Abstract
Ozonation of tannery dye Acid Black 52 in a packed bed reactor has been examined to study the effect of operating variables pH, dye concentration and contact time. In this study, the effect of operating variables on the removal of colour and chemical oxygen demand (COD) was studied using 23 central composite designs using Minitab 15 software. The operating variables were analysed based on the removal efficiency of colour and COD. Experimental results were analysed by regression analysis and analysis of variance statistical methods. The optimum conditions for maximum colour removal (100 %) and COD removal (61 %) were found to be at pH: 1.96, dye concentration: 1,159 mg/L, contact time: 10.6 min (min) and at pH: 4.8, dye concentration: 1,159 mg/L, contact time: 17 min, respectively. The experimental values were in good agreement with the predicted values and the model is found to be highly significant with the correlation coefficient of 0.94 for both colour and COD removal.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution caused by the tannery effluent is highly prevalent and is one of the perplexing problems faced by many countries. Tanning is one of the oldest industries in India with about 2,000 industrial units spread mostly across Punjab, Uttar Pradesh, West Bengal and Tamilnadu (Lefebvre et al. 2005). On the whole, India ranks eighth in the leather export trade in terms of foreign exchange earnings of the country. In International market, Indian leather goods constitute about 10 % of India’s finished leather goods. On the one hand, it occupies a place of reputation in the Indian economy, while on the other hand the process water used for the processing and the solid byproducts (Ravindranath et al. 2010) from the leather units pose major difficulties with the discharge limits on land and water surfaces set by the pollution control boards. About 30–40 m3 of wastewater is generated per ton of raw material processed (Ravindranath et al. 2004). Leather processing in a tannery generally comprises three categories: pre-treatment of skin/hide (beamhouse operations), chrome or vegetable tanning of skin/hide (tanning operation) and including dyeing and surface treatment (finishing operations) (Stoop 2003; Thanikaivelan et al. 2004). In the finishing operations, dyeing results in major contribution of colour and chemical oxygen demand (COD) in the effluent (Shamshath Begum et al. 2011; Srinivasan et al. 2012). Some dyes are innocuous while some are not. But the colour imparted by these dyes results in aesthetic pollution of water and some of the dyes are toxic and anaerobic degraded product of the dyes are found to be carcinogenic. It is imperative to remove dyes in order to develop a balanced sustainable eco-system.
There are no economically feasible treatment technologies available for colour removal (Alaton et al. 2002; Alalewi and Jain 2010; Adinew Bizuneh 2012). Commonly applied treatment methods for tannery effluents consists of integrated processes involving various combinations of physical, biological and chemical degradation methods (Dilaconi et al. 2002; Durai and Rajasimman 2011). These integrated treatment methods are efficient but not cost-effective. Existing physiochemical technologies such as membrane processes (De Gisi et al. 2009), adsorption (Shamshath Begum et al. 2006), coagulation and flocculation (Song et al. 2004; Hamidi Abdul Aziz et al. 2007) have been reported to be effective methods. These treatment methods lead to generation of sludge or solid wastes, which requires further disposal and moreover they transfer the pollutants from one form to another form and are commercially inefficient. Conventional biological treatments, the most economical and feasible ones, are not a suitable alternative when working with toxic, recalcitrant and non-biodegradable wastewater. In fact, most pollutants present in the tannery wastewater are non-biodegradable and so, they are adsorbed onto the biomass without being really degraded. The application of biological processes to industrial wastewater treatment may still face some difficulties mainly due to the presence of inhibiting compounds. These effluents cannot be released into the environment without tertiary treatment because of their toxicity. In order to allow a significantly higher biodegradability of poorly biodegradable industrial wastewaters and to breakup refractory or toxic organic matter, the integration of an advanced oxidation process (AOP) step seems to be promising (Tabrizi and Mehrvar 2004; Oller et al. 2011).
Among different dyes used in tannery industries, usage of acid dyes has been increasing to meet the demand because of the bright colour shade and simpler dyeing methods compared with the other dyes. These dyes are water soluble and are reported to be generally problematic (Robinson et al. 2001).
