Abstract
Bank filtration is presented as a barrier for cyanobacteria and their metabolites (cyanotoxins and odor compounds). Retention of cyanobacteria is supported by column and bench studies using sandy materials. Most of the removal is in the first decimeters of infiltration, where physical and biological processes take place. Cyanotoxin removal is a very complex process including sorption and/or biodegradation. Sorption depends on the type of cyanotoxin and the sediments/aquifer composition. Biodegradation depends on the type of cyanotoxin, redox conditions, previous contact with the cyanotoxin, type and content of natural organic matter, and temperature. Pilot studies at the Lake Lagoa do Peri in Brazil demonstrated the effectiveness of bank filtration in removing phytoplankton and Cylindrospermopsis raciborskii (105–106 cells/mL) before reaching a sampling well at 12-m depth located 20 m from the lakeshore. Column experiments confirmed biomass removal predominantly in the upper 2 cm of infiltration. No saxitoxins (STXs) were found in the well water and a high retention of neo-STX and STX was determined by isotherm experiments. The sorption linear coefficient (K d 13–16 L/kg) was found to better represent the sorption processes than the Freundlich coefficient (K f 6.5–12 L/kg).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cyanobacteria are photosynthetic bacteria that can grow in salty, brackish or fresh water, and in cold and hot springs (Mur et al. 1999). They cause problems in many surface waters around the world (Lahti et al. 2001) and derivative drinking water supplies. They can release compounds like geosmin and 2-methylisoborneol (MIB), which impart an earthy or musty odor to the water. In addition, the presence of single cells, colonies, or filaments can interfere with the formation of flocs during the coagulation/flocculation treatment process and can clog the filters (Logsdon 2008). Cyanobacteria cells and extracellular material contribute to the total organic carbon content and can produce disinfection byproducts (Hoehn et al. 1980; Plummer and Edzwald 2001; Huang et al. 2009). According to Hudnell (2010), cyanobacteria are the primary organism responsible for fresh water algal blooms, of concern because of the production of cyanotoxins, which are among the most potent toxins for humans. Some cyanotoxins are strong neurotoxins [anatoxin-a (ATX) and saxitoxins (STXs)], others exhibit liver toxicity [microcystins (MCs), nodularin (NOD), and cylindrospermopsion (CYN)], another group (lipopolysaccharides) can cause gastroenteritis (Bartram et al. 1999), and a last group can irritate and trigger allergic reactions in the skin (aplysiatoxins and lyngbyatoxin) (Westrick 2003). In terms of regulations, the World Health Organization (WHO) established a provisional drinking water guideline value of 1 μg/L for microcystin-RL (MC-RL), one of the most frequent variants of MCs (WHO 2011).
The cyanotoxins are likely cell bound (produced and contained within the cells) and consequently they are released during cell senescence, death, and lysis (Sivonen and Jones 1999). Therefore, removal of intact cells is the primary objective for waterworks. In general, there is no single technique or isolated treatment step capable of removing all the intact cells and the dissolved cyanotoxins at once. Therefore, the multi-barrier principle, based on several treatment steps, must be applied (Hrudey et al. 1999; Westrick et al. 2010). For example, Lahti et al. (2001) reported the effective removal of cells and MCs in Finland waterworks using (1) the three-step sequence of chemical coagulation, clarification, and activated carbon or (2) the two-step sequence of pre-ozonation and slow sand filtration.
Bank filtration is a process during which surface water is subjected to subsurface flow prior to abstraction from vertical or horizontal wells. During bank filtration, the river/lake bed and aquifer material act as filter media. Along the flow path, water quality is improved in terms of turbidity, natural organic matter (NOM), dissolved organic carbon (DOC), organic micropollutants (e.g., pesticides, pharmaceuticals, endocrine and disruptor compounds), bacteria, viruses, and protozoa. Bank filtration has been applied in Europe since the 1870s and its use in the United States started around 50 years ago (Ray et al. 2002a, b). Recent studies showed the potential of bank filtration as water supply to many cities in the Ganga Plains, in India (Sandhu et al. 2011) and the Nile Valley in Egypt (Shamrukh and Abdel-Wahab 2008). Ray (2008) suggests the potential use of bank filtration to produce drinking water in Africa, northern Asia, and South America.
Bank filtration also appears to be an efficient drinking water process as unique treatment or as a pre-treatment step to remove cyanobacteria and cyanotoxins. Good results in Finland (Kivimäki et al. 1998; Lahti et al. 2001) and Germany (Chorus et al. 2001; Grützmacher et al. 2002) illustrate its removal capacity in temperate regions. The potential to remove cyanotoxins by soils and sediments has also been reported in Australia (Miller et al. 2001, 2005) and Egypt (Mohamed et al. 2007). Despite the fact that cyanobacteria blooms are a worldwide problem, studies are rather scarce.
The aim of this paper is to present the variables and mechanisms relevant to cyanotoxin removal and the potential of bank filtration as an effective barrier against cyanobacteria and cyanotoxins based on literature data and studies at the Lagoa do Peri. The Lagoa is a natural subtropical lake located in South Brazil, in which cyanobacteria blooms occur throughout the entire year. The lake’s cyanobacteria population is dominated by Cylindrospermopsis raciborskii.
Bank filtration as an effective barrier against cyanobacteria, cyanotoxins, and odor compounds
Bank filtration is an effective technique for removing particulate (i.e., cyanobacteria cells) and dissolved pollutants (i.e., cyanotoxins). This improvement is mainly due to physical filtering, biodegradation, sorption, and dilution (Ray et al. 2002a, b). These processes occur in two zones: the biologically active clogging layer (hyporheic zone) at the river/lake bed and the subsequent aquifer passage between the surface water source and the well. Biodegradation and sorption processes firstly take place in the organic-rich clogging layer, which typically has a short average residence time. In the aquifer, further degradation and sorption occurs at lower reaction velocities, and dispersion and mixing with landside groundwater further decrease and equilibrate input concentrations (Hiscock and Grischek 2002). Recent studies by Gunkel and Hoffmann (2009) at Lake Tegel, Berlin, identified a highly active biological zone in the upper 10 cm of the lake bottom sediments. This zone is formed by a biocenosis or biological community of epipsammic algae (algae that lives attached to sand grains), bacteria, meiofauna, and biofilms/extracellular polymeric substances. These bacteria and the consuming meiofauna enable a high turnover of fresh organic matter (OM). Moreover, Gunkel et al. (2009) observed the consumption of fluorescently labeled fine particulate OM by meiofauna, including fragments of planktonic algae.
