Abstract
ZnO/CdS heteronanostructures with different shell thicknesses (20–45 nm) have been successfully synthesized by a novel thermal decomposition approach, and the synthesis involves three steps. In the first step, ZnO nanorods were synthesized by homogeneous precipitation method. Then, the surface of ZnO nanorods was functionalized using citric acid as the surface-modifying agent. Finally, the cadmium sulfide (CdS) shell was deposited on the surface-modified ZnO nanorods by the thermal decomposition of cadmium acetate and thiourea in ethylene glycol at 180 °C. The ZnO/CdS heteronanostructures were characterized using X-ray diffraction, infrared spectroscopy, thermal gravimetric analysis, scanning electron microscopy (SEM), transmission electron microscopy (TEM), diffuse reflectance spectroscopy (DRS), and photoluminescence spectroscopy. SEM and TEM results indicate the deposition of CdS shell on the ZnO nanorods, and DRS results show blue shift of CdS band gap absorption with respect to bulk CdS. PL results show evidence for synergistic interaction between ZnO and CdS. The ZnO/CdS heteronanostructures were explored as catalyst for visible-light-induced photocatalytic degradation of methylene blue in an aqueous solution. The ZnO/CdS samples show higher photocatalytic activity for the degradation of methylene blue compared with pure ZnO nanorods and CdS nanoparticles. The possible mechanism for the formation of CdS shell on the ZnO nanorods and the mechanism for photodegradation of methylene blue are explained in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Core–shell nanoparticles have potential applications in areas such as optoelectronics, catalysis, and biology (Nie et al. 2012; Wang et al. 2013a; Schladt et al. 2012; Ghosh and Paria 2012). The important reasons for coating one material over another are that the coating can alter the charge, functionality, and reactivity of the surface, and it can enhance thermal, mechanical, and chemical stability of the materials (Ghosh and Paria 2012). A number of researchers have made tremendous effort to use semiconductor materials in photocatalysis, environmental remediation, water splitting, solar cells, and sensors (Xiao et al. 2014; Latthe et al. 2013; Li et al. 2013a, 2014; Galoppini et al. 2006; Kadir et al. 2014). Metal oxide semiconductors such as ZnO (Becker et al. 2011), TiO2 (Ren et al. 2014), and α-Fe2O3 (Xia and Yin 2013) have been used as efficient photocatalysts. Among metal oxide semiconductors, ZnO is the most widely used one and it is an intrinsic semiconductor with a band gap of about 3.38 eV, and high exciton binding energy (60 meV) (Khanchandani et al. 2012). In the past few years, a lot of efforts have been made by researchers for the synthesis of ZnO nanoparticles with different morphologies and sizes. One-dimensional nanostructures (e.g., nanowires, nanorods, nanoneedles, nanotubes, columns, and helices) play an important role in many applications because they possess high surface to volume ratio with short diffusion length (Kim et al. 2008; Liao et al. 2007; Kuo et al. 2007; Wang 2004). The one-dimensional nanostructures provide a direct pathway for charge transport since they have large junction area which imparts low reflectance (Misra et al. 2013). Also, these materials reduce the recombination of excitons and thus show high energy conversion efficiency.
Since ZnO has a wide band gap, its use is restricted to the visible region. To enable the use of semiconductors in the visible region, the wide band gap semiconductors have been coupled with semiconductors with narrow band gap such as CdS, ZnS, PbS, Ag2S, Bi2S3, InP, ZnSe, and CdSe (Xu et al. 2009a; Huang et al. 2012; Zhu et al. 2011; Wu et al. 2011; Tena et al. 2007). Among the narrow band gap semiconductors, CdS is an excellent choice since it has a band gap of about 2.42 eV, and also the conduction band edge of CdS (−4.1 eV) is slightly higher than that of ZnO (−4.3 eV) (Guerguerian et al. 2011). Also, ZnO and CdS have nearly same lattice constants. These characteristics have enabled achieving good efficiency in different applications like photocatalysis, solar cells, etc. (Khanchandani et al. 2012; Guerguerian et al. 2011).
ZnO@CdS core–shell nanoparticles belong to type-II semiconductors, and charge injection from one semiconductor to another leads to an efficient charge separation due to reduction in the exciton recombination (Tak et al. 2008). One of the excitons is confined to the core, and the other one is confined to the shell. Tak et al. 2008, Khanchandani et al. 2012, and Li et al. 2013b have demonstrated applications of ZnO@CdS core–shell nanoparticles in environmental remediation such as degradation of orange-II, rhodamine B, and eriochrome black T. Gao et al. 2005, Zhai et al. 2010, and Villani et al. 2012 have explored their applications in sensing. Guerguerian et al. 2011, and Cui et al. 2013 have explored the application of ZnO@CdS core–shell nanoparticles in solar cells.
Different synthetic routes have been reported in the literature for the synthesis of ZnO@CdS core–shell nanoparticles. They include precipitation method (Khanchandani et al. 2012), successive ionic layer adsorption and reaction (Guerguerian et al. 2011; Cui et al. 2013), chemical bath deposition (Misra et al. 2013; Tak et al. 2008; Li et al. 2013c), sonochemical synthesis (Gao et al. 2005; Zhai et al. 2010; Geng et al. 2011), hydrothermal synthesis (Fang et al. 2008), electrochemical deposition (Li et al. 2013c), and sol–gel method (Panda et al. 2004). In the present study, ZnO/CdS heteronanostructures have been successfully synthesized by a simple, economical, and novel thermal decomposition approach. The CdS shell thickness in ZnO/CdS heteronanostructures could be controlled by varying the concentrations of precursors used during the thermal decomposition. The ZnO/CdS heteronanostructures were thoroughly characterized and thereafter, they were explored as visible-light photocatalysts for the degradation of methylene blue in aqueous solution.
Experimental
Reagents
Zinc nitrate (99 %, Himedia, AR), urea (99.5 %, Rankem, AR), citric acid (99 %, Himedia, LR), cadmium acetate (99 %, Himedia, AR), thiourea (99 %, Rankem, LR), ethylene glycol (99 %, Rankem, LR), methylene blue (SRL), terephthalic acid (99 %, Himedia, AR), and sodium hydroxide (98 %, Rankem, AR) were used as-received, and methanol (99 %, SD Fine) was distilled before further use. The ZnO/CdS heteronanostructures were synthesized in three steps and the detailed procedure is as follows.