Of the many oxidation processes available, ozonation is an effective method for the oxidation and degradation of refractory compounds. Ozone cleaves the conjugated bonds of azo dye chromophores, which result in colour removal and increases biodegradability as well. It usually produces oxygenated organic compounds with low molecular weight that are easily biodegradable (Chaturapruek et al. 2005; Fanchiang et al. 2009). Ozonation process has been reported in the literature for the removal of different types of dyes like acid dyes (Gao et al. 2012), basic dyes (Turhan et al. 2012), reactive dyes (Tizaoui and Grima 2011) and disperse dyes (Shin and Bae 2012) which are discharged from the various types of industries.
In order to optimize the process variables for colour and COD removal in wastewater, response surface methodology (RSM) has been reported (Srinivasan and Murthy 2009; Ayed et al. 2012). RSM is a method where the experiments are designed prior to the start up. RSM investigates the relation between a number of independent variables (input) and an output variable (response). Experiment has to be conducted at the specific operating variables as per the statistical design requirements to ascertain the optimum conditions.
In this study, this methodology (statistical design software, i.e. Minitab 15, PA, USA) has been applied for optimization of colour and COD removal of Acid Black 52 (AB 52) dye. Based on the reported literatures (Arlindo Gomes et al. 2012; Salem Abu Amr and Aziz 2012), it was observed that the main operating variables that affect the ozonation have been identified as pH, dye concentration and contact time. The main objective of the study is to investigate the effect of pH, dye concentration and contact time for maximum removal of colour and COD using RSM. The interaction effects between the variables were also studied.
Materials and methods
Analytical methods
Chemical oxygen demand was measured as per the Standard Methods (APHA 1998). The initial pH of synthetic dye solution was adjusted by addition of 0.1 N sodium hydroxide (NaOH) or sulphuric acid (H2SO4) to a desired value as per the statistical design requirements. The pH of the dye solution was measured using a pH meter (Model: 330, WTW-Germany). All the chemicals used in the experiments were of analytical grade.
Synthetic dye solution and colour measurement
The molecular weight of the dye is 1488.13 (g/mol) and its colour index number (C. I. No.) is 15711. The synthetic dye solution was prepared by dissolving a known amount of AB 52 dye in distilled water for obtaining the desired concentration. The maximum absorbance of the dye was found to be λmax = 572 nm and this wavelength was used to determine the concentration of the dye solution before and after ozonation contact time as per the statistical design requirements. The absorbance measurements were made using UV–Vis spectrophotometer (Shimadzu UV-2101PC). The dye structure, molecular weight, formula and maximum absorption wavelength are given in Table 1.
A known amount of sample was taken from packed bed reactor (PBR) after ozonation as per the statistical requirements. Residual dye concentration after ozonation in PBR was measured using spectrophotometer and was calculated from the calibration curve (absorbance vs known dye concentrations at λmax). Higher concentrations of samples were diluted in order to obey Beer–Lambert’s law. Similarly, COD concentrations of the samples after ozonation in PBR were also determined by standard methods.
Experimental setup and operation of the PBR
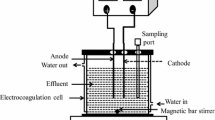
Batch experiments were carried out in a PBR of 5.5 cm diameter and 110 cm height with working volume of 0.5 L of the synthetic dye solution. A classical dielectric tubular ozone cell generator was used (Pristinus, India) in this study. Ozone was generated from pure oxygen by a silent electric discharge method at the rate of 4 g/h and was passed through the bottom of the reactor through the diffuser. Glass raschig rings of height 0.8 cm and diameter 1.8 cm was used as a packing material. The entire setup of the experiment was conducted by Teflon tubing. The process description of the experimental setup is given in Fig. 1. The unreacted ozone leaving the PBR was passed through a series of gas washing bottles filled with 2 % of potassium iodide (KI) solution. Experiments were carried out at the ambient temperature.
To study the effect of pH, dye concentration and contact time on removal of colour and COD, experiments were conducted in PBR with different variables as per the statistical design requirements and the responses in terms of colour and COD removal (%) were determined.
