Abstract
The stain resistance and intrinsic hydrophobic features of wool are the most important problems which have negative impacts on various aspects of wool and prompt scientists to find a solution over the past decades. Zinc oxide nanoparticles (ZnO-np) were successfully synthesized and stabilized on natural protein wool using the impregnation process with butane tetra carboxylic acid (BTCA) as eco-friendly cross-linker. In this research, these particles were linked to wool surface by BTCA and also sodium hypophosphite was used as a catalyst. The self-cleaning technology of ZnO-np deposited on the wool fabrics was followed by degrading of Direct Blue 71 and Disperse Red 1 as synthetic stains and also determined by degrading rate of food stains such as coffee and black mulberry under the ultraviolet irradiation. The hydrophilicity was monitored by water drop absorption time. The central composite design was used for different variables based on Design of Expert software. The analysis of variance was utilized to obtain the optimum models for wool with maximum color reductions and minimum absorption time. The presence of ZnO-np on the wool surface was confirmed with scanning electron microscopy. Further transmission and absorbance spectra validated the UV protection properties of ZnO-np-treated wool. The results showed that increasing the amount of ZnO-np leads to enhanced reduction of synthetic color stains than food color stains on the treated wool fabric, while the fabric became more hydrophilic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Animal wool is well known for its outstanding breathability, bio-degradability, dyeability, and resilience which have made it a superior fiber (Chen et al. 2009). Increasing the photo-stability and antishrinkage of natural wool and imparting new features such as self-cleaning (Tung and Daoud 2011) and hydrophilicity to wool has attracted a great deal of attention (Mansour 2010). The shrinkage in wool is due to the scales on its surface which results in fiber entanglement (Shao and Fan 2012). In order to overcome such shrinkages in wool fabric, some formaldehyde-based cross-linkers have been reported, which enhance both felt proofing (Allam et al. 2009) and binding (Reddie and Nicholls 1971). The formaldehyde, however, has now been informed as a toxin, skin irritant (De Groot et al. 2010), and confirmed human carcinogen (World Health Organization and International Agency for Research on Cancer, World Health Organization, International Agency for Research on Cancer 2006). Unfortunately, toxic compounds are required to create the protection characteristics on wool. However, due to the increasingly stringent provisions and more awareness among the customers, some researchers have started focusing on the development of novel protection finishes, and bio-friendly treatments for wool. Recently, eco-scouring (Bahtiyari and Duran 2013), waterless dyeing (Long et al. 2013), reusing (Shams-Nateri 2011), recycling (Sikdar 2007), and sustainable treatment of wastewater (Visa et al. 2011), dyeing with ultrasonic energy (Mansour and Heffernan 2011), environmental management (Sikdar and Jain 2002), and optimization of proteases pre-treatment on natural dyeing of wool (Nazari et al. 2013a) have been investigated.
Self-cleaning technology (Peplow 2004) and hydrophilicity treatment (Montazer et al. 2011) of fibers by incorporating nanoparticles is a new concept that has been introduced in recent years. Nano-sized particles illustrate high photo-activity because of their large surface area per unit mass and also due to the diffusion of electrons/holes before recombination (Bozzi et al. 2005). Although intensive research has been conducted on self-cleaning technologies of cellulosic fabric for exploring the potential utilization of nanoparticles, little research has been informed on protein materials (Tung and Daoud 2009). Probably the utilization and development of such fibers had largely been impeded due to their limited chemical, thermal, and photo-stability (Arivithamani et al. 2014). To the best of our knowledge, there is no report related to stabilizing of ZnO-np with zero formaldehyde cross-linkers and its optimization via statistical approach of response surface methodology (RSM) in order to enhance self-cleaning technology and hydrophilicity of wool natural fabric.
The photo-catalytic characteristics of semi-conductors such as ZnO and TiO2 initiate decomposition of organic pollutants (Morris et al. 2004) such as acetaldehyde (Khalilzadeh and Fatemi 2014) and textile dyes (Meenatchisundaram et al. 2014) that come into contact with the surface and thus prevent them from building up. Zinc oxide is an important alternative oxide semiconductor photo-catalyst whose mechanism of photo-catalysis has been proven to be similar to that of TiO2 (Li et al. 2009). The most remarkable advantage of ZnO in comparison with TiO2 is its absorption of a greater fraction of UV spectrum with related threshold of 425 nm. Electron mobility in ZnO is higher than that in TiO2 making the former suitable for dye-sensitized solar cells. Recently, it has become possible to form vertical nanowires of ZnO (Greene et al. 2005). ZnO as a semiconductor with a large band gap (3.3 eV), big excitation binding energy (60 meV), and n-type conductivity, is abundant in nature and environmentally friendly (Nair et al. 2011). Zinc oxide is widely utilized in various fields such as surface acoustic wave filters, UV-blocking, gas sensors, photo-detectors, self-cleaning technology, purification of either wastewater or drinking water for human use due to its distinguished photo-catalytic, optical, electrical, electronic, dermatological, and antibacterial properties (Lin 2013), the photo-degradation of organic dyes, and mothproofing (Nazari et al. 2013b).
