Abstract
Our study describes the emm type distributions of invasive and non-invasive group A streptococci (GAS) and group G streptococci (GGS) strains in one of the biggest Health Districts in Finland. A total of 571 GAS or GGS were recovered from patients with invasive or non-invasive infections during a 1-year period in 2008–2009 in Pirkanmaa Health District in Finland. We describe here the emm type distributions of GAS and GGS collected from throat (n = 246), pus (n = 217), deep tissue (n = 56) and blood (n = 52). The most common emm types among GAS were emm77, emm1, emm28, emm89 and emm12. Among GGS, the most common emm types were stG480, stG643, stG6, stC6979 and stG485. Some emm types were found to associate with certain infection focus. In GAS, emm77 associated with pus isolates, whereas emm1 and emm12 were more frequent among throat isolates. In GGS, stG480 was more commonly found from throat isolates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Group A streptococci (GAS; Streptococcus pyogenes) are well known human pathogens causing mild superficial infections and a variety of other manifestations, such as cellulitis and erysipelas, but also severe, invasive diseases, such as bacteraemia, streptococcal toxic shock syndrome and necrotising fasciitis [1]. Group G streptococci (GGS; most notably, Streptococcus dysgalactiae subsp. equisimilis) were previously considered as commensal bacteria, but they have been shown to cause similar diseases to GAS [2–5]. Some countries have recently reported an increasing incidence of invasive GGS [6–8].
Molecular markers associated to the invasiveness of GAS have been studied with various methods (emm typing, superantigen gene profiling, random amplified polymorphic DNA [RAPD] and pulsed-field gel electrophoresis [PFGE]), but those of GGS are less well known [4, 9–11]. GAS and GGS share similar virulence factors, such as the M-protein, C5a peptidase, several binding proteins and superantigens, presumably due to horizontal genetic transfer [12–15]. The M-protein is considered to be a major virulence factor in GAS, mainly because of its antiphagocytic activity. Sequence analysis of the hypervariable 5 end of the emm gene, encoding for M-protein, is used for the molecular typing of GAS and GGS. In this study, we characterised GAS and GGS strains isolated from clinical bacterial specimens originating from throat, pus, blood or other deep infections. We compared the emm type distributions in GAS and GGS strains to identify possible differences not only between invasive and non-invasive strains, but also depending on the infection site. We aimed at recognising the emm types possibly associated with either superficial or invasive diseases.
Materials and methods
Bacterial strains
GAS and GGS strains from throat, pus (mostly skin infections), deep infections (mostly abscess) and blood were collected from patients with various infectious symptoms during a 1-year period, March 2008 to February 2009, in Pirkanmaa Health District (HD), with 480,000 inhabitants. The Centre for Laboratory Medicine serves for health care centres and hospitals in the HD and all blood and cerebrospinal fluid samples and the majority of other bacteriological samples are studied there in the HD. Each month, approximately ten first clinical throat and pus isolates were selected and collected among all GAS and large colony-forming GGS isolations. Also, all isolations of GAS or GGS from blood, cerebrospinal fluid or deep tissue were collected. The isolates were sent to the reference laboratory at the National Institute for Health and Welfare (THL) for further analysis. The Lancefield serogroups were defined by latex agglutination using the Streptex latex test system (Remel Europe Ltd., Dartford, UK). Isolates were stored at −70°C.
emm typing
emm typing was performed for all isolates according to the protocol of the emm sequence database at the Centers for Disease Control and Prevention (CDC; http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm). When the strain was non-typable with primer1 and primer2, polymerase chain reaction (PCR) was performed using primers MF1/MR1, as previously described [16]. Sequencing of the emm gene was performed using primer emmseq2.
Data analysis and statistical methods
emm types that were present in >10% of the isolates were considered to be common emm types. The Chi-squared test was used to test for differences between two groups. Statistical analyses were performed using GraphPad InStat version 3.10 for Windows 95 (GraphPad Software, San Diego, CA, USA). A p-value below 0.05 was considered to be statistically significant.
Results
In total, we collected 571 GAS and GGS isolates (Table 1). Our collected study material covers 13% (125/993) of GAS throat isolates, 26% (121/467) of GGS throat isolates, 56% (104/185) of GAS pus isolates and 44% (113/258) of GGS pus isolates handled at the Centre for Laboratory Medicine during the study period. In addition, 56 GAS or GGS isolates from deep infections and 52 blood isolates were collected and analysed.