Synthesis of ZnO nanorods
The ZnO nanorods were synthesized by homogeneous precipitation method as reported in the literature (Bitenc et al. 2009a). The concentration of reagents used was 0.05 M zinc nitrate and 0.25 M urea in a mixture of ethylene glycol and water (1:1 vol%). In a typical synthesis, 100 mL of 0.05 M zinc nitrate solution was mixed with 100 mL of 0.25 M urea solution, and the contents were stirred at 90 °C. After completion of 5 h, a white precipitate was obtained. The contents were cooled to room temperature, filtered, washed with water, and allowed to dry in an oven at 70 °C for overnight.
Surface modification of ZnO nanorods
The surface modification of ZnO hexagonal nanorods was carried out using citric acid as the surface-modifying agent. Citric acid was chosen since it has been successfully used as the surface-modifying agent for ZnO (Khanchandani et al. 2012; Bishop et al. 2012). About 1 mM of ZnO powder (0.0873 g) was dispersed in 50 mL of distilled water, and then 5 mg of citric acid was added (at higher amount of citric acid (i.e., >5 mg), it was found that ZnO nanorods begin to dissolve). The contents were stirred at room temperature for 4 h. The contents were filtered, washed, and dried in the oven at 70 °C.
Synthesis of ZnO/CdS heteronanostructures
In a typical synthesis, about 50 mg of the surface modified ZnO nanorods was mixed with different molar ratios of cadmium acetate and thiourea (Table 1) in 10 mL of ethylene glycol. The contents were sonicated for 3 min to achieve good dispersion, and heated at 180 °C for 1 h. During the reaction, the color of the contents changed to yellow which indicates the formation of CdS. After completion of the reaction, the contents were cooled to room temperature, and 25 mL of methanol was added to get a precipitate. The obtained precipitate was centrifuged, washed with methanol, and dried under vacuum to get the ZnO/CdS powder samples. The nomenclature of various ZnO/CdS samples prepared in the present study is given in Table 1.
Characterization
Structural analysis of the samples was carried out using powder X-ray diffraction in the 2θ range 20°–90° on a Brucker AXS D8 diffractometer and the scan speed was 1°/min. FT-IR spectral analysis was carried out on a Thermo Nicolet Nexus Fourier transform infrared spectrometer in the wavelength range 400–4,000 cm−1 using KBr pellets. Thermogravimetric analysis was carried out on EXSTAR TG/DTA 6300 under air in the temperature range 25–1,000 °C, and the heating rate was 10°/min. Morphological studies of the samples were carried out using FE-SEM and transmission electron microscopy (TEM) techniques. FE-SEM analysis was carried out on a FEI Quanta 200F scanning electron microscope operating at 20 kV. For the FE-SEM studies, the powder samples were smeared on a conducting carbon tape and then gold sputtered for about 100 s. Elemental composition of the samples was analyzed using an energy dispersive X-ray analysis facility coupled with the FE-SEM. TEM analysis was carried out using a FEI TECNAI G2 transmission electron microscope operating at 200 kV. For the TEM analysis, a few mg of the sample powder was dispersed in ethanol and sonicated for about 15 min. Then, a drop of the suspension was put on carbon-coated copper grid followed by drying in air. For the band gap measurements, diffuse reflectance spectra (DRS) were recorded on a Shimadzu UV-2450 UV–Visible spectrophotometer in the wavelength range 300–700 nm, and BaSO4 was used as the reference. The sample and barium sulfate powders were mixed in 1:100 ratio for the DRS measurements. Photoluminescence (PL) studies were carried out on a Shimadzu RF 5301-PC photoluminescence spectrometer. For the PL studies, about 5 mg of the sample powder was dispersed in 5 mL of methanol and sonicated for 10 min. The PL spectra were recorded for the suspensions in the wavelength range 350–700 nm. The excitation wavelength was 325 nm and the excitation and emission slit widths were both 5 nm.
Photocatalytic experiments
The photocatalytic activity of the ZnO/CdS heteronanostructures was investigated by the photodecomposition of methylene blue under sunlight. All the photocatalytic reactions in the present study were carried out under direct sunlight between 12:00 noon and 14:00 pm at IIT Roorkee campus in the month of February 2014 when the solar intensity fluctuation is minimal. The intensity of sunlight at Roorkee (the latitude and longitude are 29°52′N and 77°53′E, respectively) in the month of February is 166 Watt/m2 (Ramachandra et al. 2011). In a typical experiment, 20 mg of the photocatalyst was dispersed in 80 mL methylene blue aqueous solution (1 × 10−5 M) and stirred in dark for about 15 min to allow adsorption equilibrium between the dye and the catalyst. The beaker was kept under sunlight for irradiation, and aliquots (5 mL each) were withdrawn at periodic time intervals, centrifuged, and the supernatant solutions were analyzed using a UV–Vis spectrophotometer (Shimadzu UV-2450). The catalytic experiments were carried out under similar conditions using pure ZnO nanorods and CdS nanoparticles. A blank reaction was also performed without using any catalyst. The degradation efficiency of methylene blue was calculated using the formula given below.
where C o is the concentration of methylene blue at its adsorption equilibrium and C is the concentration of methylene blue at different illumination times.
To understand the mechanism of photodegradation of methylene blue better, and to prove the involvement of hydroxyl radicals in the photodegradation in the presence of ZnO/CdS as the catalyst, terephthalic acid was used as a probe molecule (Yu et al. 2009; Surendar et al. 2014). In a typical experiment, 20 mg of the ZnO/CdS sample (ZC3) was dispersed in 50 mL of an aqueous solution of terephthalic acid (5 × 10−4 M) containing sodium hydroxide (2 × 10−3 M). The contents were kept under sunlight for 2 h. During the irradiation, aliquots (5 mL each) were taken and analyzed by PL spectroscopy (λexcitation = 315 nm) at regular intervals (10 min) after removing the solid residue by centrifuging.
Results and discussion
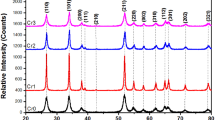
Figure 1 shows the XRD patterns of pure ZnO, surface-modified ZnO, pure CdS and ZnO/CdS heteronanostructures (ZC1, ZC2, and ZC3). Pure ZnO and surface-modified ZnO show reflections due to only ZnO (JCPDS file No: 36-1541). The observed reflections at 31.75°, 34.41°, 36.25°, 47.54°, 56.55°, 62.82°, 67.92°, and 69.17° are attributed to (100), (002), (101), (102), (110), (103), (112), and (201) planes of ZnO, respectively. The intense peak at 2θ = 36.25° confirms the preferred orientation of ZnO facet along the (101) plane. Pure CdS shows reflections at 26.44°, 43.80°, and 52.00° corresponding to (111), (220), and (311) planes of cubic CdS (JCPDS file no: 75-1546). In the XRD patterns of ZnO/CdS heteronanostructures, reflections due to both ZnO and CdS are observed. The XRD peaks due to CdS in the heteronanostructures are less intense and broad and they are shown in the inset of Fig. 1. The crystallite size of ZnO and CdS in the ZnO/CdS samples was calculated using Debye–Scherrer equation using the XRD peaks at 2θ = 36.25° for ZnO ((101)) and at 2θ = 26.44° for CdS ((111)). The crystallite size of pure ZnO is higher (50 nm) compared to that in the ZnO/CdS heteronanostructures (36–45 nm). The crystallite size of ZnO decreases after the formation of CdS shell on the ZnO. It was difficult to calculate the crystallite size of CdS in the ZnO/CdS samples due to less intense and broad XRD peaks of CdS. This suggests the presence of small CdS nanoparticles in the ZnO/CdS samples.