The multifunctional properties of poly carboxylic acids (PCA)-based zero formaldehyde cross-linkers such as environment friendly and high accessibility lead to their utilization on the textile surfaces (Kim et al. 2003). The cross-linking, antibacterial activity, and the simultaneous antimicrobial and cross-linking finish are among these uses (Nazari et al. 2009). Carboxylic groups of PCA bio-friendly cross-linkers are potential sites for binding of TiO2-np (Lugovskoy et al. 2008). The surfaces of wool fabrics should undergo an enrichment process that causes the introduction of these extra groups. Subsequently TiO2-np was increasingly attached to modified fabric surface by an electrostatic bond more than before. The results reported by Daoud confirmed that the introduction of additional carboxylic groups to natural wool surface allowed for efficient binding of TiO2 on the fabric surface which resulted in an enhancement of self-cleaning technologies (Tung and Daoud 2011). Bio-friendly cross-linker, BTCA, exhibited excellent multifunctional characteristics such as enhancing the adsorption, stability (Montazer and Seifollahzadeh 2011), and hydrophilicity (Montazer et al. 2011) of TiO2-np treated surface. This caused the effective increase of the efficiency of TiO2-np on characteristics such as self-cleaning technology, hydrophilicity, and UV protection. However, little valid researches have been published about the stabilization of ZnO-np on the textile surfaces through BTCA bio-cross-linker. Montazer et al. presented that by applying TiO2-np on wool fabrics, the rate of photo-yellowing decreased (Montazer and Pakdel 2010). In other words, because of the presence of TiO2 on the fabric surface, wool stability against UV irradiation increases (Cao et al. 2013). Semi-conductors such ZnO and TiO2-np are powerful UV absorbers that are able to protect fabrics against adverse effects of UV irradiation.
In a previous study (Nazari et al. 2013a), we described the natural wool fabrics pre-treated with protease enzyme at different concentrations during various times. The dyeing process is carried out on the treated fabrics with madder and cochineal natural dyes. This could significantly enhance madder and cochineal natural uptake. Therefore, the enhanced mothproofing activity of bio-degradable wool fabric was optimized with madder as a natural dye through one factor statistical analysis (Nazari et al. 2014). Lack of scientific reports on statistical modeling of ZnO-np along with environmentally friendly cross-linkers without formaldehyde on natural wool self-cleaning technology motivated the researchers of this research toward optimizing and stabilizing of ZnO-np on wool fabric through surface modification using BTCA bio-cross-linker. The purpose of this study was to optimize and enhance the influences of both ZnO-np and bio-cross-linker (BTCA) concentration on durable self-cleaning technologies and hydrophilicity of natural wool fabrics through evaluating color reduction and drop absorption time. RSM was used in order to conduct an exact analysis and the impact of each independent variable was reported on response surfaces.
Experimental
Materials
Sodium hypophosphite (SHP), sodium hydroxide, BTCA, isopropanol, zinc acetate, lithium hydroxide, and sodium carbonate were supplied by Merck Chemical Co., Germany. Non-ionic detergent (Rucogen DEN) composed of fatty alcohol ethoxylated was purchased from Rudolf Chemie Co., (Tehran, Iran). The 100 % raw wool fabric with twill structure, 374 g/m2, yarn count of 20/2 Nm, and wrap/weft density of 18/16 yarn/cm was purchased from Iran Merinous Co (Iran).
Instrument
The contents of finishing baths were prepared using an ultrasonic bath (200 V, 50 W, 40 kHz). The observations of scanning electron microscopic (SEM) on treated fabrics were performed using an EM-3200 electron microscope (KYKY, China). The transmission and absorbance spectra were recorded at UV region (200–400 nm) using UV/VIS spectrophotometer (CARRY 500 scan, Varian, Australia). Thermal oven was used to dry and cure the samples. The stained and treated samples were exposed to UV-A (320–400 nm) irradiation of an HPA 400S lamp (400 W, Philips, Belgium). The CIE color coordinates of samples were measured using reflectance spectrophotometer (color-guide sphere, D/8° spin, Germany).
Method
The wool fabric samples were prepared in 12 × 6 cm2 swatches and scoured with 1.5 % non-ionic detergent at 40 °C for 50 min at liquor to goods ratio of 30:1. The preparation process of employed ZnO-np has been described by researchers (Farouk et al. 2009). A two-neck round bottom distillation flask was used to suspend 2.8 g (zinc acetate 2H2O) in 100 mL isopropanol by reflux heating for 3 h. 0.75 g lithium hydroxide was dissolved in 100 mL isopropanol at room temperature by magnetic stirring. The zinc acetate suspension was cooled to 0 °C before the lithium hydroxide solution was added drop wise under vigorous stirring. The mixture was treated in an ultrasonic bath (200 V, 50 W, 40 kHz) at room temperature for about 2 h (Abd El-Hady et al. 2013).
The different aqueous solutions of BTCA bio-cross-linker with different concentrations of BTCA (5.00–10.00 % w/v) and SHP (3.00–6.00 % w/v) were prepared based on weight of bath: O.W.B (Table 1) using required distilled water. The scoured natural wool fabrics were impregnated in freshly prepared aqueous solutions at 55 °C for 50 min. The BTCA cross-linked fabrics were dried at 80 °C for 6 min and then cured at 140 °C for 4 min. Treated fabrics were rinsed with hot water and cold water and finally dried at room temperature. The suspended solutions of ZnO-np were firstly prepared by suspension of different concentration of ZnO-np prepared previously in isopropanol (0.1–1.50 % w/v based on O.W.B) and sonicated for 20 min (Table 1). The BTCA bio-cross-linked wool fabrics were impregnated in suspended solutions of ZnO-np at 55 °C for 20 min. The treated samples were then dried at 75 °C for 8 min and cured at 115 °C for 6 min. The cured fabrics were ultrasonically washed at 55 °C for 15 min in a solution of 1.0 g/L Na2CO3 and 1.0 g/L non-ionic detergent (Rucogen DEN) based on O.W.B in order to remove ZnO-np having no bonding reaction with wool fabrics and then dried at room temperature.