Removal of cyanobacteria cells
One of the first studies published was performed in a laboratory using soil and lake sediments column studies in Finland (Lahti et al. 1998). In this study, lake water inoculated with two toxic and one non-toxic species (i.e., Mycrocystis sp. strain 98, Anabaena sp. strain 90, and Oscillatoria sp. strain 18G, respectively) was filtered through columns filled with soil (two columns in series, height 0.25 m each) and lake sediment (one column, height 1.1 m). The column detention times were 5.25 and 6.2 h in soil and sediment columns, respectively. During 9–14 days of the flow-through experiment, the cyanobacteria cells were retained efficiently in both column systems with a 98.4–99.9 % cyanobacteria biomass reduction. Furthermore, the removal of filamentous cyanobacteria (Anabaena and Oscillatoria) was more effective than that of cyanobacteria occurring as single cells (Microcystis).
Kloep and Röske (2004) studied advective transport of algae in the hyporheic zone of the bank filtration waterworks Dresden-Saloppe on the Elbe River, Germany and column experiments. The analysis of sediment samples taken in the field showed that the majority of the penetrated algae are retained in the hyporheic sediments. In the column experiments, more than 90 % of the algal cells were retained within the upper 10 cm of sediment. Moreover, the results proved that the retention capacity depends on the permeability of the sediment and the morphotype of the algae. For example, in the case of Scenedesmus acuminatus 98.6 % of the cells were retained when the permeability was 2.7 × 10−5 m/s and the retention was reduced to 83.6 % with an increase in permeability to 1.4 × 10−4 m/s. Smaller cells moved more readily than larger cells through the sediments. The authors also asserted that stingers (small protrusions) on the cells of the species Desmodesmus communis promote pore bridging and clogging of pores.
The studies cited show that cyanobacteria cell removal via bank filtration depends principally on the permeability of the filter material and the morphotype of the species present in surface water. The results indicate that most of the removal occurs in a relatively short infiltration distance, the so-called clogging layer.
Therefore, it is easy to interpret the results from actual production wells located several meters away from surface water sources. For example, Lahti et al. (1998, 2001) only occasionally observed individual cells and filaments in wells located 100 m from two lakes situated in an esker area in Finland. Even though the uppermost layer in both lakes consisted of sand with silty and clayey material, the sand and gravel formation of the esker formation might have permitted the migration of a few cyanobacteria cells.
Clancy and Gollnitz (2003) compared the removal of microorganisms for six engineered waterworks and six bank filtration sites in the United States and Canada. The systems were monitored for periods ranging from 1 to 11 years. Determination, as accomplished using microscopic particulate analysis, was based on the filtration of 100 gallons (378.5 L) of water through a cartridge filter. After filtration and re-suspension, the biological material of a portion of the filter was counted through a microscope. Algae and diatoms, used as microorganism indicators, varied from 102 to 109 per 100 gallons (0.26–2.6 × 106 microorganism per liter) in the raw waters. The engineered waterworks removal ranged from non-reduction to greater than 6 log10. The bank filtered water showed a reduction of at least 4 log10 to greater than 7 log10. In summary, all previous studies demonstrate the effectiveness of bank filtration as a barrier against cyanobacteria cells.
Removal of cyanotoxins
The main processes that remove soluble organic compounds during bank filtration are adsorption and biodegradation. The most relevant studies concerning those mechanisms at both bench scale and full scale related to cyanotoxins are summarized in the following paragraphs.
An overview of sorption studies for several cyanotoxins is given in Table 1. The cyanotoxins most often studied are the MCs and to a lesser extent NOD. The low sorption parameters (K d, K f, or K L stand for the distribution coefficients, as per the linear, Freundlich and Langmuir models, respectively) indicate that for those cyanotoxins sorption is very low. A Freundlich exponent (n) lower than one shows high sorbent loadings at low concentrations (Worch 2012) and weaker sorption as concentration increases (Schwarzenbach et al. 2003). Interestingly, studies with River Nile and canal sediments in Egypt showed higher adsorption parameters for MC-LR (Mohamed et al. 2007). However, differences in the physicochemical parameters of the sediments and pH of water can influence the adsorption of a compound. The first studies have shown that sorption increases with the amount of clay and/or OM in the sorbents (Miller et al. 2001, 2005) with a reduced impact of the last factor (Dillon et al. 2002). Wu et al. (2011) concluded that the OM content controls the adsorption after evaluating the sorption of MC-LR and MC-RR in 15 sediments of eutrophic lakes in China and four clay minerals. At high OM (i.e., >8 %) content, adsorption depends on OM whereas at low level OM (i.e., <8 %) clay content dominates the adsorption. In the case of high OM, the interactions of MCs and OM are favored, while in the case of low OM the MCs and dissolved OM compete for the adsorption sites. Another important aspect that influences the adsorption of NOD and MCs is the water pH, since a higher pH is associated with decreased adsorption (Miller et al. 2001; Wu et al. 2011). At lower pH cyanotoxins have no charge, while in the pH range 4–10 they have one negative charge. Similarly, the surface of natural adsorbents is typically positively charged at low pH and negatively charged at high pH. Moreover, fulvic and humic acids that form the major portion of OM become more negatively charged as pH increases. Therefore, repulsion between the cyanotoxins and the sorbents hinders sorption. A small difference in sorption between MC-RR compared with MC-LR and MC-YR is explained as a difference in dissociation between the amino acids groups of the compounds. MC-LR and MC-YR possess one lysine and one tyrosine group, respectively, which are dissociated at neutral pH, increasing repulsion with the negative charge of the sorbent (Grützmacher et al. 2010). In addition, the MC-RR structure possesses one more arginine residue that favors the formation of cation bridges and hydrogen bonds with clays (Wu et al. 2011).