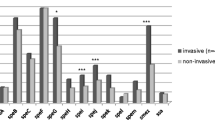
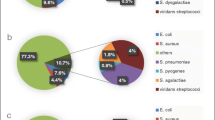
Among 279 GAS isolates, 26 different emm types were found (Table 2). Five strains remained non-typable (NT). For GAS, five different emm types (emm77, emm1, emm28, emm89 and emm12) were present in >10% of the isolates and were included in the statistical analysis. emm77 was significantly associated with skin infections (pus) than with other sample sites (40% (42/104) vs. 13% (22/175), p < 0.0001). emm1 was significantly more commonly found in throat than in other sample sites (27% (34/125) vs. 11% (17/154), p < 0.001). The same was true for emm12 (18% (23/125) vs. 4% (6/154), p < 0.0001).
Among the 292 GGS strains studied, we found 26 different emm types (Table 3). Fifteen strains remained non-typable. Three emm types (stG480, stG643 and stG6) were present in >10% of the GGS isolates and were included in the statistical analysis. stG480 was significantly associated with throat infections than with other sample sites (32% (39/121) vs. 15% (26/171), p < 0.01).
Seasonal occurrence of the five most common emm types in GAS and the three most common emm types in GGS were studied. Differences in the seasonal occurrence of GAS emm77 strains were detected (all isolates, regardless of the isolation site). emm77 isolates were found to be more common between September and February than between March and August (31% (48/157) vs. 13% (16/122), p < 0.001) (Fig. 1). In GGS, stG6 was more common between September and February than between March and August (21% (32/158) vs. 8% (11/158), p < 0.005) (Fig. 2).
The distribution of strains to common (>10% of the isolates) and rare (<10% of the isolates) emm types differed in GAS and GGS. The common emm types covered 83% of the GAS strains, but only 70% of the GGS strains (231/279 vs. 205/292, p < 0.001). emm types stG485 and stG643 were present in both GAS and GGS isolates.
Discussion
This is the first study to describe and compare the emm type distributions of invasive and non-invasive GAS and GGS strains during a 1-year period in a defined population in Finland. In both GAS and GGS, 26 different emm types were discovered. We observed associations between certain emm types and infection foci. Our finding of emm12 to be common in throat isolates is in line with those previously reported [10, 17, 18]. Our finding of emm1 strains as a cause for pharyngitis is also commonly known, although emm1 is often considered to cause especially invasive disease [10, 17–19]. In our study material, emm77 was found especially in skin and wound swabs, but it was also the most common emm type in deep infection and blood isolates. emm77 seems to have features that enable these strains to cause especially skin infections, rather than pharyngitis, since emm77 was rare in throat isolates. A recent study in Germany compared emm types among invasive and non-invasive isolates (throat and skin) and found emm77 to be a predictor of non-invasiveness [9]. It would be essential to study the virulence factors and clonality of these emm77 strains in more detail.
The emm types of invasive GAS have been extensively studied in Finland and Europe [10, 20, 21]. In Finland, the most common emm types in 2008 were emm1, emm12, emm28 and emm119 [22]. In our study material, among the 29 GAS blood isolates, the most common emm types were emm77, emm1, emm28 and emm89. The emm type distribution of invasive GAS seems to vary geographically in Finland, since the emm type distribution of the whole country and Pirkanmaa HD differ. It is noteworthy that emm3, which has been shown often to associate with invasive diseases in other Western hemisphere countries, was absent in our study material [21, 23].
Recent studies on GGS have concentrated mainly on invasive strains and their emm type distributions also seem to vary geographically [24–26]. The emm type distribution of 128 invasive GGS isolates in Pirkanmaa HD has been previously described in a population-based study, and an association between rare emm types and the severity of disease was then discovered [27]. At that time, the most common emm types in invasive GGS were stG480, stG6, stG485, stG643 and stC6979 [27]. Similarly, the same five types were the most common emm types in our study. Also, among blood isolates, these types covered 83% of the strains (24/29). In Pirkanmaa HD, GGS isolates have also been studied in patients with acute bacterial non-necrotising cellulitis in 2004–2005 [3]. The most common emm types associated with these infections were stG6, stG480 and stG643, which we also found in our study. Based on these findings, we can conclude that stG480, stG6, stG485, stG643 and stC6979 have been the most common emm types among GGS in Pirkanmaa HD in recent years, and they cause both invasive and non-invasive diseases.