FT-IR spectra of pure ZnO, surface-modified ZnO, pure CdS and ZnO/CdS heteronanostructures (ZC1, ZC2, and ZC3) are shown in Fig. 2 and the assignments are given in Table 2. All the samples show bands at 3,435 and 1,630 cm−1 attributed to stretching and bending modes of hydroxyl groups of water molecules physisorbed on the surface of ZnO (Wahab et al. 2010). They show a weak band at 2,922 cm−1 and another band at 1,120 cm−1 attributed to asymmetrical stretching of −CH2 group and symmetrical stretching of C–OH due to ethylene glycol molecules, respectively (Wang et al. 2010; Imali and Vicki 2010). All the samples show a band corresponding to Zn–O vibration at about 570 cm−1 (Rahman et al. 2013; Bitenc and Crnjak 2009b). In ZnO, the band at 1,382 cm−1 is attributed to symmetrical stretching of carbonate groups (Bitenc and Crnjak 2009b). Surface-modified ZnO shows a band at 1,397 cm−1 attributed to symmetrical stretching of COO− due to citrate ions used during the surface modification of ZnO (Imali and Vicki 2010). Pure CdS shows a band at 649 cm−1 attributed to Cd–S stretching (Yang et al. 2013). Pure CdS and the ZnO/CdS heteronanostructures (ZC1, ZC2, and ZC3) show bands at 1403, 1397, 1395, and 1395 cm−1, respectively, which are attributed to symmetrical stretching of COO− group (Imali and Vicki 2010). In the TGA analysis, pure ZnO showed a weight loss of 1.8 % and surface-modified ZnO showed a higher weight loss of 2.5 % in the region 35–800 °C. This indicates the presence of more organic content in the surface-modified ZnO compared to pure ZnO (Feng et al. 2014; Singh et al. 2009).
The FE-SEM images of pure ZnO, CdS, and ZnO/CdS samples are shown in Fig. 3. Pure ZnO shows rods with hexagonal facets and smooth surface. The length of ZnO rods is about 2 μm and the diameter is 250 ± 20 nm. Pure CdS shows agglomerated small particles. In the ZnO/CdS samples, the surface of ZnO is rough which indicates the deposition of CdS on ZnO, and the diameter of the ZnO nanorods (250 nm) increases in the ZnO/CdS samples (ZC1, ZC2 and ZC3) to about 290, 330, and 370 nm, respectively. The elemental composition of ZnO/CdS heteronanostructures was analyzed using EDX analysis (Fig. 4). The standard deviation of elemental composition (cadmium and sulfur) from the data measured at different spots on the samples is given in Table 3. All the samples show the presence of cadmium and sulfur on the surface of ZnO nanorods. An increase in the weight percent of cadmium and sulfur is observed according to the concentrations of CdS precursors (cadmium acetate and thiourea) used during the synthesis ([Cd2+]:[S2−]=[0.075]:[0.075], [0.100]:[0.100], and [0.125]:[0.125]). The effects of reaction temperature, thermal decomposition time, and surface modification of ZnO nanorods on the quality of CdS coating in the ZnO/CdS heteronanostructures were studied and the results are discussed below.
Since sample ZC3 shows more uniform deposition of CdS on ZnO compared to the other samples (ZC1 and ZC2) as evidenced by SEM results, the thermal decomposition reactions were carried out for this sample at 150 and 180 °C while the heating time was fixed (1 h). The sample prepared at 150 °C shows poor deposition of CdS on ZnO (Fig. 5a). In the case of 180 °C, the ZnO/CdS sample clearly shows good deposition of CdS. Two CdS deposition reactions were carried out using surface unmodified ZnO nanorods and with surface modified ZnO nanorods at 180 °C for 1 h. In the case of surface unmodified ZnO, poor deposition of CdS and extra CdS particles are observed in the ZnO/CdS heteronanostructures (Fig. 5b). In the case of surface functionalized ZnO, uniform deposition of CdS nanoparticles on the surface of ZnO nanorods is observed. To understand the effect of thermal decomposition time on the deposition of CdS shell on the ZnO nanorods, the reactions were carried out for sample ZC3 at 180 °C for different times, e.g., 30, 60 and 90 min. Deposition of CdS shell is observed in all the cases but in the case of 30 and 90 min, extra CdS particles are observed in the ZnO/CdS heteronanostructures (Fig. 6a, c). In the case of 60 min, uniform CdS shell on the ZnO nanorods with no extra CdS particles is observed (Fig. 6b).
The TEM and SAED images of pure ZnO, CdS and ZnO/CdS heteronanostructures (ZC1, ZC2 and ZC3) are shown in Fig. 7. Pure ZnO nanorods show very smooth surface, and the length of ZnO nanorods is about 2 μm and diameter about 200 nm. Pure CdS nanoparticles show aggregated particles. The ZnO/CdS samples show clearly the deposition of CdS shell on the ZnO nanorods. Compared to samples ZC1 and ZC2, sample ZC3 shows uniform CdS shell on the ZnO nanorods. The SAED patterns for pure ZnO, CdS and ZnO/CdS heteronanostructures are shown as insets in the corresponding TEM images (Fig. 7). The SAED pattern of a single-ZnO nanorod shows a set of diffraction spots due to hexagonal structure, and this confirms the single-crystalline nature of the nanorods. The distance between two adjacent spots was measured as 0.27 nm which is attributed to (002) plane of hexagonal ZnO (Zhong et al. 2009; Hu et al. 2013). The SAED pattern of pure CdS show rings confirming the polycrystalline nature of CdS, and the rings are attributed to (311), (220), and (111) planes of cubic CdS (Guerguerian et al. 2011). The SAED patterns for ZnO/CdS samples ZC1 and ZC2 show both spots and ring patterns due to ZnO and CdS but sample ZC3 shows only a ring pattern due to CdS which suggests thicker CdS shell on the ZnO nanorods.