Another group of cyanotoxins includes the positively charged STX (pK a1 = 8.22 and pK a2 = 11.28) and ATX (pK a = 9.2) and the zwitterion CYN that simultaneously exhibits both a positive and a negative charge at neutral pH. Sorption of both positively charged cyanotoxins shows a strong correlation with the clay content (Table 1), most probably due to the interaction with the negatively charged sediment particles or clay surfaces. This idea is supported by a good correlation with the cation exchange capacities (CEC) of the sorbents (Burns et al. 2009; Klitzke et al. 2011). The zwitterion CYN is highly hydrophilic and might be sorbed to OM by a non-specific sorption mechanism and the cation exchange might therefore be limited (Klitzke et al. 2011).
In summary, sorption of the cyanotoxins depends on the type and constitution of the sorbent (i.e., clay and OM content) and the chemical structure and properties of the cyanotoxins. If the toxin is positively charged it would be strongly sorbed, if it is negative or neutral and highly hydrophilic it would be maintained in the water phase. If the cyanotoxins are adsorbed, they will not reach the production wells and could be degraded by sessile microorganisms. However, changes in pH or ionic strength can cause desorption of cationic compounds. The cyanotoxins would then be re-dissolved in the water and transported to the wells.
Studies about degradation of cyanotoxins have been focused on poorly retained cyanotoxins such as NOD, MCs, and CYN. Lahti et al. (1998) found in soil and sediment column experiments—mentioned in the previous section—that biodegradation accounted for 60–77 and 44–61 % of removal in sediments and soil, respectively. The total removal was 75–95 % in both types of column tests. Later on, during batch experiments Miller and Fallowfield (2001) and Dillon et al. (2002) reported quick degradation of MC-LR and NOD as observed in the half-life values in Table 2. Soils with high OM (2.9 %) and clay content (16.1 %) showed higher degradation rates and sandy soils (98.5 %) presented no degradation. According to Dillon et al. (2002), the sandy soil used in the experiment contained insufficient nutrients to support the growth of toxin degraders. These batch experiments were conducted under oxic conditions. Later, sediment tests proved that MC biodegradation can also occur during anoxic conditions (Holst et al. 2003; Chen et al. 2010; Grützmacher et al. 2010). The half-life data indicate that oxic degradation is more favorable. Grützmacher et al. (2010), using lower concentrations of MC-LR, MC-RR and MC-YR, obtained similar results among the cyanotoxins studied during oxic conditions, but variable results during anoxic conditions. These results exhibited higher rates than those obtained by Chen et al. (2010) for MC-LR at higher concentrations. Grützmacher et al. (2010) postulated that in the case of realistic concentrations (10 μg/L) of MCs, under both redox conditions, a 50-day travel time in the subsurface would insure complete removal. An increase in the biodegradation rate at higher temperatures was also observed (Chen et al. 2010).
Klitzke et al. (2010) and Klitzke and Fastner (2012) studied CYN degradation under both oxic and anoxic conditions (Table 2). Even though biodegradation under oxic conditions is relatively fast, the authors proposed that 50 days of travel time are not enough for complete removal because of a lower degradation rate under anoxic conditions, which often arise along bank filtration flow paths. They also observed a lag phase if no previous contact exists between the sediments and the cyanotoxins and a possible substrate competition with the OM present.
Biodegradation studies of STX and ATX are scarce. Donovan et al. (2008) observed the activity of seven unidentified bacteria isolated from the digestive tract of blue mussels capable of reducing the toxicity of a STX mixture by 90 % within 1–3 days. No studies were found about biodegradation of STX and ATX in drinking water systems, sediments, or under any other circumstances relevant to bank filtration systems. By contrast, while the transformation of the less toxic variants (C1 and C2) of STX into the more toxic ones (GTX2, GTX3, and STX) has been observed in a biologically active sand filter containing anthracite, no variant concentration changes were observed in a parallel filter containing only sand (Kayal et al. 2008). In a different study using wastewater polluted with STXs, Ho et al. (2012a) reported a transformation of C2 variant into GTX2 and consequently a toxicity increase in the effluent after treatment by biological sand filters. Moreover, Ho et al. (2012c), using water from an Australian reservoir, found that under oxic conditions and two temperatures (14 and 24 °C), STXs (5 μg STX-eq/L) were not biodegraded by the water’s indigenous microbes during batch experiments. By contrast, MCs (10 μg/L) and geosmin (0.1 μg/L) were effectively biodegraded in less than 25 days.
In summary, the degradation of cyanotoxins depends on the type of cyanotoxin, previous contact with the cyanotoxin, OM present, and composition of sediment/aquifer material. In addition, redox conditions and temperature can play an important role in the degradation velocity.
With respect to the removal of cyanotoxins at bank filtration sites, only very few reports exist. Lahti et al. (1998, 2001) reported only traces of MCs (below 0.1 μg/L MC-LR equivalents) in four bank filtration plants in Finland while the maximum raw water concentration was 1.9 μg/L. Chorus et al. (2001) also observed a strong reduction in MC from 18.5 μg/L cell bond and 0.51 μg/L dissolved to less than 0.06 μg/L in a bank filtration system at Radeburg near Dresden, Germany. MC removal from 20 μg/L to less than 0.1 μg/L has also been observed during bank filtration at Lake Wannsee, Berlin, Germany (Grützmacher et al. 2002).