The self-cleaning technology of synthetic and food color stains was conducted through discoloration analysis of color stains after exposing to UV-A radiation for 25 min. The treated natural wool samples were placed on flat surface and then one drop of coffee, black mulberry, Direct Blue 71, and Disperse Red 1 (1 %) was dripped vertically on the surface using burette (50 mL), form 1 cm above the wool fabric. The color stain on the wool surfaces was created with approximate diameter of 1.50 cm. The color stained wools were then dried at room temperature. Virtually, the amounts of ΔE* for the blank and treated different wool samples were measured. Total color difference (ΔE*) as color reduction is determined by Eq. 1 on the basis of measured CIE color coordinates:
where ΔL * is the color lightness difference; Δa * is red/green difference; and Δb * is yellow/blue difference. Hence, the ΔE T * of stained samples were measured before and after exposing to UV-A (400 W). The degree of self-cleaning technology of different samples is compared by Eq. 2.
A drop of water from specific distance was placed on the surface of fabrics in order to evaluate the hydrophilic features of treated samples, and then spreading time of water drops was recorded by a stopwatch. This experiment was evaluated based on British standard 4554:1970 test method. The burning method was utilized to determine the actual ZnO-np loading on weight of fabric (O.W.F.). Weight-treated samples according to ASTM 5630, D 2584 were placed in a porcelain crucible and kept in an oven with a range of room temperature to 600 °C for 180 min. The calculations were conducted in such a manner that the remainder weight was subtracted from the remainder weight of the blank sample, and then the percentage of ZnO-np loading on wool based on O.W.F was obtained. Washing durability of treated samples was conducted according to AATCC test method 61(2A)-2003. In this approach, each cycle of washing process is equivalent to five home laundries in ambient conditions at 38 ± 3 °C.
Design of experimental (DOE)
The CCD was used for experimental plan with three variables. These variables included the amounts of SHP catalyst, BTCA bio-cross-linker, and ZnO-np co-catalyst concentrations. The ranges of these variables are: SHP (3.00–6.00 % w/v), BTCA (5.00–10.00 % w/v), and ZnO-np (0.10–1.50 % w/v) using trial version of Design Expert 8.0.1.0 software from Stat-Ease, Inc. (USA). The details of the design of natural wool samples with BTCA bio-cross-linker and SHP catalyst in the presence of ZnO-np are presented in Table 1 (Run 1–17). Also the influence of the variables on the results Y 1 (ΔE *) and Y 2 (time of water absorption) was adjusted using the following third-order polynomial function:
In this equation, b 0 is an independent term according to the mean value of the experimental plan, b i is regression coefficients that explain the influence of the variables in their linear form, b ij is regression coefficients of the interaction terms between variables, and ci is the coefficients of quadratic form of variables.
Results and discussion
Hydrophilicity features
The results indicate that the water drop absorption time for the ZnO-np/BTCA wool samples decreased significantly compared with control sample (Table 1). Some researches which have been mostly evaluated in papers published relating to wool and its surface modification has been summarized in Table 2.
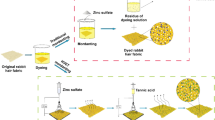
In this study, the hydrophilicity of natural wool samples increased through ZnO-np and BTCA bio-friendly treatment. It can also be obviously seen in Table 1 that by increasing the amount of ZnO-np up to 0.80 %, the amounts of water absorption time decreased (as Run: 8 and 9 or 2 and 17), while with constant concentration of ZnO-np and gradual increase of BTCA in impregnated bath, the amounts of water absorption time increase (as Run: 7, 8, 11, 17). In this research, the ZnO-np were bonded to wool through free carboxylates anions of BTCA bio-cross-linker. ZnO-np have high affinity towards hydroxyl and carboxyl groups of carboxylic acids. The pH of impregnated bath was around 4.5 which led to positive charges of ZnO-np; subsequently their tendency towards carboxyl groups of BTCA with negative charge increased. This caused a higher absorption of ZnO-np on the wool surface as illustrated in Scheme 1 (Nazari 2014). Although the mechanism of the suggested Scheme 1 has not been investigated experimentally, but other similar mechanisms (Behzadnia et al. 2014) may also be presented in the literature (Yuranova et al. 2007).
Similarly, the surface hydroxyl molecules density had an excellent effect on improving the hydrophilicity producing more hydrogen bonds with molecules of waters (Euvananont et al. 2008). Therefore, it is reported that the contact angles can be decreased through light irradiation and water can be absorbed faster on the surface of wool fabric (Montazer et al. 2011).