The HRTEM images of the ZnO/CdS samples (Fig. 8) show clearly the interface between the ZnO core and the CdS shell. The images also reveal that there is no amorphous region at the interface; a crystalline interface facilitates the injection of photo excited electrons from the CdS shell to the ZnO nanorods (Guerguerian et al. 2011). The thickness of CdS shell in the ZnO/CdS heteronanostructures was estimated, and the thickness values for the samples ZC1, ZC2 and ZC3 are about 20, 33 and 45 nm, respectively. The CdS shell thickness increases with increasing concentration of CdS precursors ([Cd2+]:[S2−] = [0.075]:[0.075], [0.100]:[0.100], and [0.125]:[0.125]) used during the synthesis. From the HRTEM images, the lattice spacing was calculated for CdS shell as 0.36 nm which corresponds to (111) plane of cubic CdS. This indicates that the growth of CdS shell on the ZnO nanorods is along the (111) direction.
The DRS spectra of ZnO/CdS heteronanostructures are shown in Fig. 9. The band gap values of pure ZnO, CdS and the ZnO/CdS heteronanostructures were calculated using the Tauc equation (Misra et al. 2013; Li et al. 2013b), and the Tauc plots were obtained by plotting (αhν)2 versus hν. Pure ZnO and CdS have direct bulk band gap values of 3.38 and 2.42 eV, respectively (Gao et al. 2005; Fan et al. 2007). In the present study, pure ZnO nanorods possess a band gap of 3.31 eV, and this is in good agreement with the reported band gap for hexagonal ZnO nanorods (Guerguerian et al. 2011; Li et al. 2013c). No quantum confinement effect is observed in the case of ZnO nanorods as their diameter (250 nm) is much larger than the Bohr exciton radius for ZnO (a B = 1.8 nm) (Guerguerian et al. 2011; Ozgur et al. 2005). Pure CdS nanoparticles exhibit a band gap of 2.53 eV with a blue shift of about 0.1 eV with respect to bulk CdS which is attributed to quantum size effect (Chang et al. 1994). The ZnO/CdS heteronanostructures show a band gap of about 3.25 eV for ZnO core and between 2.61 and 2.66 eV for the CdS shell. The ZnO in the ZnO/CdS heteronanostructures show about 0.13 eV red shift with respect to bulk ZnO (E g = 3.38 eV), and the CdS in the ZnO/CdS samples shows about 0.24 eV blue shift with respect to bulk CdS. The blue shift in the band gap of CdS is attributed to quantum size effect due to the presence of small CdS nanoparticles on the ZnO nanorods; small size limits the volume occupied by the electron–hole pairs (Chang et al. 1994). In the DRS spectra of ZnO/CdS heteronanostructures, the intensity of ZnO band gap absorption decreases and the intensity of CdS band gap absorption increases. This also indicates the formation of CdS shell on the ZnO nanorods. The particle size of CdS nanoparticles on the ZnO nanorods was calculated using the Brus equation (Brus 1986). For CdS, m * e = 0.19m o , m * h = 0.80m o , m o = 9.11 × 10−28 g and ε = 5.7. The calculated band gap and particle size values for CdS are given in Table 4. The calculated particle size of CdS nanoparticles using the Brus equation is smaller than the Bohr radius of CdS (2.5 nm). The blue shift of the band gap absorption of CdS in the ZnO/CdS heteronanostructures is attributed to quantum size effect (Chang et al. 1994).
The room temperature PL spectra of pure ZnO, CdS and ZnO/CdS heteronanostructures are shown in Fig. 10. Pure ZnO nanorods exhibit emission bands at 383, 452, 469, 485, and 494 nm and the observed spectrum is in good agreement with results reported for ZnO nanorods (Zhong et al. 2009; Chong et al. 2012). The strong UV emission centered at 383 nm is attributed to excitonic recombination corresponding to the near band edge emission of ZnO (Xu et al. 2009b). The emission in the blue region at about 452 nm is associated with the electronic transition between excitonic level and interstitial oxygen (Oi). The emission in the blue-green region at 469 nm is attributed to the electron transition between a shallow donor (Zni) and a deep acceptor (VZn) (Behera and Acharya 2008). The two emission bands in the green region (485 and 494 nm) are attributed to stoichiometry-related defects and these are generally attributed to zinc vacancies as well as interstitial zinc and structural defects (Chong et al. 2012; Wu et al. 2007). Pure CdS shows a broad emission band between 450 and 630 nm which is attributed to surface defects associated with the cadmium and sulfur vacancies (Xiao and Xiao 2009).
In the case of PL spectra of ZnO/CdS heteronanostructures (ZC1, ZC2 and ZC3), the luminescence in the UV region has vanished. They show luminescence in the visible range between 450 and 630 nm. The emission bands in the ZnO/CdS heteronanostructures are attributed to the following transitions: (i) from conduction band to deep acceptor levels (Oi or OZn), (ii) from deep donor levels (VOZni) to valence band, and (iii) from shallow donor levels (Zni) to shallow acceptor levels (VZn and Oi) (Khanchandani et al. 2012; Villani et al. 2012; Zhong et al. 2009). The emission intensity related to defect states in the visible region in the case of ZnO/CdS samples is relatively lower compared to pure ZnO. In the ZnO/CdS heteronanostructures, the CdS nanoparticles (shell) act as the sulfur dopant, which reduces the defect emission from the surface of ZnO nanorods (Misra et al. 2013). The absence of luminescence in the UV region and reduced intensity in the visible region in the ZnO/CdS samples is also attributed to the synergistic interaction between ZnO core and CdS shell. In the case of ZnO/CdS heteronanostructures, the emission maximum (495 nm) is blue shifted by about 40 nm compared to pure CdS nanoparticles (535 nm). This is again the evidence for electron transfer from CdS shell to the ZnO core on excitation. The band alignment of the ZnO core and the CdS shell in ZnO/CdS is shown in Scheme 1. The excitons are generated in the CdS shell on visible-light excitation, and the photogenerated electrons in the conduction band of CdS migrate to the conduction band of ZnO. This is because the conduction band of CdS is higher than that of ZnO (Khanchandani et al. 2012). The photogenerated holes in the valence band of ZnO migrate to that of CdS. The interfacial charge transfer lowers the direct transition probability between conducting band to valence band and reduces the emission intensity related to defect states (Fang et al. 2008) and the same is observed in the present study.