Removal of odor compounds
The odor metabolites MIB and geosmin are readily biodegradable by microorganisms found in water treatment systems (Ho et al. 2012b). Geosmin and other odorous compounds have been attenuated during bank filtration (Jüttner 1995; Chorus et al. 1992). Recently, Maeng et al. (2012) obtained complete removal of both metabolites from spiked water (70–293 ng/L of geosmin and MIB) using batch experiments with silica sand previously acclimated for 30 days with river water. Column studies demonstrated that adsorption, followed by biodegradation, is the most important removal mechanism of both substances. Biodegradation accounted for 23 and 31 % of the total removal of geosmin and MIB, respectively. The authors conclude that managed aquifer recharge (including riverbank filtration, artificial recharge, and soil aquifer treatment) is a robust barrier against taste- and odor-causing compounds such as MIB and geosmin.
Lagoa do Peri Lake: case study
Site study
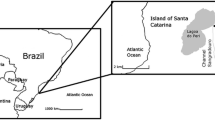
The Lagoa do Peri Lake is located in the south of the Island of Santa Catarina, in the state of Santa Catarina, Brazil (Fig. 1). The lake is part of a national park that protects the lake and its catchment area of 5.2 and 20.1 km2, respectively. The perimeter of the lake is about 11 km, the length 4 km, minimum width 1.5 km, maximum depth 11 m, and medium depth 7 m (Oliveira 2002). The climate of the region is subtropical with an average temperature during summer of 24.3 °C and of 16.4 °C in the winter. The precipitation falls mostly during spring and summer (October–March) and averages around 1,519 mm/year (Simonassi 2001; Mondardo 2004). The wind also intensifies during that period from the N-NE and S-SE (Hennemann and Petrucio 2011).
The vegetation around the lake is typical of the Atlantic Rain Forest, consisting of small and medium trees and shrubs and protected by national park status; therefore anthropogenic influence is relatively small and limited to recreation during the summer. Probably, this is one of the main reasons that the Santa Catarina’s Sanitation Company (CASAN in Portuguese) decided to construct a waterworks to supply water to the south of the island in the year 2000. The waterworks operates a direct filtration system with capacity to supply water for up to 100,000 people during peak demand. However, the filters become quickly clogged because of the natural occurrence of phytoplankton during the whole year with dominance of the cyanobacteria species C. raciborskii. The filters must therefore be cleaned regularly, creating a high demand for manpower, problems with sludge disposal and high treatment costs. Besides this, the risk of breakthrough of cyanobacteria cells and cyanotoxins is also present. Specific properties of C. raciborskii including survival at low phosphorus concentrations, use of ammonium as a nitrogen source, tolerance of a wide range of temperature and low light environments permit its dominance in the Lagoa do Peri (Hennemann and Petrucio 2011).
Considering that the lake terminates in the east with a coastal plain area formed mainly by marine deposits, bank filtration was considered as an alternative to treat or pre-treat the lake water. This region, 600-m wide and about 1.6 km2 in area, is formed by sandy materials with some silt, clay, and peat lenses to a maximum depth of 20 m. In the region, several experiments at pilot and bench scale were conducted to assess the feasibility of bank filtration. Data about water quality parameters, NOM removal and redox conditions as well as groundwater flow modeling can be found in other under evaluation studies (Romero et al., unpublished manuscript; Soares et al. 2014). Therefore, only the results from experiments regarding cyanobacteria and cyanotoxins are presented.
Materials and methods
Bank filtration well
A 100-mm diameter and 12-m deep well was installed 20 m away from the lakeshore. The well was made of PVC tube and had a 2-m long filter screen located at a depth between 10 and 12 m below ground (about 9.5 m below groundwater level). Results from the drilling confirmed the presence of mainly marine sediments with an upper sand layer of 1-m thickness with a high OM content and dark color. From a depth of 1–4 m, clean sand with some occurrence of OM lenses was found. From a depth of 4–18 m, the aquifer consists of fine sand, underlined by a clay layer from a depth of 18–23 m. Thus, the total aquifer thickness is about 18 m. A centrifugal pump (Schneider BCR 2000) installed in 2005 was operated at a pumping rate of 24 m3/day. Phytoplankton, cyanobacteria, and C. raciborskii cell contents were determined in lake and well water monthly between February 2005 and January 2006. STX, neo-STX, GTX1, GTX2, GTX3, and GTX4 were analyzed in water samples from June to September 2006.
Column experiments for study of cyanobacteria removal
To simulate wave movement at the lake bank, which reduces the clogging during bank filtration, a flow channel was used. The model is based on a concept by Beyer and Banscher (1976) and a newer version used by Grischek et al. (2002). A storage tank is filled continuously with fresh lake water (Fig. 2). The water flows with a controlled flow velocity through a channel. Four PVC columns (diameter 0.1 m and length 0.5 m) filled with 15 cm of gravel on the bottom and 29 cm of lake bottom sediment on top are fixed at the bottom of the channel, allowing infiltration of lake water. Four piezometers are installed at different depths in the columns (1, 10, 21, and 29 cm) to measure the hydraulic head relative to the water level in the channel. A constant hydraulic head of 70 cm was maintained for all columns. The system was operated from July to December 2004. The filter velocity, initially between 1.8 and 6.5 m/day in all columns, dropped quickly in the first 2 days of infiltration and then remained steady at values close to 0.1 m/day during the rest of the experiment, in all columns.
Column system used to study phytoplankton removal (Grischek et al. 2002)
Phytoplankton content in the raw water and the column effluent was measured monthly from August to December. By the end of this period, sediment samples were taken and studied through a microscope (400×) from 0–2, 2–5, 5–10, 10–20, and 20–30 cm from columns 1 and 3. A sample from the first 2 cm of the lake bottom was examined as well.