Analysis on response surface methodology
Response surface methodology, a statistical tool was used to calculate the relationship between the designed variables and response obtained based on the experimental results. The levels (actual and coded) of three significant variables like pH (P), dye concentration (C) and time (T) are given in Table 2. Low (−1) and high levels (+1) of these process variables were chosen and the set of experiments to be carried at various conditions were obtained from the software Minitab. It comprises 23 = 8 factorial design plus six centre points and six axial points. Centre points constitute the intermediate level (0), whereas the axial points constitute the others: lowest (−1) and the highest (+1) levels. Thus, 20 experimental runs were performed to elucidate the linear, quadratic and two-way interactive effects of the process variables on the removal % of colour and COD. For statistical calculations, the variables X i were coded as x i according to the following relationship:
Based on 20 sets, experimental conditions were carried out as shown in Table 3 with varying independent variables and response in terms of colour and COD removal were determined. Response obtained (colour and COD removal) experimentally were fed into the software and various statistical tests were carried out. The optimized condition for the colour and COD removal was obtained from the contour, surface plots graphically and as well as response surface analysis using polynomial regression equation. The three significant variables were approximated by the quadratic model equation as follows:
where Y is the predicted response; X 0 is a constant; X P, X C and X T are the linear coefficients; X PP, X CC and X TT are the quadratic coefficients and X PC, X CT and X PT are the cross-coefficients. P, C and T are the input variables (coded values). From the experiments carried out, 10 coefficients of second-order polynomial equation for colour and COD removal were determined. Based on the polynomial expression, the predicted responses were estimated using the set experimental conditions. Predicted values and experimental responses were analysed using ANOVA to find whether the polynomial expression is able to predict the responses statistically. The statistical significance was analysed by Fisher’s F test and Student’s t test. The fitness of the model is expressed by the value of R 2 (coefficient of determination).
Results and discussion
In this study, the central composite experimental statistical design was applied to optimize the removal of AB 52 dye from synthetic dye solution by varying the parameters pH, dye concentration and contact time using AOP, i.e. ozonation in PBR.
Effect of process variables on colour and COD removal of AB 52 dye
The main effect plots (MEP) that describes the effect of each variables on removal of colour and COD are shown in Figs. 2 and 3, respectively. From Fig. 2, it was observed that maximum removal of colour of 94.11, 96.32 and 95.81 % were observed at pH 1.96, dye concentration: 1,159 mg/L, contact time: 15 min, respectively. It was observed that lower concentration (1,159 mg/L) resulted in maximum colour removal (96.32 %) and higher concentration (2,841 mg/L) resulted in lesser colour removal efficiency (85.68 %). Contact time is significant factor in ozonation and same was observed in this study also. It was observed from MEP (Fig. 2) that the contact time of 10 min is sufficient for maximum colour removal of about 94 %. This indicates that higher the contact time between ozone and the dye, higher the removal rate and further increase in contact time does not show much impact on the removal rate (Sanjay Singh and Dikshit 2012).
In case of COD removal, maximum removal efficiency of 39.96, 57.86 and 49.98 % were obtained at pH: 1.96, dye concentration: 1,159 mg/L, contact time: 18.41 min, respectively and same is shown in Fig. 3. It was observed that the lower concentration of dye (1,159 mg/L) resulted in maximum COD removal (57.86 %), while higher concentration (2,841 mg/L) resulted in lesser COD removal (22.97 %). From the MEP, it was observed that the increase in contact time shows corresponding increase in COD removal (Houshyar et al. 2012).
The estimated regression coefficient values for second-order polynomial regression equation for the removal of colour and COD during ozonation process in PBR of AB 52 dye are given in Table 4. Equations 4 and 5 give polynomial regression equation with coefficients for colour and COD removal, respectively. The Student’s t test was used to estimate the significance of each parameter on the responses. This test is a sample test used to test the significance of regression coefficients in linear, quadratic and interaction effects. P value (probability level) is related to statistical significance and gives the percentage chance that the response of a statistical test is due to random error. Student’s t test and P value were used to relate the factors which are significantly related to the response. P values are always positive even for a negative T value. T values can be negative. The P value for a negative T value is the same as that for the positive account of that T value. In general, P value <0.05 indicates that the component has a significant effect on the variable (Montgomery 1991). It was observed from the analysis (Table 4) that the coefficients for the linear effect of time (T) were highly significant (P = 0.000) for both the colour and COD removal and for concentration (C) in case of colour removal is not as highly as significant (P = 0.084). Whereas it was observed that the linear effect of concentration (C) was found to be highly significant (P = 0.000) for COD removal. Both colour (P = 0.872) and COD (P = 0.957) removal were found to be insignificant with respect to pH and no correlation was also observed. The validity of this analysis was also observed in MEP Figs. 2 and 3. From MEP for colour and COD removal (%) (Figs. 2, 3), it was observed that the time and concentration plot shows almost a linear effect. The quadratic effect of time (T 2) were considered to be highly significant in colour (P = 0.000) and COD (P = 0.001) removals, whereas other quadratic factors showed very little significance. While for all other factors, significance was not observed in Table 4 with the higher P values >0.05. Similarly, significance for the interaction effects (PC, PT and CT) was not found having P values >0.05.