Self-cleaning technologies
The influence of BTCA bio-cross-liker for stabilizing ZnO-np on the surfaces of wool fabric on color reductions (ΔE *T ) of the treated wool samples under UV-A (400 W) irradiation is summarized in Table 1. Figure 1 demonstrated that degradation activity was more in ZnO/BTCA treated samples than in control ones. In samples treated with moderated concentrations of ZnO, the color stains seemed lighter after 25 min of UV-A irradiation (Run: 2, 3, 5, 6) and were almost completely removed (Run: 8) because the amount of ZnO-np on the surface of treated sample is enough, whereas in control samples, the color stains were clearly observed on the fabric surface. This result showed that ZnO-np can degrade synthetic and food color stains on wool fabrics. Conversely this feature was not significant in control wools.
Therefore, the low reduction of natural and synthetic color stains under UV-A irradiation appeared for control ones (Table 1). Similar behavior was obtained for variation of color differences (ΔE *T ) for each of natural and synthetic color stains based on different concentrations of ZnO-np and BTCA bio-cross-liker. ΔE *T values of finished fabrics gradually increased with the increase of ZnO-np at constant BTCA as it could be presented for set of each following Runs (Run: 2, 3, 10, 12, 14, 17, Run: 5, 7, 13, Run: 4, 6, and Run: 1, 8, 9, 15,16). This could be due to the presence of more ZnO-np on the natural wool samples that absorb UV irradiations more effectively. In other words, ZnO-np are capable of efficiently absorbing UV wavelengths and also more production of active radicals (•OH) and consequently effective degrading the color stains produced by Disperse Red 1, Direct Blue 71, black mulberry, and coffee. This may be attributed to the suitable energy produced by UV irradiations for transforming the electrons on ZnO-np surfaces from valence band to the conduction band. The famous reactions for the production of electrons and holes created on the ZnO-np as a result of UV irradiation and consequently the oxidation and reduction reactions for color photo-degradation and also self-cleaning technology mechanism were illustrated by researchers (Kandula and Jeevanandam 2014).
The color reduction activity of ZnO-np on wool fiber was attributed to the dispersed ZnO-np which were satisfactorily stabilized on the fiber surface. Conduction-band electrons and valence-band holes would be generated on its surface during photo-catalysis when ZnO-np was illuminated by UV irradiations with energy greater than its band gap energy Eq. (4). Holes could then react with water molecules adhering to the surface of the photo-catalyst to form highly reactive hydroxyl radicals (•OH) Eq. (5). Here Oxygen acted as an electron acceptor by forming a super-oxide radical anion (•O2 −) on the catalyst surface Eq. (6). The super-oxide radical anions may act as oxidizing agents or as an additional source of hydroxyl radicals via the subsequent formation of hydrogen peroxide. The powerful oxidants associated with hydroxyl radicals were able to oxidize color materials Eq. (7).
The performance of ZnO-np slightly decreased at 1.50 % ZnO-np. This could be due to the satiating of functional groups existed in the BTCA bio-cross-liker, the agglomeration of ZnO-np on the surface of wool fabric or in the finishing bath and consequently decreased the efficiency of ZnO-np photo-degradation. Therefore, using experimental design to find out the effects of each variable and their interactions and also obtaining the optimum color reduction conditions on ZnO-np/BTCA bio-cross-linked wool fabrics is very beneficial.
In addition, Table 1 represented the increase of color reduction values at constant ZnO-np and more BTCA bio-cross-linker as it could be seen for set of each following Runs (Run: 1, 3, 5, 12, 15, Run: 2, 9, 10, 13, 14, 16, and Run: 7, 8, 11, 17). The BTCA bio-cross-linking led to the higher adsorption and stabilization of ZnO-np and lower release of ZnO-np from the cross-linked wool surface of fabric. This possibly increased the degradation activity of ZnO/BTCA cross-linked wool fabrics for discoloring of food and synthetic color stains. Therefore, the formation of anionic multifunctional structure resulted from the function of BTCA bio-cross-linker acted as a stabilizing agent of ZnO-np on the surface of natural wool fabric. Interestingly, the performance of ZnO-np in the degradation of coffee color stains was slightly decreased at concentrations greater than 7.50 % BTCA as the decrease of ΔE *T values was observed. Protein fibers capable of changing light to self-cleaning technology for degrading its color stains, dirt, and harmful microorganisms in a process of photo-catalytic purification are very interesting materials for various applications (Tung and Daoud 2009). So BTCA bio-cross-linker not only restricted the self-cleaning technology but also had a noticeable influence on the degradation of natural and synthetic color stains. Consequently BTCA-based zero formaldehyde cross-linkers accompanying with ZnO-np had a complementary effect on the treated wool fabrics that exhibited a considerable increase in the durable self-cleaning technologies. The self-cleaning technology developed in this research, eliminated the necessity of frequent washing of wool fabric in order to remove synthetic and natural color pollutants. In a way that frequent washing along with increasing temperature had detrimental effect on the strength and appearance of woolen textiles; also it is assumed as high cost and time-consuming process. Consequently, the obtained durable self-cleaning technology had accessibility for industrial applications, while eliminating the utilization of formaldehyde cross-linkers and providing antishrinkage and self-cleaning suitable technologies for woolen fabric. However, economical processes relating to this research require more examining and optimizing based on the economic cost and benefit accordance with industrial scales.