Batch experiments for study of cyanotoxins adsorption
STX and neo-STX sorption tests were performed with sediments from the first 50 cm of the lake bottom within the catchment area of the abstraction well. The collected sediments were stored in the dark at 4 °C until used in the experiments. The OM content determined as loss of ignition of the sediment was 0.20 %. Sieving analysis showed 15.4 % medium and coarse sand (0.2–2 mm), 80.6 % fine sand (0.2–0.05 mm), and 4 % fines (clay and silt <0.063 mm). The sorption tests were performed as it follows: 50 mL of the cyanotoxins solutions was placed in contact with sediment subsamples, previously dried at 105 °C for 24 h, of 1, 3, 6, 8, and 10 g in Erlenmeyer flasks. The solutions were shaken for 24 h at 19 ± 3 °C in an incubator shaker. Other researchers (Burns et al. 2009) reported equilibrium conditions for STX sorption in clay and sediment samples after 16 h, but the present studies were performed using 24 h as a convenient time schedule. Two experiments were assayed, each one with a different initial concentration of both cyanotoxins. Because a cultivated source of cyanobacteria was used, each test started with a different amount of both cyanotoxins: 46.36, 11.06 μg/L and 48.93, 11.06 μg/L of neo-STX and STX, respectively. After the shaken period, the samples were filtered through a 1.22-μm glass fiber filter and stored at 4 °C until analysis of cyanotoxins.
The adsorption processes were analyzed using Freundlich (1) and linear sorption isotherms (2).
where C s is the equilibrium toxin concentration in the sediment (μg/kg), C w is the equilibrium toxin concentration in the water (μg/L), K f is the Freundlich sorption coefficient (L/kg), n is the Freundlich exponent, and K d is the linear sorption coefficient (L/kg)
All data were plotted for both isotherms and the one showing the best fit was chosen as the descriptive model for the sorption process under the experimental conditions.
Determination of cyanobacteria and cyanotoxins
Phytoplankton cells were counted using an inverted microscope (Diavert model, Leitz, 400X) and the Utermöhl technique as described by Hasle (1978). The results are reported in individual/mL in the case of the column study and in cell/mL for the pilot abstraction well. The photographs of the pore water were obtained using fluorescence in situ hybridization (FISH) in the same microscope.
The cyanotoxins STX and neo-STX were obtained from a harvested culture of C. raciborskii, since using pure toxin standards would have been too expensive. Cultures of C. raciborskii (obtained from the Laboratory of Ecophysiology and Ecotoxicology of Cyanobacteria of the Federal University of Rio de Janeiro) strain T3 were grown at 22 ± 2 °C in ASM-1 medium (Gorham et al. 1964), sparged with air at 12-h periods of 50 μmol photon/m2 s irradiance. Cells were harvested when the cell density reached about 107 cells/mL. STX extract was obtained after two cycles of freezing and thawing and filtration through GF/92 filters. The STX working solutions were obtained by diluting the extracts with Milli-Q water and maintaining pH at 7.2 with a buffer solution.
The STX analysis for both the extracts and the water samples was performed with high-pressure liquid chromatography (HPLC) following the procedure described by Oshima (1995). The method consists of the ionic separation by HPLC, post-column derivatization, and fluorescence detection. A Shimadzu LC-10AD chromatograph and a Shimadzu RF 551 fluorescence apparatus were used. The Shimadzu HPLC system used a Merck Lichrospher (125 × 4.6 mm i.d.) column. The mobile phase to determine STX and neo-STX was a mixture of 2 mM sodium 1-heptanesulfonate in 30 mM ammonium phosphate buffer solution (pH 7.1) and acetonitrile (10:5). The GTXs variants were separated using only 2 mM sodium 1-heptanesulfonate in 30 mM ammonium phosphate buffer solution (pH 7.1). The temperature of the column was kept constant at 30 °C and a flow rate of 0.6 mL/min. The eluate was continuously mixed with 7 mM periodic acid in 10 mM potassium phosphate buffer (0.45 mL/min, pH 9.0) while passing through a Teflon® tube (0.5 mm i.d., 10 m long, 85 °C), and then mixed with 500 mM acetic acid (0.2 mL/min) before entering the detector. The fluorescence detector was set at an excitation wavelength of 330 nm and an emission wavelength of 390 nm. The standards were purchased from the Institute for Marine Bioscience, National Research Council of Canada in Halifax. The detection limits of STX, neo-STX, GTX1, GTX2, GTX3, and GTX4 were 0.72, 1.56, 2.95, 0.73, 0.65, and 3.78 μg/L, respectively.
Results and discussion
Bank filtration well
The phytoplankton content in the lake water did not show any specific seasonal variation. Total phytoplankton averaged (1.4 ± 0.3) × 106 cells/mL from January 2005 to January 2006. Total cyanobacteria were (1.3 ± 0.3) × 106 cells/mL and C. raciborskii dominated with an average concentration of (1.2 ± 0.4) × 106 cells/mL. During the whole monitoring period no cells, filaments, or parts of phytoplankton or cyanobacteria were found in the well water, proving that bank filtration was an effective barrier. The results obtained were similar and even better than previous reports in Finland, where residues of cyanobacteria were found in the well water (Lahti et al. 1998, 2001). It is likely that the aquifer material at Lagoa do Peri, consisting primarily of medium to fine sand, has a higher filtration capacity than the gravel aquifer material at the lake bank filtration site in Finland.
The raw lake water only yielded positive results in one sample for dissolved neo-STX (4.1 μg/L) and in two different samples for cell bond GTX-4 (9.8 and 10.0 μg/L). The results for STX, neo-STX, GTX1, GTX2, GTX3, and GTX4 in the water from the abstraction well were below detection limits in all samples.
The results agree with the local legislation for both cyanobacteria cells and cyanotoxins. Brazilian law requires monthly monitoring for Water Treatment Plants (WTP) with a 10,000 cells/mL limit value (BMH 2011). If that value is exceeded, and up to 20,000 cells/mL and toxicity is evidenced through bioassays using mice, weekly analysis of cyanotoxins becomes mandatory. The guideline establishes a limit of 3.0 μg equivalents/L for STX. STX equivalents are obtained by multiplying the concentration of each STX variant by a toxicity factor. In this case, lake water samples had 3.78, 7.11, and 7.26 μg Eq/L, and bank filtrate was always below detection limits. Therefore, bank filtrate clearly meets the requirement in terms of both cyanobacteria cells and cyanotoxins.