ANOVA was carried out to find the significance of the predicted values. ANOVA results for the colour and COD removal are shown in Table 5. The F test is used to compare the two sets of responses to test the exactness of the response is similar or dissimilar. The reliability of the model can be explained by the coefficient of determination (R 2). The higher the R 2, the better the prediction of responses based on experimental values. The coefficient of determination R 2 value was found to be 0.94. This clearly indicates that 94 % variation of response can be explained effectively and also confirms that 6 % of the variations occur while performing the experiments which were not explained by the model. The value shows that the prediction of the experimental data is quite satisfactory and Eqs. 4, 5 can be used effectively to predict the response of removal of colour and COD (%) with the combination of three coded variables (P, C and T). The calculated F statistics values (F colour removal = 17.45; F COD removal = 17.47) was found to be greater than the tabulated value F 9, 10 at 95 % confidence level as given in Table 5 with low P values (COD: P = 0.000; colour: P = 0.000). It clearly decodes that obtained Eqs. 4 and 5 are highly significant which gives the relationship between the response and the variables. It was observed that the residual sum of the squares (SS) and the lack of fit were small with a pure error of zero. This shows that the model explains the variables on colour and COD removal % in a more appropriate way. As shown in Table 5, interaction effects were found to be less significant with higher P values for colour removal (P = 0.993) and for COD removal (P = 0.477), respectively.
Response surface methodology was analysed with three variables to ascertain the colour and COD removal of AB 52 dye. Response contour (two-dimensional plane) and surface (three-dimensional plane) were plotted to ascertain the response with respect to the variables taken for the study. In a contour plot, the response can be analysed and convenient to find the interactions between the two variables keeping others as constant.
With respect to the change in three parameters, the contour and surface response plots were obtained for both colour and COD removal. The response contour plots and surface plots for the colour and COD removal of AB 52 dye are given in Figs. 4, 5 and 6, respectively. The contour plots show that there is a slight significance between dye concentration and time for COD removal which is also observed in Table 4 for interaction effect of dye concentration and time (CT) with P value of 0.15. The response surface plot gives the clear idea about the maximum removal % (colour and COD) at particular conditions.
Optimum conditions and validation
The optimum values of the process variables were obtained by response surface analysis method and same are shown in Table 6. Maximum colour removal (100 %) was obtained at the operating conditions, i.e. pH: 1.96, dye concentration: 1,159 mg/L and contact time: 10.6 min. In case of COD, the maximum removal (61 %) was obtained at pH: 4.8, dye concentration: 1,159 mg/L and contact time: 17.1 min.
The values which were obtained from the contour plots analysis was found to be almost similar with the optimum values. This elucidates that the experiments carried out based on central composite designs (CCD) can be used effectively to optimize the parameters. The obtained optimum conditions were checked by running the experiments for validation and the validation results are shown in Table 6. Colour and COD removal based on validation experiments were found to be 99.7 and 59.8 %, respectively. The values obtained from validation were in good agreement with the optimization values showing the accuracy of the experiments carried out.
Figure 7 shows the experimentally obtained and theoretically predicted values for colour as well as COD removal of AB 52 dye. The graph shows a linear pattern and justifies that the model is adequate enough to predict the colour and COD removal of AB 52 dye within the CCD variable values. The value of coefficient of determination R 2 (both colour and COD; R 2 = 0.94) shows good agreement of the model with the experimental data obtained.
Conclusions
The effect of three important variables namely viz., pH, dye concentration and contact time of AB 52 dye on colour and COD removal was studied. A statistical approach, 23 response surface CCD was successfully employed for experimental design and analysis of results, i.e. to study the linear, quadratic and interaction effects of each of the variables and also to optimize those variables for maximum removal of colour and COD. It was concluded that dye concentration and time were significant factors in colour and COD removal during ozonation in PBR. Appropriate regression model equations were developed for predicting the colour and COD removal for AB 52 dye and satisfactorily predicted the experimental values. Graphical surface and contour response plots were used to obtain the optimum points. The optimum conditions for maximum colour removal (100 %) and COD removal (61 %) were obtained at pH: 1.96; dye concentration: 1,159 mg/L; contact time: 10.6 min and at pH: 4.8; dye concentration: 1,159 mg/L; contact time: 17.1 min, respectively. Optimum values were confirmed by validation experiments.