Proposed mechanism for the formation of ZnO/CdS heteronanostructures
The proposed mechanism for the formation of ZnO/CdS heteronanostructures is shown in Scheme 2. On surface modification of ZnO nanorods with citric acid, the citrate groups adsorb on the surface of ZnO nanorods (Khanchandani et al. 2012). Cadmium acetate and thiourea form a complex [Cd(Tu)2]2+ in ethylene glycol which prevents the release of a large amount of S2− ions in the solution (Khatamian et al. 2014). The cadmium ions in the complex ([Cd(Tu)2]2+) form an ionic bond with the citrate groups present on the surface of ZnO nanorods. When the temperature is increased about to 180 °C, the complex undergoes decomposition leading to the formation of uniform nanocrystalline CdS shell on the surface of ZnO nanorods (Khatamian et al. 2014; Yu et al. 1999; Vadivel et al. 2001). In the case of 30 and 90 min, the amount of CdS seems to be higher compared to that at 60 min due to irregular non-uniform deposition of CdS on the surface of ZnO at these timings. In the case of 30 min, very few CdS nuclei are generated in the solution and the growth of CdS is faster. Hence, the CdS nanoparticles deposit irregularly on the surface of ZnO which leads to agglomeration. As the reaction time is increased to 60 min, stable CdS nuclei are formed in large numbers. This leads to decrease in the concentration of CdS precursors in the solution with a uniform deposition of CdS. In the case of 90 min, further growth of CdS occurs, which results in agglomeration.
Photocatalytic studies
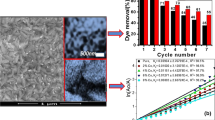
The photocatalytic activity of ZnO/CdS samples towards methylene blue degradation in aqueous solution under sunlight was tested and the results are shown in Fig. 11a–d. The kinetics of methylene blue degradation was also studied (Fig. 11a). The ZnO/CdS samples show higher photocatalytic activity compared to pure ZnO nanorods and CdS nanoparticles (Fig. 11b).
a Kinetics of photodegradation of methylene blue using ZnO/CdS (ZC3) as the catalyst, b comparison of photocatalytic performance of ZnO/CdS samples with pure ZnO nanorods and CdS nanoparticles, c plots of ln(Co/C) versus irradiation time for various photocatalysts and d comparison of methylene blue degradation efficiencies of ZnO nanorods, CdS nanoparticles, and ZnO/CdS samples (ZC1, ZC2 and ZC3)
The ZnO/CdS samples show linear relationship between ln(Co/C) and irradiation time (Fig. 11c) which indicates that the photodegradation of methylene blue follows first-order kinetics (Zhang et al. 2013; Zhao et al. 2010). The apparent first-order rate constant (k) for the degradation of methylene blue was calculated as 2.72 × 10−2, 1.96 × 10−2, 1.33 × 10−2, 9.43 × 10−3, and 6.58 × 10−3 min−1 for ZC3, ZC2, ZC1, ZnO, and CdS, respectively. Among all the samples, ZC3 shows the highest rate constant and the degradation efficiency follows the following order: ZC3 > ZC2 > ZC1 > ZnO > CdS (Fig. 11d). The higher photocatalytic efficiency of ZC3 is attributed to efficient charge separation and good synergistic interaction between ZnO core and the CdS shell in this sample. ZC3 consists of thicker shell of CdS compared to ZC1 and ZC2, and the increased photocatalytic efficiency of ZC3 is attributed to uniform coverage of CdS shell on the ZnO nanorods. The above results suggest that the synthesized ZnO/CdS heteronanostructures can be used as good catalyst for the degradation of organic pollutants in aqueous solutions under natural sunlight.
The possible mechanism for the photodegradation of methylene blue is given in Scheme 3. In type–II semiconductors such as ZnO/CdS, the photogenerated excitons are confined in the core and the shell. On illumination of ZnO/CdS samples under sunlight, the photogenerated electrons in the conduction band of CdS are effectively transferred to that of ZnO which facilitates the charge separation of the electron–hole pairs before they recombine (Kim et al. 2009). The conduction band of ZnO possesses more electrons, and no free holes are available in the valence band of ZnO for the recombination. The holes remain in the valence band of CdS, and they are not transferred to the corresponding band of ZnO since the valence band of CdS is more cathodic than that of ZnO, as shown in Scheme 1. The electrons in the conduction band of ZnO readily react with the dissolved oxygen in water and produce highly reactive superoxide radical anions (O •−2 ). The electrons in the conduction band of ZnO also react with dissolved oxygen and H+ ions present in the aqueous solution leading to the formation of hydroxy radicals (OH•). The active species such as holes existing in the valence band of CdS (h+), superoxide radical anions (O •−2 ), and hydroxyl radicals (OH•) are responsible (Scheme 3) for the photodegradation of methylene blue (Zhu et al. 2008; Khanchandani et al. 2012; Pawar and Lee 2014).
Determination of hydroxyl radicals
Since hydroxyl radicals play an important role in the degradation of organic pollutants, the hydroxyl radicals generated on the surface of the photocatalyst (ZnO/CdS, ZC3) were determined by photoluminescence spectroscopy, and terephthalic acid was used as the probe molecule (Xiao et al. 2008; Chen et al. 2010; Yu et al. 2010; Wang et al. 2013a, b). The hydroxyl radicals produced in the aqueous solution on irradiation readily react with terephthalic acid leading to the formation of highly fluorescent hydroxy terephthalic acid (λmax = 430 nm). The measured photoluminescence intensity of hydroxy terephthalic acid increases linearly with the irradiation time (Fig. 12). This suggests that the amount of hydroxyl radicals formed on the surface of photocatalyst is proportional to the irradiation time (Yu et al. 2010; Wang et al. 2013b).
Conclusions
ZnO/CdS heteronanostructures with different CdS shell thicknesses (20–45 nm) have been successfully synthesized by a novel thermal decomposition approach. Uniform deposition of cadmium sulfide nanoparticles on the surface-modified ZnO nanorods is possible at 180 °C. Surface modification of ZnO nanorods is necessary for the uniform deposition of CdS on ZnO nanorods. TEM/SEM images clearly show the uniform deposition of cadmium sulfide nanoparticles on the surface-functionalized ZnO nanorods. SAED/HRTEM studies confirm the crystalline nature of hexagonal ZnO nanorods and cubic cadmium sulfide nanoparticles. DRS results show a red shift of the band gap of ZnO and a blue shift of the band gap of CdS in the ZnO/CdS heteronanostructures compared to pure ZnO and CdS nanoparticles. The blue shift in the band gap of CdS shell is attributed to quantum size effect. Photoluminescence spectroscopy results show disappearance of UV emission band and reduction in the intensity of visible emission in the ZnO/CdS heteronanostructures. These observations are explained on the basis of synergistic interaction between ZnO and the CdS in the ZnO/CdS heteronanostructures. The synthesized ZnO/CdS samples show higher photocatalytic activity towards the photodegradation of methylene blue in aqueous solution compared to pure ZnO and CdS nanoparticles. The higher catalytic activity of ZC3 is attributed to efficient charge separation and also the synergistic interaction between ZnO and CdS. The present synthetic method is inexpensive, and the synthesized ZnO/CdS heteronanostructures are useful in photocatalysis applications.