Column experiments
The column study showed that most of phytoplankton removal takes place in the first centimeters of infiltration. Total phytoplankton and C. raciborskii removal after passing 30 cm in the columns during the study period were between 94 and 97 %, in all columns (Fig. 3). Moreover, microscopic observations at different depths in two of the columns showed that most of the cells and filaments were retained in the first 2 cm of the sediments. As seen in Fig. 4, the amount of phytoplankton was lower between 2 and 5 cm and was not found after 20–30 cm. A sediment sample taken from the lake bank beneath the water table showed a similar density of phytoplankton in the first centimeters of sediment. In addition, the average head loss in the upper 10 cm of sediment in the columns was higher than in the deeper parts of the columns, confirming the clogging layer on top.
Batch experiments
The sorption of both cyanotoxins neo-STX and STX was between 40 and 80 % in the batch experiments. The linear sorption coefficients of both cyanotoxins variants fit better than the Freundlich model as the r 2 indicates (Table 3). The positively charged cyanotoxins are retained in the sediments mainly because the sediment contained a small amount of fines (4 % clay and silt) in which cation exchange can occur. As expected, the sorption parameters are similar, since the only structural difference is a hydroxyl group in neo-STX instead of a hydrogen atom in the case of STX. Moreover, the n value for neo-STX reflects a lower affinity to the sorbent than for STX, probably because its extra hydroxyl group makes it more water soluble.
The sorption coefficients calculated are much lower than those reported by Burns et al. (2009) in clay and sediments (Table 1). They used clay minerals and sediments containing very high amounts of clay and silt (8–42 % silt; 5–30 % clay and 59–89.6 % silt; 2.8–22.1 % clay) and much higher concentrations of toxins. Moreover, the results presented here involved the two cyanotoxins in the same solution together with residuals of NOM from their cultures. Therefore, sorption interference among both cyanotoxins and the cell OM likely occurred. The results of this study are slightly higher than those of Klitzke et al. (2011) for ATX (Table 1). Regardless of the differences between the sediments tested, the cyanotoxins evaluated exhibit two positive charges (STX: pK a1 = 8.22 and pK a2 = 11.28) rather than one in the case of ATX (pK a = 9.2). Similarly, ATX studies (Klitzke et al. 2011) indicate that the sorption of this type of cyanotoxin can be an important removal mechanism, however, there is a risk of desorption in case of pH and ionic strength variation. In the case of the well system in the Lagoa do Peri, the pH of the raw water and abstraction well water was 7.10 ± 0.55 and 7.73 ± 0.26, respectively (Romero et al., unpublished manuscript). Therefore, no strong increase in pH is expected, which would affect the adsorption capacity.
Conclusions
The attenuation of cyanobacteria during bank filtration depends on the type of sediments and aquifer material. Although the morphotype of the cyanobacteria species influences advective transport, given a sufficiently long flow path, bank filtration in sand and gravel aquifers is a reliable barrier for the removal of cyanobacteria.
The attenuation of cyanotoxins is based on sorption and biodegradation. Sorption depends on the cyanotoxins properties and the composition of the sediment/aquifer material. The content of fine materials (mainly clay) and OM facilitates sorption. However, some cyanotoxins exhibit positive or negative charges depending on the pH, in such cases sorption can be enhanced or reduced if the sorbent material is also charged. Dissolved OM can also compete for the sorption sites. Therefore, biodegradation is the preferable removal mechanism for most of the cyanotoxins. The cyanotoxin degradation depends mainly on the type of cyanotoxin, redox conditions, temperature, and the presence of OM.
The studies at the Lagoa do Peri confirmed that bank filtration is an effective method of cyanobacteria removal. Cell removal was about 95 % within the first 2 cm of infiltration. STX toxin removal by sorption in the lake sediments is likely and no desorption is expected to occur, since no relevant changes in pH are expected along the flow path to the abstraction well. Nevertheless, regular monitoring is recommended when using bank filtration for drinking water treatment.
Abbreviations
- C 0 :
-
Concentration at time t = 0 (μg/L)
- C s :
-
Equilibrium concentration in sediment (μg/kg)
- C w :
-
Equilibrium concentration in water (μg/L)
- K f :
-
Freundlich sorption coefficient (L/kg)
- K d :
-
Linear sorption coefficient (L/kg)
- K L :
-
Langmuir coefficient (L/μg)
- q m :
-
Maximum sorbent loading (μg/kg)
- n :
-
Freundlich exponent
- pK a :
-
−logK a
- t 1/2 :
-
Half-life time (days)
- r 2 :
-
Correlation coefficient
References
Bartram J, Carmichael WW, Chorus I et al (1999) Introduction. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. The World Health Organization, E&FN Spon, London, pp 12–25
Beyer W, Banscher E (1976) Zur Erkundungsmethodik der Uferfiltratgewinnung (Exploration methodology for bank filtration sites) (in German). Z Angew Geol 22(4):149–154
BMH (2011) Act. No. 2.914. Brazilian Ministry of Health, 12.12.2011. Official Gacette. 14.12.2011, pp 39–46
Burns JM, Hall S, Ferry JL (2009) The adsorption of saxitoxin to clays and sediments in fresh and saline waters. Water Res 43:1899–1904. doi:10.1016/j.watres.2009.02.004
Chen X, Yang X, Yang L et al (2010) An effective pathway for the removal of microcystin LR via anoxic biodegradation in lake sediments. Water Res 44:1884–1892. doi:10.1016/j.watres.2009.11.025