Little is known of the association of emm types with invasiveness in GGS. In our study, none of the emm types presented an association with invasiveness (blood or deep infection). In a Portuguese study, emm types stG2078 and stG10 were found to be more common in invasive isolates [4]. These two emm types were absent in our blood isolates and, overall, we found these in very small numbers. We found stG480 more often in throat than in other sample sites. No other association was found between emm types and infection site in GGS. In a Norwegian study, emm types stG6, stG643 and stG485 were the most common emm types among GGS and GAS throat and skin isolates, but these types were also found in invasive strains and, therefore, no association of these types to non-invasiveness was found [28]. These types were also common in our throat and pus isolates. In an Australian study, the most common emm types among throat and skin GGS isolates were stC1400, stG4831, stG480, stG6792 and stC74a [29]. Apart from stG480, these emm types were present in very low numbers in our study material (n ≤ 5), suggesting that the emm distribution in non-invasive GGS varies geographically.
A seasonal difference of genotype distribution was detected in GAS emm77 and GGS stG6 isolates: both were more common between September and February than between March and August. Our finding of emm77 being especially prevalent during the winter months has not been reported previously. It is not clear if this is a true sign of seasonal fluctuation. It has been shown that previously unknown or rare emm types can rapidly emerge and cause invasive GAS disease [10, 30]. A similar phenomenon may have occurred in Pirkanmaa HD with emm77 emerging during our study period, which would explain the large number of emm77 during these winter months, rather than their specific association with the winter season.
emm types StG485 and stG643 were found in both GAS and GGS, although they were more common in GGS. Lateral genetic transfer has been shown to occur between GAS and GGS, and it may be one interpretative factor for these shared emm types [31]. It is also possible that GAS strains that harbour emm types typical to GGS belong to Streptococcus dysgalactiae subsp. equisimilis species and not Streptococcus pyogenes. GAS bearing Streptococcus dysgalactiae subsp. equisimilis strains have been reported previously [32].
Limitations of our study are that no patient data were collected and that species identification was not performed for the strains.
We conclude that emm77 associates to skin and invasive infections in GAS, but is rare in throat isolates. More detailed studies of the clonality of emm77 strains would be of interest. The over-representation of emm12 and emm1 among throat isolates is in congruence with findings from previous studies. In GGS, stG480 was associated with throat isolates. emm typing is a useful tool in epidemiological surveys on GAS and GGS, but a more detailed study of several virulence factors and clonality would be essential in order to further investigate the invasiveness of the isolates.
References
Cunningham MW (2000) Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 13(3):470–511
Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, Hasegawa T, Ohta M (2004) Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol 42(1):186–192
Siljander T, Karppelin M, Vähäkuopus S, Syrjänen J, Toropainen M, Kere J, Vuento R, Jussila T, Vuopio-Varkila J (2008) Acute bacterial, nonnecrotizing cellulitis in Finland: microbiological findings. Clin Infect Dis 46(6):855–861. doi:10.1086/527388
Pinho MD, Melo-Cristino J, Ramirez M (2006) Clonal relationships between invasive and noninvasive Lancefield group C and G streptococci and emm-specific differences in invasiveness. J Clin Microbiol 44(3):841–846. doi:10.1128/JCM.44.3.841-846.2006
Liao CH, Liu LC, Huang YT, Teng LJ, Hsueh PR (2008) Bacteremia caused by group G Streptococci, Taiwan. Emerg Infect Dis 14(5):837–840. doi:10.3201/eid1405.070130
Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J (2009) Clinical presentations and epidemiology of beta-haemolytic streptococcal bacteraemia: a population-based study. Clin Microbiol Infect 15(3):286–288. doi:10.1111/j.1469-0691.2008.02672.x
Sylvetsky N, Raveh D, Schlesinger Y, Rudensky B, Yinnon AM (2002) Bacteremia due to beta-hemolytic Streptococcus group G: increasing incidence and clinical characteristics of patients. Am J Med 112(8):622–626. doi:10.1016/S0002-9343(02)01117-8
Wong SS, Lin YS, Mathew L, Rajagopal L, Sepkowitz D (2009) Increase in group G streptococcal infections in a community hospital, New York, USA. Emerg Infect Dis 15(6):991–993. doi:10.3201/eid1506.080666