Statistical analysis
RSM is a powerful statistical technique for testing multiple variables (Box et al. 1978) because of fewer required experimental trials compared to the ‘‘one-factor-at-a-time’’ method (Montgomery 2004). This research was conducted based on CCD and RSM. Seventeen experimental CCD designed runs were conducted according to Table 1. In this model, the impacts of independent variables including ZnO-np, SHP, and BTCA concentration on response surfaces were assessed. It explained water drop absorption time and color differences (ΔE *T ) of ZnO-np/BTCA cross-linked wool samples stained with Direct Blue 71 and Disperse Red 1 as color synthetic stains and coffee and black mulberry as food color stains under the ultraviolet irradiation. Several statistical models were obtained based on results of relations between surface responses and independent factors whose related responses were functions of its independent variables.
Statistical models related to water drop absorption time and color reductions (ΔE *T ) of stained wool fabrics with Direct Blue 71, Disperse Red 1, coffee, and black mulberry are presented in Eqs. (8–12):
Response surfaces were drawn via achieved statistical models (Eqs. 8–12) and the relation between independent variables and accordingly drop absorption time and color reductions (ΔE *T ) of synthetic and food color stains were found. Therefore, the response surfaces of drop absorption time and ΔE *T of stained wool fabrics with Direct Blue 71, Disperse Red 1, coffee, and black mulberry are suggested in Fig. 2a–e. It is recognized that BTCA bio-cross-linker plays a greater role in increasing of color reductions (ΔE *T ) in comparison with ZnO-np.
Figure 2a–e represented the response surfaces of model for (a) absorption time and color reductions (ΔE *) of stained wool samples with (b) Direct Blue 71, (c) Disperse Red 1, (d) black mulberry, and (e) coffee. The optimum condition of hydrophilicity and self-cleaning each of synthetic and food color stains was evaluated by Design of Expert software and are shown in Table 3. Thus, a significant lightening of color was observed in optimized samples after 25 min of UV-A irradiation (Fig. 3). In a way that obtained results from the performance of ZnO-np at optimized condition for color reduction and self-cleaning is nearly equal with using TiO2-np (Montazer and Pakdel 2011) and lower than results obtained from TiO2/Ag nanocomposite (Montazer et al. 2012) accompany with poly carboxylic cross-linking agent on wool fabrics.
ANOVA was used to analyze the data and obtain the interaction between process of independent variables and responses. The results were then evaluated by ANOVA to assess the ‘‘goodness of fit’’ (Tables 4, 5, 6, 7, 8).
The lack of fit described the variation of data around the fitted model (Sugashini and Meera Sheriffa Begum 2013). If the model did not fit the data well, this would not be significant (Vedaraman et al. 2013). It was observed that designed model for water absorption time of treated wool samples was statistically significant at F value of 36.63 and values of prob > F (<0.0001) (Table 4). Therefore, it was concluded that designed models for self-cleaning technology of stained wool with Direct Blue 71 and Disperse Red 1 were statistically significant at F value of 67.45 and 51.05 and values of prob > F (<0.0001), respectively (Tables 5, 6). Also it was observed that chosen models of stained wool samples for self-cleaning technology with black mulberry and coffee were significant at F value of 600.09 and 153.53 and values of prob > F (<0.0001), respectively (Tables 7, 8).
The fit of the model could be evaluated through R-squared coefficients. The results of Table 4 indicated that the only 2.08 % of total variables for water absorption time model of treated wool samples could not be explained by this model (Navid et al. 2014). Also the results of Tables 5 and 6 indicated that the only 1.14 and 1.50 % of total variables for self-cleaning technology models of Direct Blue 71 and Disperse Red 1 stained wool samples, respectively, could not be explained by these models (Navid et al. 2014). Therefore, R-squared of self-cleaning technology models of black mulberry and coffee stained wool samples could not explain 0.13 and 0.50 % of total variables, respectively (Tables 7, 8). A lower value of coefficient of variation (CV %) showed that conducted experiments were precise and reliable especially for the self-cleaning technology model of black mulberry stained samples (CV = 2.74 %) which indicated a good precision and reliability of experiments (Rashid et al. 2013).
Durability of washing
The washing durability of ZnO-np/BTCA wool fabrics treated after washing test was measured. The color reduction (ΔE *T ) results are summarized in Table 9 and color variations are presented in Fig. 3.
The self-cleaning technology obtained in the optimized conditions exhibits a higher washing durability. It is because of sufficient existence of BTCA bio-cross-linker on fabric surface which was capable of stabilizing ZnO-np on the bio-cross-linked wool samples and consequently caused the increase of their durability against wash cycles. There existed an ionic interaction between groups of carboxylate anionic related to BTCA poly carboxylic acid and ZnO-np cation. Similar kind of interaction has been previously reported in the presence of TiO2-np photo-catalyst and poly carboxylic acid (Yuranova et al. 2007). It turned out that partial discoloration of CI Basic Blue 9 as a stain on the cross-linked cationized and conventional cotton with BTCA bio-cross-linker under UV and daylight irradiation presented a long-term stable performance for TiO2-np (Nazari et al. 2011). The BTCA was bio-cross-linker that had the ability to improve the absorption of more ZnO-np on the natural wool surface since they contained four functional groups. The mechanism of electrostatic linkages between ZnO-np with BTCA and ester linkages between BTCA with wool was suggested in Scheme 1. Therefore, the reason of the small decrease in self-cleaning technology efficiency after washing durability test could be attributed to the removal of the few ZnO-np available on the cross-linked wool surface as a result of evaluating washing durability. Therefore, the ZnO-np/BTCA cross-linked wool contained the great durability property including ideal self-cleaning technology in the optimized conditions. Also the percentage of actual content of ZnO-np (based on O.W.F) loaded on wool samples was measured using burning test and the results are presented in Table 10. It is clear that actual percentage of created weight increase for treated wool sample is 2.13 % at optimized condition (before washing durability test) but after the washing durability test, insignificant decrease is observed in actual content percentage of ZnO-np (2.08 %). In a way that main part of ZnO-np at optimized condition preserved themselves on treated wool sample with strong connection. This consideration confirms that using BTCA with statistical optimized concentration is capable of absorption and stabilization increase of ZnO-np on wool surfaces. Consequently it was observed that BTCA bio-cross-linker could perform as ZnO-np stabilizer and preserved more ZnO-np on the surface of the cross-linked wool fabrics.