Chorus I, Klein G, Fastner J, Rotard W (1992) Off-flavors in surface waters, how efficient is bank filtration for their abatement in drinking water? Water Sci Technol 25:251–258
Chorus I, Schlag G, Heinze R et al (2001) Elimination of microcystins through bank filtration at the Radeburg Reservoir. In: Chorus I (ed) Cyanotoxins: occurrence, causes, and consequences. Springer, Berlin, pp 226–228
Clancy J, Gollnitz W (2003) Using microscopic particulate analysis for riverbank filtration. In: Melin G (ed) Riverbank filtration: the future is now! Proceedings of the second international riverbank filtration conference. National Water Research Institute, Cincinnati, OH, pp 111–113
Dillon PJ, Miller M, Fallowfield H, Hutson J (2002) The potential of riverbank filtration for drinking water supplies in relation to microsystin removal in brackish aquifers. J Hydrol 266:209–221
Donovan CJ, Ku JC, Quilliam MA, Gill TA (2008) Bacterial degradation of paralytic shellfish toxins. Toxicon 52:91–100
Gorham PR, McLachlan J, Hammer UT, Kim WK (1964) Isolation and culture of toxic strains of Anabaena flos-aquae (Lyngb.) de Bréb. Verh Int Ver Theor Angew Limnol 15:796–804
Grischek T, Macheleidt W, Nestler W (2002) River bed specifics and their effect on bank filtration efficiency. In: Dillon P (ed) Management of aquifer recharge for sustainability. Swets and Zeitlinger, Lisse, pp 59–64
Grützmacher G, Bottcher G, Chorus I et al (2002) Cyanobacterial toxins in bank filtered water from Lake Wannsee, Berlin. In: Dillon P (ed) Management of aquifer recharge for sustainability. Swets and Zeitlinger, Lisse, pp 175–179
Grützmacher G, Wessel G, Klitzke S, Chorus I (2010) Microcystin elimination during sediment contact. Environ Sci Technol 44:657–662. doi:10.1021/es9016816
Gunkel G, Hoffmann A (2009) Bank filtration of rivers and lakes to improve the raw water quality for drinking water supply. In: Gertsen N, Sønderby L (eds) Water purification. Nova Science Publishers Inc., New York, pp 137–169
Gunkel G, Beulker C, Hoffmann A, Kosmol J (2009) Fine particulate organic matter (FPOM) transport and processing in littoral interstices: use of fluorescent markers. Limnol Ecol Manage Inland Waters 39:185–199. doi:10.1016/j.limno.2008.11.001
Hasle GR (1978) The inverted microscope method. In: Sournia A (ed) Phytoplankton manual. UNESCO, Paris, pp 88–96
Hennemann MC, Petrucio MM (2011) Spatial and temporal dynamic of trophic relevant parameters in a subtropical coastal lagoon in Brazil. Environ Monit Assess 181:347–361. doi:10.1007/s10661-010-1833-5
Hiscock KM, Grischek T (2002) Attenuation of groundwater pollution by bank filtration. J Hydrol 266:139–144
Ho L, Hoefel D, Grasset C et al (2012a) Removal of cyanobacterial metabolites through wastewater treatment plant filters. Water Sci Technol 65:1244–1251. doi:10.2166/wst.2012.002
Ho L, Sawade E, Newcombe G (2012b) Biological treatment options for cyanobacteria metabolite removal: a review. Water Res 46:1536–1548. doi:10.1016/j.watres.2011.11.018
Ho L, Tang T, Monis PT, Hoefel D (2012c) Biodegradation of multiple cyanobacterial metabolites in drinking water supplies. Chemosphere 87:1149–1154. doi:10.1016/j.chemosphere.2012.02.020
Hoehn R, Barnes D, Thompson B et al (1980) Algae as sources of trihalomethane precursors. J AWWA 72:344–349
Holst T, Jørgensen NOG, Jørgensen C, Johansen A (2003) Degradation of microcystin in sediments at oxic and anoxic, denitrifying conditions. Water Res 37:4748–4760. doi:10.1016/S0043-1354(03)00413-5
Hrudey S, Burch M, Drikas M, Gregory R (1999) Remedial measures. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. The World Health Organization, E&FN Spon, London, pp 267–301
Huang J, Graham N, Templeton MR et al (2009) A comparison of the role of two blue-green algae in THM and HAA formation. Water Res 43:3009–3018. doi:10.1016/j.watres.2009.04.029
Hudnell HK (2010) Within water-body management: a needed but neglected complement to watershed management. Clean Technol Environ Policy 12:205–207. doi:10.1007/s10098-010-0304-6
Jüttner F (1995) Elimination of terpenoid odorous compounds by slow sand and river bank filtration of the Ruhr River, Germany. Water Sci Technol 31:211–217
Kayal N, Newcombe G, Ho L (2008) Investigating the fate of saxitoxins in biologically active water treatment plant filters. Environ Toxicol 23:751–755. doi:10.1002/tox
Kivimäki A, Lahti K, Hatva T et al (1998) Removal of organic matter during bank filtration. In: Peter J et al (eds) Artificial recharge of groundwater. Balkema, Rotterdam, pp 107–112
Klitzke S, Fastner J (2012) Cylindrospermopsin degradation in sediments: the role of temperature, redox conditions, and dissolved carbon. Water Res 46:1549–1555. doi:10.1016/j.watres.2011.12.014
Klitzke S, Apelt S, Weiler C et al (2010) Retention and degradation of the cyanobacterial toxin cylindrospermopsin in sediments: the role of sediment preconditioning and DOM composition. Toxicon 55:999–1007. doi:10.1016/j.toxicon.2009.06.036
Klitzke S, Beusch C, Fastner J (2011) Sorption of the cyanobacterial toxins cylindrospermopsin and anatoxin-a to sediments. Water Res 45:1338–1346. doi:10.1016/j.watres.2010.10.019
Kloep F, Röske I (2004) Transport of algal cells in hyporheic sediments of the River Elbe (Germany). Int Rev Hydrobiol 89:88–101. doi:10.1002/iroh.200310662
Lahti K, Vaitomaa J, Kivimäki A, Sivonen K (1998) Fate of cyanobacterial hepatotoxins in artificial recharge of groundwater and in bank filtration. In: Peters JH et al (eds) Artificial recharge of groundwater. Balkema, Rotterdam, pp 211–216
Lahti K, Rapala J, Kivimäki A et al (2001) Occurrence of microcystins in raw water sources and treated drinking water of Finnish waterworks. Water Sci Technol 43:225–228