References
Becker J, Raghupathi KR, Pierre J, Zhao D, Koodali RT (2011) Tuning of the crystallite and particle sizes of ZnO nanocrystalline materials in solvothermal synthesis and their photocatalytic activity for dye degradation. J Phys Chem C 115:3844–13850. doi:10.1021/jp2038653
Behera D, Acharya BS (2008) Nano-star formation in Al-doped ZnO thin film deposited by dip-dry method and its characterization using atomic force microscopy, electron probe microscopy, photoluminescence and laser Raman spectroscopy. J Lumin 128:1577–1586. doi:10.1016/j.jlumin.2008.03.006
Bishop LM, Yeager JC, Chen X, Wheeler JN, Torelli MD, Benson MC, Burke SD, Pedersen JA, Hamers RJ (2012) A citric acid-derived ligand for modular functionalization of metal oxide surfaces via “click” chemistry. Langmuir 28:1322–1329. doi:10.1021/la204145t
Bitenc M, Crnjak OZ (2009) Synthesis and characterization of crystalline hexagonal bipods of zinc oxide. Mater Res Bull 44:381–387. doi:10.1016/j.materresbull.2008.05.005
Bitenc M, Podbrscek P, Crnjak OZ, Cleveland MA, Paramo JA, Peters RM, Strzhemechny YM (2009) Correlation between morphology and defect luminescence in precipitated ZnO nanorod powders. Cryst Growth Des 9:997–1001. doi:10.1021/cg8008078
Brus L (1986) Electronic wave functions in semiconductor clusters: experiment and theory. J Phys Chem 90:2555–2560
Chang SY, Liu L, Asher SA (1994) Preparation and properties of tailored morphology, monodisperse colloidal silica-cadmium sulfide nanocomposites. J Am Chem Soc 116:6739–6744
Chen S, Zhang H, Yu X, Liu W, Wang J, Liu Q, Chen L (2010) Preparation, characterization and activity evaluation of heterojunction ZrTi2O6/TiO2 photocatalyst. Mater Chem Phys 124:1057–1064. doi:10.1016/j.matchemphys.2010.08.031
Chong X, Li L, Yan X, Hu D, Li H, Wang Y (2012) Synthesis, characterization and room temperature photoluminescence properties of Al doped ZnO nanorods. Physica E 44:1399–1405. doi:10.1016/j.physe.2012.03.001
Cui Q, Liu C, Wu F, Yue W, Qiu Z, Zhang H, Gao F, Shen W, Wang M (2013) Performance improvement in polymer/ZnO nanoarray hybrid solar cells by formation of ZnO/CdS-core/shell heterostructures. J Phys Chem C 117:5626–5637. doi:10.1021/jp312728t
Fan D, Thomas PJ, O’Brien P (2007) Deposition of CdS and ZnS thin films at the water/toluene interface. J Mater Chem 17:1381–1386. doi:10.1039/b616004b
Fang F, Zhao DX, Li BH, Zhang ZZ, Zhang JY, Shen DZ (2008) The enhancement of ZnO nanowalls photoconductivity induced by CdS nanoparticle modification. Appl Phys Lett 93:233115/1–233115/3. doi:10.1063/1.3045952
Feng Y, Feng N, Wei Y, Zhang G (2014) An in situ gelatin-assisted hydrothermal synthesis of ZnO-reduced graphene oxide composites with enhanced photocatalytic performance under ultraviolet and visible light. RSC Adv 4:7933–7943. doi:10.1039/c3ra46417b
Galoppini E, Rochford J, Chen H, Saraf G, Lu Y, Hagfeldt A, Boschloo G (2006) Fast electron transport in metal organic vapor deposition grown dye-sensitized ZnO nanorod solar cells. J Phys Chem B 110:16159–16161. doi:10.1021/jp062865q
Gao T, Li Q, Wang T (2005) Sonochemical synthesis, optical properties, and electrical properties of core/shell-type ZnO nanorod/CdS nanoparticle composites. Chem Mater 17:887–892. doi:10.1021/cm0485456
Geng J, Jia XD, Zhu JJ (2011) Sonochemical selective synthesis of ZnO/CdS core/shell nanostructures and their optical properties. CrystEngComm 13:193–198. doi:10.1039/c0ce00180e
Ghosh CR, Paria S (2012) Core/shell nanoparticles: classes, properties, synthesis, mechanisms, characterization, and applications. Chem Rev 112:2373–2433. doi:10.1021/cr100449n
Guerguerian G, Elhordoy F, Pereyra CJ, Marotti RE, Martin F, Leinen D, Ramos B, Jose R, Dalchiele EA (2011) ZnO nanorod/CdS nanocrystal core/shell-type heterostructures for solar cell applications. Nanotechnology 22:505401/1–505401/9. doi:10.1088/0957-4484/22/50/505401
Hu J, Fan Y, Pei Y, Qiao M, Fan K, Zhang X, Zong B (2013) Shape effect of ZnO crystals as cocatalyst in combined reforming-hydrogenolysis of glycerol. ACS Catal 3:2280–2287. doi:10.1021/cs400526s
Huang X, Wang M, Willinger MG, Shao L, Su DS, Meng XM (2012) Assembly of three- dimensional hetero- epitaxial ZnO/ZnS core/shell nanorod and single crystalline hollow ZnS nanotube arrays. ACS Nano 6:7333–7339. doi:10.1021/nn3024514
Imali AM, Vicki HG (2010) Citric acid adsorption on TiO2 nanoparticles in aqueous suspensions at acidic and circumneutral pH: surface coverage, surface speciation, and its impact on nanoparticle–nanoparticle interactions. J Am Chem Soc 132:14986–14994. doi:10.1021/ja106091q
Kadir RA, Li Z, Sadek AZ, Abdul RR, Zoolfakar AS, Field MR, Ou JZ, Chrimes AF, Kalantar-zadeh K (2014) Electrospun granular hollow SnO2 nanofibers hydrogen gas sensors operating at low temperatures. J Phys Chem C 118:3129–3139. doi:10.1021/jp411552z
Khanchandani S, Kundu S, Patra A, Ganguli AK (2012) Shell thickness dependent photocatalytic properties of ZnO/CdS core-shell nanorods. J Phys Chem C 116:23653–23662. doi:10.1021/jp3083419
Khatamian M, Saket OM, Haghighi M (2014) Photocatalytic hydrogen generation over CdS–metalosilicate composites under visible light irradiation. New J Chem. doi:10.1039/c3nj01348k