References
Adinew Bizuneh (2012) Chemistry: textile effluent treatment and decolourisation techniques—a review. Bul J Sci Educ 21(3):434–456
Alalewi A, Cuiling Jain (2010) Overview of some colour removal technologies in the textile wastewater. In: 4th International conference on bioinformatics and biomedical engineering (iCBBE), Chengdu, China. 18–20 June: 1–4. ISBN No: 978-1-4244-4713-8. doi:10.1109/ICBBE.2010.5518300
Alaton IA, Balcioglu IA, Bahnemann DW (2002) Advanced oxidation of a reactive dyebath effluent: comparision of O3, H2O2/UV-C and TiO2/UV-A processes. Water Res 36(5):1143–1154. doi:10.1016/S0043-1354(01)00335-9
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. APHA, AWWA, Washington, DC, New York
Arlindo Gomes C, Luis Fernandes R, Rogerio Simoes MS (2012) Oxidation rates of two textile dyes by ozone: effect of pH and competitive kinetics. Chem Eng J 180–190:175–181. doi:10.1016/j.cej.2012.02.051
Ayed L, Ksibi I, Cheref A, Bakhrouf A (2012) Response surface methodology for optimization of the treatment of textile wastewater by a novel bacterial consortium: enzymes and metabolites characterization. Afr J Biotechnol 11(59):12339–12355. doi:10.5897/AJB11.3506
Aziz HA, Alias S, Adlan MN, Asaari AH, Faridah, Zaharim MS (2007) Colour removal from landfill leachate by coagulation and flocculation. Bioresour Technol 98(1):218–220. doi:10.1016/j.biortech.2005.11.013
Chaturapruek A, Visvanathan C, Ahn KH (2005) Ozonation of membrane bioreactor effluent for landfill leachate treatment. Environ Technol 26(1):65–73. doi:10.1080/09593332608618583
ChemicalBook (2010) http://www.chemicalbook.com/ChemicalProductProperty_EN_CB7300112.htm. Accessed 16 Nov 2011
De Gisi S, Galasso M, De Feo G (2009) Treatment of tannery wastewater through the combination of a conventional activated sludge process and reverse osmosis with a plane membrane. Desalination 249(1):337–342. doi:10.1016/j.desal.2009.03.014
Dilaconi C, Lopez A, Ramadori R, Di Pinto AC, Passino R (2002) Combined chemical and biological degradation of tannery wastewater by a periodic submerged filter. Water Res 36(9):2205–2214. doi:10.1016/S0043-1354(01)00445-6
Durai G, Rajasimman M (2011) Biological treatment of tannery wastewater—a review. J Environ Sci Technol 4(1):1–17. doi:10.3923/jest.2011.1.17
Fanchiang Jen-Mao, Tseng Dyi-Hwa, Guo Gia-Luen, Chen Hsi-Jien (2009) Ozonation of complex industrial park wastewater: effects on the change of wastewater characteristics. J Chem Technol Biotechnol 84(7):1007–1014. doi:10.1002/jctb.2124
Gao M, Zeng Z, Sun B, Zou H, Chen J, Shao L (2012) Ozonation of azo dye acid red 14 in a microporous tube-in-tube microchannel reactor: decolourisation and mechanism. Chemosphere 89(2):190–197. doi:10.1016/j.chemosphere.2012.05.083
Houshyar Z, Khoshfetrat AB, Fatehifara E (2012) Influence of ozonation process on characteristics of pre-alkalized tannery effluents. Chem Eng J 191:59–65. doi:10.1016/j.cej.2012.02.053
Lefebvre O, Vasudevan N, Torrijos M, Thanasekaran K, Moletta R (2005) Halophilic biological treatment of tannery soak liquor in a sequencing batch reactor. Water Res 39(8):1471–1480. doi:10.1016/j.watres.2004.12.038
Montgomery DC (1991) Design and analysis of experiments, 3rd edn. Wiley, New York
Oller I, Malato S, Sanchez-Perez JA (2011) Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Sci Total Environ 409(20):4141–4166. doi:10.1016/j.scitotenv.2010.08.061
Ravindranath E, Chitra Kalyanaraman, Suthanthararajan R, Umamaheswari B, Shamshath Begum S, Bhuvaneswari R, Rajamani S (2004) A scientific remedy for disposal of fleshing generated from tanneries. In: Proceedings of the national symposium on recent trends in industrial waste management for sustainable development (Annamalai University, Chidambaram, Tamilnadu, India). March 17–18, pp 111–115