SEM images
Figure 4a–d demonstrated the SEM images of (a) control wool, (b) sample Run: 2, treated at optimized condition (c) before, and (d) after wash durability test. SEM analysis of control and treated samples (Fig. 4a–d) confirmed that the surfaces of treated samples were covered by ZnO-np. Because of small dimension properties, high surface activity, and large specific surface area ZnO-np had a tendency to be agglomerated easily (Zhang and Yang 2009). Bigger particles could be easily removed from the fabric surface, while the smaller particles could penetrate into the fabric matrix and adhered stronger to the fabric surface. These images showed the more ZnO-np loading on the treated sample at optimized condition (Fig. 4c) than the other samples. Therefore, the small decrease of ZnO-np presence was observed on the surface of sample Fig. 4 d than the sample Fig. 4c. This could be due to the removal of some ZnO-np from the wool surface after the process of wash durability evaluation. BTCA bio-cross-linker significantly maintained the majority of ZnO-np on the cross-linked wool surfaces even after completing the evaluation of washing durability test and this showed achieving of durable self-cleaning technology and hydrophilicity wool fabrics. Also SEM image of sample (Run: 1) with the highest amount of ZnO-np (1.50 %) shows the agglomeration of ZnO-np on the wool fabric which leads to the reduction of their efficiency (Fig. 4e). However, BTCA-based zero formaldehyde cross-linkers not only cross-linked between protein chains of wool structure as a poly carboxylic, but also caused the stabilization of ZnO-np on the surface of natural wool fabric.
UV transmission and absorbance spectra
The UV transmission, absorbance spectra of control, treated wool with 0.10 % ZnO-np, 5.00 % BTCA, and 3.00 % SHP (Run: 2), and 0.89 % ZnO-np, 10.00 % BTCA, and 6.00 % SHP (optimized condition) in the UV region (200–400 nm) are presented in the Figs. 5, and 6, respectively. The wool samples treated with BTCA and ZnO-np had low UV transmission especially in UVB region (290–315 nm) and UVA region (315–400 nm) due to the UV absorption of ZnO-np. The lowest transmission was related to the treated wool at the optimized condition. Therefore, the absorbance extents of the treated wool samples (Figs. 5b and 5c) were more than control sample (Fig. 5a). This image clearly demonstrated that the most absorption through ZnO-np/BTCA-treated wools occurred in the UV regions (200–400 nm). Therefore, ZnO-np absorbed the light with the wavelengths lower than 400 nm and the obtained treated wool sample indicated UV absorption property. ZnO-np acted primarily as a UV absorber resulting in a slower UV transmission rate, and the results are consistent with previous findings.
Conclusion
This research intended to modify the bio-degradable wool surface to enhance ZnO-np adsorption and stabilize them on the fabric surface using BTCA as an eco-friendly cross-linker to produce a fabric with enhanced self-cleaning technology and hydrophilicity property. The increase in the bond ability of ZnO-np on the wool surface was investigated by surface modification induced by introducing extra hydroxyl and carboxyl groups using BTCA bio-cross-linker and SHP as a catalyst. According to the statistical analysis, the bio-cross-linked fabrics with BTCA and ZnO-np easily enable degradation of the synthetic and food color stains, while the treated wools became more hydrophilic. It can be concluded that at a given concentration of ZnO-np, BTCA bio-cross-linker, and catalyst, the color reduction (ΔE *) values of stained wool samples with the Direct Blue 71 and Disperse Red 1 are higher than those of coffee, black mulberry. Consequently BTCA bio-cross-linker with ZnO-np had a complementary effect on treated wool samples which demonstrated a noticeable increase in the durable self-cleaning technology and hydrophilicity property. It is also completely formaldehyde free, easily available, and soluble.