Kim YJ, Yoo J, Kwon BH, Hong YJ, Lee CH, Yi GC (2008) Position-controlled ZnO nanoflower arrays grown on glass substrates for electron emitter application. Nanotechnology 19:315202/1–315202/5. doi:10.1088/0957-4484/19/31/315202
Kim H, Tak Y, Senthil K, Joo J, Jeon S, Yong K (2009) Novel heterostructure of CdS nanoparticle/WO3 nanowhisker: synthesis and photocatalytic properties. J Vac Sci Technol, B 27:2182–2186. doi:10.1116/1.3212913
Kuo TJ, Lin CN, Kuo CL, Huang MH (2007) Growth of ultralong ZnO nanowires on silicon substrates by vapor transport and their use as recyclable photocatalysts. Chem Mater 19:5143–5147. doi:10.1021/cm071568a
Latthe SS, An S, Jin S, Yoon SS (2013) High energy electron beam irradiated TiO2 photoanodes for improved water splitting. J Mater Chem 1:13567–13575. doi:10.1039/c3ta13481d
Li C, Ahmed T, Ma M, Edvinsson T, Zhu J (2013a) A facile approach to ZnO/CdS nanoarrays and their photocatalytic and photoelectrochemical properties. Appl Catal B 138–139:175–183. doi:10.1016/j.apcatb.2013.02.042
Li H, Yao C, Meng L, Sun H, Huang J, Gong Q (2013b) Photoelectrochemical performance of hydrogenated ZnO/CdS core-shell nanorod arrays. Electrochim Acta 108:45–50. doi:10.1016/j.electacta.2013.06.074
Li L, Liu X, Zhnag Y, Salvador PA, Rohrer GS (2013c) Heterostructured (Ba, Sr)TiO3/TiO2 core/shell photocatalysts: influence of processing and structure on hydrogen production. Int J Hydrogen Energy 38:6948–6959. doi:10.1016/j.ijhydeene.2013.03.130
Li L, Salvador PA, Rohrer GS (2014) Photocatalysts with internal electric fields. Nanoscale 6:24–42. doi:10.1039/c3nr03998f
Liao L, Lu HB, Li JC, He H, Wang DF, Fu DJ, Liu C, Zhang WF (2007) Size dependence of gas sensitivity of ZnO nanorods. J Phys Chem C 111:1900–1903. doi:10.1021/jp065963k
Misra M, Kapur P, Ghanshyam C, Singla ML (2013) ZnO@CdS core-shell thin film: fabrication and enhancement of exciton life time by CdS nanoparticle. J Mater Sci 24:3800–3804. doi:10.1007/s10854-013-1321-0
Nie T, Chen ZG, Wu Y, Guo Y, Zhang J, Fan Y, Yang X, Jiang Z, Zou J (2012) Fabrication of crystal α-Si3N4/Si–SiOx core-shell/Au–SiOx peapod-like axial double heterostructures for optoelectronic applications. Nanotechnology 23:305603/1–305603/9. doi:10.1088/0957-4484/23/30/305603
Ozgur U, Alivov YI, Liu C, Teke A, Reshchikov MA, Dogan S, Avrutin V, Cho SJ, Morkoc H (2005) A comprehensive review of ZnO materials and devices. J Appl Phys 98:041301/1–041301/103. doi:10.1063/1.1992666
Panda SK, Chakrabarti S, Satpati B, Satyam PV, Chaudhuri S (2004) Optical and microstructural characterization of CdS–ZnO nanocomposite thin films prepared by sol-gel technique. J Phys D Appl Phys 37:628–633. doi:10.1088/0022-3727/37/4/014
Pawar RC, Lee CS (2014) Single-step sensitization of reduced graphene oxide sheets and CdS nanoparticles on ZnO nanorods as visible-light photocatalysts. Appl Catal B 144:57–65. doi:10.1016/j.apcatb.2013.06.022
Rahman QI, Ahmad M, Misra SK, Lohani MB (2013) Hexagonal ZnO nanorods assembled flowers for photocatalytic dye degradation: growth, structural and optical properties. Superlattices Microstruct 64:495–506. doi:10.1016/j.spmi.2013.10.011
Ramachandra TV, Rishabh J, Krishnadas G (2011) Hotspots of solar potential in India. Renew Sust Energy Rev 15:3178–3186. doi:10.1016/j.rser.2011.04.007
Ren L, Li Y, Hou J, Zhao X, Pan C (2014) Preparation and enhanced photocatalytic activity of TiO2 nanocrystals with internal pores. ACS Appl Mater Interfaces 6:1608–1615. doi:10.1021/am404457u
Schladt TD, Koll K, Pruefer S, Bauer H, Natalio F, Dumele O, Raidoo R, Weber S, Wolfrum U, Schreiber LM, Radsak MP, Schild H, Tremel W (2012) Multifunctional superparamagnetic MnO@SiO2 core/shell nanoparticles and their application for optical and magnetic resonance Imaging. J Mater Chem 22:9253–9262. doi:10.1039/c2jm15320c
Singh J, Im J, Whitten JE, Soares JW, Steeves DM (2009) Encapsulation of zinc oxide nanorods and nanoparticles. Langmuir 25:9947–9953. doi:10.1021/la9010983
Surendar T, Santosh K, Syam K, Vishnu S (2014) Fe-doped and -mediated graphitic carbon nitride nanosheets for enhanced photocatalytic performance under natural sunlight. J Mater Chem A 2:6772–6780. doi:10.1039/c3ta15358d
Tak Y, Kim H, Lee D, Yong K (2008) Type-II CdS nanoparticle-ZnO nanowire heterostructure arrays fabricated by a solution process: enhanced photocatalytic activity. Chem Comm 38:4585–4587. doi:10.1039/b810388g
Tena ZR, Katty A, Bastide S, Levy CC (2007) Annealing effects on the physical properties of electrodeposited ZnO/CdSe core–shell nanowire arrays. Chem Mater 19:1626–1632. doi:10.1021/cm062390f
Vadivel MA, Sonawane RS, Kale BB, Apte SK, Kulkarni AV (2001) Microwave-solvothermal synthesis of nanocrystalline cadmium sulfide. Mater Chem Phys 71:98–102
Villani M, Calestani D, Lazzarini L, Zanotti L, Mosca R, Zappettini A (2012) Extended functionality of ZnO nanotetrapods by solution-based coupling with CdS nanoparticles. J Mater Chem 22:5694–5699. doi:10.1039/c2jm16164h
Wahab R, Kim YS, Lee K, Shin HS (2010) Fabrication and growth mechanism of hexagonal zinc oxide nanorods via solution process. J Mater Sci 45:2967–2973. doi:10.1007/s10853-010-4294-x
Wang ZL (2004) Zinc oxide nanostructures: growth, properties and applications. J Phys Condens Matter 16:R829–R858. doi:10.1088/0953-8984/16/25/R01