Ravindranath E, Kalyanaraman Chitra, Shamshath Begum S, Gopalakrishnan N (2010) Effect of recirculation rate on anaerobic treatment of fleshing using UASB reactor with recovery of energy. J Sci Ind Res India 69(10):790–793
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255. doi:10.1016/S0960-8524(00)00080-8
Salem Abu Amr S, Aziz HA (2012) New treatment of stabilized leachate by ozone/Fenton in the advanced oxidation process. Waste Manage 32(9):1693–1698. doi:10.1016/j.wasman.2012.04.009
Sanjay Singh S, Dikshit AK (2012) Decolourization of polyaluminium chloride and fungal sequencing batch aerobic reactor treated molasses spentwash by ozone. Am J Environ Eng 2(3):45–48. doi:10.5923/j.ajee.20120203.01
Shamshath Begum S, Miranda LR, Srinivasan SV (2006) Adsorption of remazol brilliant red (5B) by activated carbon produced from rubber wood. Indian J Environ Prot 6:769–775
Shamshath Begum S, Hariganesh B, Vedaraman N (2011) Studies on degradation of dyeing wastewater generated from tanneries. In: Proceedings 3rd international conference on science, engineering and technology (3rd SET) (Vellore Institute of Technology University, Vellore, Tamilnadu, India). 17–18 November, 3:244–250. ISBN No:13 978-81-923320-3-1
Shin Choon-Hwan, Bae Jun-Seok (2012) A stability study of an advanced co-treatment system for dye wastewater reuse. J Ind Eng Chem 18(2):775–779. doi:10.1016/j.jiec.2011.11.120
Song Z, Williams CJ, Edyvean RGJ (2004) Treatment of tannery wastewater by chemical coagulation. Desalination 164(3):249–259. doi:10.1016/S0011-9164(04)00193-6
Srinivasan SV, Murthy DVS (2009) Statistical optimization for decolorization of textile dyes using Trametes versicolor. J Hazard Mater 165:909–914. doi:10.1016/j.jhazmat.2008.10.072
Srinivasan SV, Prea Samitha Mary G, Kalyanaraman Chitra, Sureshkumar PS, Sri Balakameshwari K, Suthanthararajan R, Ravindranath E (2012) Combined advanced oxidation and biological treatment of tannery effluent. Clean Technol Environ Policy 14:251–256. doi:10.1007/s10098-011-0393-x
Stoop MLM (2003) Water management of production systems optimized by environmentally oriented integral chain management: case study of leather manufacturing in developing countries. Technovation 23(3):265–278. doi:10.1016/S0166-4972(01)00117-1
Tabrizi GB, Mehrvar M (2004) Integration of advanced oxidation technologies and biological processes: recent developments, trends, and advances. J Environ Sci Health A Tox Hazard Subst Environ Eng 39(11–12):3029–3081. doi:10.1081/ESE-200034939
Thanikaivelan P, Jonnalagadda Rao R, Balachandran Nair U, Ramasami T (2004) Progress and recent trends in biotechnological methods for leather processing. Trends Biotechnol 22(4):181–188. doi:10.1016/j.tibtech.2004.02.008
Tizaoui C, Grima N (2011) Kinetics of the ozone oxidation of reactive orange 16 azo-dye in aqueous solution. Chem Eng J 173(2):463–473. doi:10.1016/j.cej.2011.08.014
Turhan K, Durukan I, Arda Ozturkcan S, Turgut Z (2012) Decolourisation of textile basic dye in aqueous solution by ozone. Dyes pigments 92(3):897–901. doi:10.1016/j.dyepig.2011.07.012
Acknowledgments
The authors wish to acknowledge the financial support granted from Council of Scientific and Industrial Research (CSIR), New Delhi, India (CSIR letter No. dated: 16.03.2006 :31/6/(260)/2006-EMR-I) to conduct this investigation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vedaraman, N., Shamshath Begum, S. & Srinivasan, S.V. Response surface methodology for decolourisation of leather dye using ozonation in a packed bed reactor. Clean Techn Environ Policy 15, 607–616 (2013). https://doi.org/10.1007/s10098-012-0544-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-012-0544-8