References
Abd El-Hady MM, Farouk A, Sharaf S (2013) Flame retardancy and UV protection of cotton based fabrics using nano ZnO and polycarboxylic acids. Carbohydr Polym 92:400–406
Allam OG, El-Sayed A, Kantouch A, Haggag K (2009) Use of sericin in felt proofing of wool. J Nat Fibers 6:14–26
Arivithamani N, Mary SA, Kumar MS, Giri Dev VR (2014) Keratin hydrolysate as an exhausting agent in textile reactive dyeing process. Clean Technol Environ Policy 16:1207–1215
Bahi A, Jones JT, Carr CM, Ulijin RV, Shao J (2004) Surface characterization of chemically modified wool. Text Res J 77:937–945
Bahtiyari MI, Duran K (2013) A study on the usability of ultrasound in scouring of raw wool. J Clean Prod 41:283–290
Behzadnia A, Montazer M, Rashidi A, Rad MM (2014) Sonosynthesis of nano TiO2 on wool using titanium isopropoxide or butoxide in acidic media producing multifunctional fabric. Ultrason Sonochem 21:1815–1826
Box GEP, Hunter WG, Hunger JS (1978) Statistics for experimenters: an introduction to design, data analysis and model building, 1st edn. Wiley, New York
Bozzi A, Yuranova T, Guasaquillo I, Laub D, Kiwi J (2005) Self-cleaning of modified cotton textiles by TiO2 at low temperatures under daylight irradiation. J Photochem Photobiol A Chem 174:156–164
Brown AE, Hornstein LR, Harris M (1951) The chemical modification of wool-treatment with formaldehyde solutions. Text Res J 21:222–227
Cao Q, Yu Q, Connell DW, Yu G (2013) Titania/carbon nanotube composite (TiO2/CNT) and its application for removal of organic pollutants. Clean Technol Environ Policy 15:871–880
Chen D, Tan L, Liu H, Hu J, Li Y, Tang F (2009) Fabricating superhydrophilic wool fabrics. Langmuir 26:4675–4679
De Groot A, Le Coz C, Lensen G, Flyvholm M, Maibach H, Coenraads P (2010) Formaldehyde-releasers: relationship to formaldehyde contact allergy. Formaldehyde-releasers in clothes: durable press chemical finishes. Part 1. Contact Dermatitis 62:259–271
Demir A, Karahan HA, Ozdogan E, Oktem T, Sventekin N (2008) The synergetic effects of alternative methods in wool finishing. Fibers Text East Eur 16:89–94
Euvananont C, Juinin C, Inpor K, Limthonkul P, Thanachayanont C (2008) TiO2 optical coating layers for self-cleaning applications. Ceram Int 34:1067–1071
Farouk A, Textor T, Schollmeyer E, Tarbuk A, Marija A (2009) Sol–gel derived inorganic–organic hybrid polymer filled with nano-ZnO as ultraviolet protection finish for textiles. Autex Res J 9:114–120
Greene LE, Law M, Tan DH, Montano M, Goldberger J, Somorjai G, Yang P (2005) General route to vertical ZnO nanowire arrays using textured ZnO seeds. Nano Lett 5:1231–1236
Hsieh SH, Huang ZK, Huang ZZ, Tseng AZ (2004) Antimicrobial and physical properties of woolen fabrics cured with citric acid and chitosan. J Appl Polym Sci 94:1999–2007
Kandula S, Jeevanandam P (2014) Visible-light-induced photodegradation of methylene blue using ZnO/CdS heteronanostructures synthesized through a novel thermal decomposition approach. J Nanopart Res 16:2452–2459
Kantouch A, Bendak A, Sadek M (1978) Studies on the shrink-resist treatment of wool with potassium permanganate. Text Res J 48:619–624
Khalilzadeh A, Fatemi S (2014) Modification of nano-TiO2 by doping with nitrogen and fluorine and study acetaldehyde removal under visible light irradiation. Clean Technol Environ Policy 16:629–636
Kim YH, Nam CW, Choi JW, Jang J (2003) Durable antimicrobial treatment of cotton fabrics using N-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride and polycarboxylic acids. J Appl Polym Sci 88:1567–1572
Li YS, Jiao ZH, Yang N, Gao H (2009) Regeneration of nano-ZnO photocatalyst by the means of soft-mechanochemical ion exchange method. J Environ Sci 21:S69–S72
Lin YC (2013) Applying Ag–TiO2/functional filter for abating odor exhausted from semiconductor and opti-electronic industries. Clean Technol Environ Policy 15:359–366
Long J, Cui C, Wang L, Xu H, Yu Z, Bi X (2013) Effect of treatment pressure on wool fiber in supercritical carbon dioxide fluid. J Clean Prod 43:52–58
Lugovskoy S, Nisnevitch M, Zinigrad M, Wolf D (2008) Mechanochemical synthesis of salicylic acid–formaldehyde chelating co-polymer. Clean Technol Environ Policy 10:279–285
Mansour HF (2010) Environment and energy efficient dyeing of woollen fabric with sticta coronate. Clean Technol Environ Policy 12:571–578
Mansour HF, Heffernan S (2011) Environmental aspects on dyeing silk fabric with sticta coronata lichen using ultrasonic energy and mild mordants. Clean Technol Environ Policy 13:207–213
Meenatchisundaram S, Devaraj M, Rai CL, Nadarajan KM (2014) An integrated approach for enhanced textile dye degradation by pre-treatment combined biodegradation. Clean Technol Environ Policy 16:501–511
Montazer M, Pakdel E (2010) Reducing photoyellowing of wool using nano TiO2. Photochem Photobiol 86:255–260
Montazer M, Pakdel E (2011) Self-cleaning and color reduction in wool fabric by nano titanium dioxide. J Tex Ins 102:343–352
Montazer M, Seifollahzadeh S (2011) Enhanced self-cleaning, antibacterial and UV protection properties of nano TiO2 treated textile through enzymatic pretreatment. Photochem Photobiol 87:877–883