Logsdon G (2008) Filtration water filtration practice: including slow sand filters and precoat filtration, 1st edn. American Water Works Association, Denver
Maeng SK, Abel CDT, Sharma SK, Park NS, Amy GL (2012) Removal of geosmin and 2-methylisoborneol during managed aquifer recharge: batch and column studies. J Water Supply Res Technol AQUA 61:220–227. doi:10.2166/aqua.2012.096
Miller MJ, Fallowfield HJ (2001) Degradation of cyanobacterial hepatotoxins in batch experiments. Water Sci Technol 43:229–232
Miller MJ, Critchley MM, Hutson J, Fallowfield HJ (2001) The adsorption of cyanobacterial hepatotoxins from water onto soil during batch experiments. Water Res 35:1461–1468. doi:10.1016/S0043-1354(00)00419-X
Miller MJ, Hutson J, Fallowfield HJ (2005) The adsorption of cyanobacterial hepatoxins as a function of soil properties. J Water Health 3:339–347. doi:10.2166/wh.2005.049
Mohamed ZA, El-Sharouny HM, Ali WS (2007) Microcystin concentrations in the Nile River sediments and removal of microcystin-LR by sediments during batch experiments. Arch Environ Contam Toxicol 52:489–495. doi:10.1007/s00244-006-0140-1
Mondardo RI (2004) Influência da Pré-oxidação na Tratabilidade das Águas Via Filtração Direta Descendente em Manancial com elevadas Concentrações de Microalgas e Cianobactérias (influence of pre-oxidation on rapid sand filtration treatment of water with high concentration of micro algae and cyanobacteria). M.Sc. thesis, Federal University of Santa Catarina, Florianópolis, Brazil
Mur LR, Skulber OL, Utkilen H (1999) Cyanobacteria in the environment. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. The World Health Organization, E&FN Spon, London, pp 25–54
Oliveira JS (2002) Analise sedimentar em zonas costeiras: subsidio ao diagnostico ambiental da Lagoa do Peri: Ilha de Santa Catarina, Brasil. (Sediment analysis in coastal zones: subside for an environmental diagnosis from Peri Lake). M.Sc. thesis, Federal University of Santa Catarina, Florianópolis, Brazil
Oshima Y (1995) Post-column derivatization liquid chromatographic method for paralytic shellfish toxins. J AOAC lnt 78:528–532
Plummer JD, Edzwald JK (2001) Effect of ozone on algae as precursors for trihalomethane and haloacetic acid production. Environ Sci Technol 35:3661–3668. doi:10.1021/es0106570
Ray C (2008) Worldwide potential of riverbank filtration. CleanTechnol Environ Policy 10:223–225. doi:10.1007/s10098-008-0164-5
Ray C, Grischek T, Schubert J, Wang J, Speth T (2002a) A perspective of riverbank filtration. JAWWA 94:149–160
Ray C, Soong TW, Lian YK, Roadcap GS (2002b) Effect of flood-induced chemical load on filtrate quality at bank filtration sites. J Hydrol 266:235–258
Sandhu C, Grischek T, Kumar P, Ray C (2011) Potential for riverbank filtration in India. Clean Technol Environ Policy 13:295–316. doi:10.1007/s10098-010-0298-0
Schwarzenbach RP, Gschwend PM, Imboden DM (2003) Environmental organic chemistry, 2nd edn. Wiley, New Jersey
Shamrukh M, Abdel-Wahab A (2008) Riverbank filtration for sustainable water supply: application to a large-scale facility on the Nile River. Clean Technol Environ Policy 10:351–358. doi:10.1007/s10098-007-0143-2
Simonassi J (2001) Caracterização da Lagoa do Peri, através da análise de parâmetros físico-químicos e biológicos, como subsídio ao gerenciamento dos recursos hídricos da Ilha de Santa Catarina, SC, Brasil. (Characterization of the Lagoa do Peri by physico-chemical and biological parameters as a tool for water resources management at the Island of Santa Catarina, Brazil). M.Sc. thesis, Federal University of Santa Catarina, Florianópolis, Brazil
Sivonen K, Jones G (1999) Cyanobacterial toxins. In: Chorus I, Bartram J (eds) Toxic cyanobacteria in water: a guide to their public health consequences, monitoring and management. The World Health Organization, E&FN Spon, London, pp 55–124
Soares M (2014) Sediment clogging characterization of water bodies in temperate regions and its effects on new bank filtration sites in tropical climate. Ph.D. thesis, Technical University Berlin and University of Applied Sciences Dresden, Dresden, Germany
Westrick J (2003) Manager to manager: everything a manager should know about algal toxins but was afraid to ask. JAWWA 95:26–34
Westrick JA, Szlag DC, Southwell BJ, Sinclair J (2010) A review of cyanobacteria and cyanotoxins removal/inactivation in drinking water treatment. Anal Bioanal Chem 397:1705–1714. doi:10.1007/s00216-010-3709-5
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Worch E (2012) Adsorption technology in water treatment. Fundamentals, processes, and modeling. De Gruyter, Berlin
Wu X, Xiao B, Li R et al (2011) Mechanisms and factors affecting sorption of microcystins onto natural sediments. Environ Sci Technol 45:2641–2647. doi:10.1021/es103729m
Acknowledgments
This work was supported by the Federal Ministry of Education and Research (BMBF), project 03FH042PX2, and the German Academic Exchange Service DAAD. We would like to acknowledge the National Council for Scientific of Costa Rica (CONICIT), the Development Council Science and Technology of Brazil (CNPq), and the Instituto Tecnológico de Costa Rica by the doctoral scholarship awarded to Luis G. Romero. Furthermore, the authors are grateful to T. Voltz for editing the English.
Conflict of interest
The authors declare that there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romero, L.G., Mondardo, R.I., Sens, M.L. et al. Removal of cyanobacteria and cyanotoxins during lake bank filtration at Lagoa do Peri, Brazil. Clean Techn Environ Policy 16, 1133–1143 (2014). https://doi.org/10.1007/s10098-014-0715-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-014-0715-x