Lasers Med Sci 32, 189–200 (2017). https://doi.org/10.1007/s10103-016-2101-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-016-2101-x