Montazer M, Pakdel E, Bameni Moghadam M (2011) The role of nano colloid of TiO2 and butane tetra carboxylic acid on the alkali solubility and hydrophilicity of proteinous fibers. Colloids Surf A 375:1–11
Montazer M, Pakdel E, Bameni Moghadam M (2012) Superior self-cleaning features on wool fabric using TiO2/Ag nanocomposite optimized by response surface methodology. J Appl Polym Sci 125:E356–E363
Montgomery DC (2004) Design and analysis of experiments, 6th edn. Wiley, New York
Morris RE, Krikanova E, Shadman F (2004) Photocatalytic membrane for removal of organic contaminants during ultra-purification of water. Clean Technol Environ Policy 6:96–104
Nair G, Nirmala M, Rekha K, Anukaliani A (2011) Structural, optical, photo catalytic and antibacterial activity of ZnO and Co doped ZnO nanoparticles. J Mater Sci Lett 65:1797–1800
Navid P, Khoshgoftar Manesh MH, Marigorta AMB (2014) Optimal design of cogeneration system based on exergoenvironmental analysis. Clean Technol Environ Policy 16:1045–1065
Nazari A (2014) Proteases pretreatment on wool to enhance durable antimicrobial and UV protection with nano TiO2 and polycarboxylic acids using RSM. J Tex Ins 105:620–630
Nazari A, Montazer M, Rashidi A, Yazdanshenas M, Anary-Abbasinejad M (2009) Nano TiO2 photo-catalyst and sodium hypophosphite for cross-linking cotton with poly carboxylic acids under UV and high temperature. Appl Catal A Gen 371:10–16
Nazari A, Montazer M, Bameni-Moghadam M, Anary-Abbasinejad M (2011) Self-cleaning properties of bleached and cationized cotton using nanoTiO2: A statistical approach. Carbohydr Polym 83:1119–1127
Nazari A, Montazer M, Afzali F, Sheibani A (2013a) Optimization of proteases pretreatment on natural dyeing of wool using response surface methodology. Clean Technol Environ Policy 16:1081–1093
Nazari A, Montazer M, Dehghani-Zahedani M (2013b) Mothproofing of wool fabric utilizing ZnO nanoparticles optimized by statistical models. J Ind Eng Chem. doi:10.1016/j.jiec.2013.12.112
Nazari A, Montazer M, Dehghani-Zahedani M (2014) Simultaneous dyeing and mothproofing of wool against Dermestes maculatus with madder optimized by statistical model. Clean Technol Environ Policy. doi:10.1007/s10098-014-0745-4
Peplow M (2004) Self-cleaning clothes. Nature 429:620
Rashid K, Suresh Kumar Reddy K, Al Shoaibi A, Srinivasakannan C (2013) Sulfur-impregnated porous carbon for removal of mercuric chloride: optimization using RSM. Clean Technol Environ Policy 15:1041–1048
Reddie RN, Nicholls CH (1971) Some reactions between wool and formaldehyde. Text Res J 41:841–852
Shams-Nateri A (2011) Reusing wastewater of madder natural dye for wool dyeing. J Clean Prod 19:775–781
Shao J, Fan Q (2012) Eco dyeing, finishing and green chemistry. Adv Mat Res 441:33–37
Sikdar S (2007) Sustainability and recycle–reuse in process systems. Clean Technol Environ Policy 9:167–174
Sikdar S, Jain R (2002) Environmental management is inherently multi-disciplinary. Clean Technol Environ Policy 3:335
Sugashini S, Meera Sheriffa Begum KM (2013) Optimization using central composite design (CCD) for the biosorption of Cr(VI) ions by cross linked chitosan carbonized rice husk (CCACR). Clean Technol Environ Policy 15:293–302
Tung WS, Daoud WA (2009) Photocatalytic Self-cleaning Keratins: a feasibility study. Acta Biomater 5:50–56
Tung WS, Daoud WA (2011) Self-cleaning fibers via nanotechnology: a virtual Reality. J Mater Chem 21:7858–7869
Vedaraman N, Shamshath Begum S, Srinivasan SV (2013) Response surface methodology for decolourisation of leather dye using ozonation in a packed bed reactor. Clean Technol Environ Policy 15:607–616
Visa M, Pricop F, Duta A (2011) Sustainable treatment of wastewaters resulted in the textile dyeing industry. Clean Technol Environ Policy 13:855–861
World Health Organization, International Agency for Research on Cancer (2006) Monographs on the evaluation of carcinogenic risks to humans, vol 88. World Health Organization, International Agency for Research on Cancer, Lyon, p 277–280
Yuranova T, Laub D, Kiwi J (2007) Synthesis, activity and characterization of textiles showing self-cleaning activity under daylight irradiation. Catal Today 122:109–117
Zhang F, Yang J (2009) Preparation of nano-ZnO and its application to the textile on antistatic finishing. Int J Chem 1:18–22
Acknowledgments
I would like to express my appreciation to Yazd Branch, Islamic Azad University for its support of research project under the title of enhancing the stability of ZnO-np in photo-degradation of natural and synthetic stains of wool fabrics using cross-linking agent.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shirgholami, M.A., Nazari, A. & Mirjalili, M. Statistical optimization of self-cleaning technology and color reduction in wool fabric by nano zinc oxide and eco-friendly cross-linker. Clean Techn Environ Policy 17, 905–919 (2015). https://doi.org/10.1007/s10098-014-0842-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0842-4