Wang Y, Sun X, Li H (2010) Synthesis, characterization and room temperature photoluminescence properties of briers-like ZnO nanoarchitectures. Mater Sci Eng, B 167:177–181. doi:10.1016/j.mseb.2010.02.001
Wang C, Cao M, Wang P, Ao Y (2013a) Preparation, characterization of CdS-deposited graphene-carbon nanotubes hybrid photocatalysts with enhanced photocatalytic activity. Mater Lett 108:336–339. doi:10.1016/j.matlet.2013.06.102
Wang Q, Jia W, Liu B, Dong A, Gong X, Li C, Jing P, Li Y, Xu G, Zhang J (2013b) Hierarchical structure based on Pd(Au) nanoparticles grafted onto magnetite cores and double layered shells: enhanced activity for catalytic applications. J Mater Chem 1:12732–12741. doi:10.1039/c3ta12814h
Wu YL, Tok AIY, Boey FYC, Zeng XT, Zhang XH (2007) Surface modification of ZnO nanocrystals. Appl Surf Sci 253:5473–5479. doi:10.1016/j.apsusc.2006.12.091
Wu Z, Zhang Y, Zheng J, Lin X, Chen X, Huang B, Wang H, Huang K, Li S, Kang J (2011) An all-inorganic type-II heterojunction array with nearly full solar spectral response based on ZnO–ZnSe core-shell nanowires. J Mater Chem 21:6020–6026. doi:10.1039/c0jm03971c
Xia Y, Yin L (2013) Core-shell structured α-Fe2O3@TiO2 nanocomposites with improved photocatalytic activity in the visible light region. Phys Chem Chem Phys 15:18627–18634. doi:10.1039/c3cp53178c
Xiao Q, Xiao C (2009) Surface-defect-states photoluminescence in CdS nanocrystals prepared by one-step aqueous synthesis method. Appl Surf Sci 255:7111–7114. doi:10.1016/j.apsusc.2008.12.032
Xiao Q, Si Z, Zhang J, Xiao C, Tan X (2008) Photoinduced hydroxyl radical and photocatalytic activity of samarium-doped TiO2 nanocrystalline. J Hazard Mater 150:62–67. doi:10.1016/j.jhazmat.2007.04.045
Xiao FX, Miao J, Liu B (2014) Layer-by-Layer self-assembly of CdS quantum dots/graphene nanosheets hybrid films for photoelectrochemical and photocatalytic applications. J Am Chem Soc 136:1559–1569. doi:10.1021/ja411651e
Xu F, Lu Y, Xie Y, Liu Y (2009a) Synthesis and photoluminescence of assembly-controlled ZnO architectures by aqueous chemical growth. J Phys Chem C 113:1052–1059. doi:10.1021/jp808456r
Xu F, Volkov V, Zhu Y, Bai H, Rea A, Valappil NV, Su W, Gao X, Kuskovsky IL, Matsui H (2009b) Long electron-hole separation of ZnO–CdS core–shell quantum dots. J Phys Chem C 113:19419–19423. doi:10.1021/jp903813h
Yang G, Yang B, Xiao T, Yan Z (2013) One-step solvothermal synthesis of hierarchically porous nanostructured CdS/TiO2 heterojunction with higher visible light photocatalytic activity. Appl Surf Sci 283:402–410. doi:10.1016/j.apsusc.2013.06.122
Yu SH, Yang J, Han ZH, Zhou Y, Yang RY, Qian YT, Zhang YH (1999) Controllable synthesis of nanocrystalline CdS with different morphologies and particle sizes by a novel solvothermal process. J Mater Chem 9:1283–1287
Yu J, Dai G, Huang B (2009) Fabrication and characterization of visible-light-driven plasmonic photocatalyst Ag/AgCl/TiO2 nanotube arrays. J Phys Chem C 113:16394–16401. doi:10.1021/jp905247j
Yu J, Wang B (2010) Effect of calcination temperature on morphology and photoelectrochemical properties of anodized titanium dioxide nanotube arrays. Appl Catal B 94:295–302. doi:10.1016/j.apcatb.2009.12.003
Zhai J, Wang D, Peng L, Lin Y, Li X, Xie T (2010) Visible-light-induced photoelectric gas sensing to formaldehyde based on CdS nanoparticles/ZnO heterostructures. Sens Actuators B 147:234–240. doi:10.1016/j.snb.2010.03.003
Zhang CF, Qiu LG, Ke F, Zhu YJ, Yuan YP, Xu GS, Jiang X (2013) A novel magnetic recyclable photocatalyst based on a core–shell metal-organic framework Fe3O4@MIL-100(Fe) for the decolorization of methylene blue dye. J Mater Chem A 1:14329–14334. doi:10.1039/c3ta13030d
Zhao W, Bai Z, Ren A, Guo B, Wu C (2010) Sunlight photocatalytic activity of CdS modified TiO2 loaded on activated carbon fibers. Appl Surf Sci 256:3493–3498. doi:10.1016/j.apsusc.2009.12.062
Zhong K, Xia J, Li HH, Liang CL, Liu P, Tong YX (2009) Morphology evolution of one-dimensional-based ZnO nanostructures synthesized via electrochemical corrosion. J Phys Chem C 113:15514–15523. doi:10.1021/jp9017794
Zhu J, Yang D, Geng J, Chen D, Jiang Z (2008) Synthesis and characterization of bamboo-like CdS/TiO2 nanotubes composites with enhanced visible-light photocatalytic activity. J Nanopart Res 10:729–736. doi:10.1007/s11051-007-9301-z
Zhu YF, Zhou GH, Ding HY, Liu AH, Lin YB, Dong YW (2011) Synthesis and characterization of highly-ordered ZnO/PbS core/shell heterostructures. Superlattices Microstruct 50:549–556. doi:10.1016/j.spmi.2011.08.017
Acknowledgments
Financial support from the Ministry of Human Resource Development, Government of India is gratefully acknowledged. We are thankful to the Institute Instrumentation Centre, Indian Institute of Technology Roorkee for providing the XRD, TGA, FE-SEM, and TEM facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandula, S., Jeevanandam, P. Visible-light-induced photodegradation of methylene blue using ZnO/CdS heteronanostructures synthesized through a novel thermal decomposition approach. J Nanopart Res 16, 2452 (2014). https://doi.org/10.1007/s11051-014-2452-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11051-014-2452-9