Lintges M, van der Linden M, Hilgers RD, Arlt S, Al-Lahham A, Reinert RR, Plücken S, Rink L (2010) Superantigen genes are more important than the emm type for the invasiveness of group A Streptococcus infection. J Infect Dis 202(1):20–28. doi:10.1086/653082
Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalén C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH (2007) Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 45(4):450–458. doi:10.1086/519936
Rogers S, Commons R, Danchin MH, Selvaraj G, Kelpie L, Curtis N, Robins-Browne R, Carapetis JR (2007) Strain prevalence, rather than innate virulence potential, is the major factor responsible for an increase in serious group A streptococcus infections. J Infect Dis 195(11):1625–1633. doi:10.1086/513875
Igwe EI, Shewmaker PL, Facklam RR, Farley MM, van Beneden C, Beall B (2003) Identification of superantigen genes speM, ssa, and smeZ in invasive strains of beta-hemolytic group C and G streptococci recovered from humans. FEMS Microbiol Lett 229(2):259–264. doi:10.1016/S0378-1097(03)00842-5
Ahmad Y, Gertz RE Jr, Li Z, Sakota V, Broyles LN, Van Beneden C, Facklam R, Shewmaker PL, Reingold A, Farley MM, Beall BW (2009) Genetic relationships deduced from emm and multilocus sequence typing of invasive Streptococcus dysgalactiae subsp. equisimilis and S. canis recovered from isolates collected in the United States. J Clin Microbiol 47(7):2046–2054. doi: 10.1128/JCM.00246-09
Towers RJ, Gal D, McMillan D, Sriprakash KS, Currie BJ, Walker MJ, Chhatwal GS, Fagan PK (2004) Fibronectin-binding protein gene recombination and horizontal transfer between group A and G streptococci. J Clin Microbiol 42(11):5357–5361. doi:10.1128/JCM.42.11.5357-5361.2004
Kalia A, Bessen DE (2003) Presence of streptococcal pyrogenic exotoxin A and C genes in human isolates of group G streptococci. FEMS Microbiol Lett 219(2):291–295. doi:10.1016/S0378-1097(03)00022-3
Siljander T, Toropainen M, Muotiala A, Hoe NP, Musser JM, Vuopio-Varkila J (2006) emm typing of invasive T28 group A streptococci, 1995–2004, Finland. J Med Microbiol 55(Pt 12):1701–1706. doi:10.1099/jmm.0.46690-0
Espinosa LE, Li Z, Gomez Barreto D, Calderon Jaimes E, Rodriguez RS, Sakota V, Facklam RR, Beall B (2003) M protein gene type distribution among group A streptococcal clinical isolates recovered in Mexico City, Mexico, from 1991 to 2000, and Durango, Mexico, from 1998 to 1999: overlap with type distribution within the United States. J Clin Microbiol 41(1):373–378. doi:10.1128/JCM.41.1.373-378.2003
Mijac V, Ranin L, Marković M, Heeg C, Reinert RR, Opavski N (2010) Distribution of emm types among group A streptococcal isolates from Serbia. Clin Microbiol Infect 16(3):295–298. doi:10.1111/j.1469-0691.2009.02823.x
Muotiala A, Seppälä H, Huovinen P, Vuopio-Varkila J (1996) Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis 175(2):392–399
Siljander T, Lyytikäinen O, Vähäkuopus S, Snellman M, Jalava J, Vuopio J (2010) Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis 29(10):1229–1235. doi:10.1007/s10096-010-0989-9
Luca-Harari B, Darenberg J, Neal S, Siljander T, Strakova L, Tanna A, Creti R, Ekelund K, Koliou M, Tassios PT, van der Linden M, Straut M, Vuopio-Varkila J, Bouvet A, Efstratiou A, Schalén C, Henriques-Normark B, Jasir A; Strep-EURO Study Group (2009) Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 47(4):1155–1165. doi:10.1128/JCM.02155-08
Hulkko T, Lyytikäinen O, Kuusi M, Möttönen T, Ruutu P (eds) (2009) THL Report. Infectious Diseases in Finland 2008. National Institute for Health and Welfare (THL), Helsinki, 48 pp
Montes M, Ardanuy C, Tamayo E, Domènech A, Liñares J, Pérez-Trallero E (2011) Epidemiological and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998–2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis 30(10):1295–1302. doi:10.1007/s10096-011-1226-x
Broyles LN, Van Beneden C, Beall B, Facklam R, Shewmaker PL, Malpiedi P, Daily P, Reingold A, Farley MM (2009) Population-based study of invasive disease due to beta-hemolytic streptococci of groups other than A and B. Clin Infect Dis 48(6):706–712. doi:10.1086/597035
Ikebe T, Murayama S, Saitoh K, Yamai S, Suzuki R, Isobe J, Tanaka D, Katsukawa C, Tamaru A, Katayama A, Fujinaga Y, Hoashi K, Watanabe H; Working Group for Streptococci in Japan (2004) Surveillance of severe invasive group-G streptococcal infections and molecular typing of the isolates in Japan. Epidemiol Infect 132(1):145–149. doi:10.1017/S0950268803001262
Lopardo HA, Vidal P, Sparo M, Jeric P, Centron D, Facklam RR, Paganini H, Pagniez NG, Lovgren M, Beall B (2005) Six-month multicenter study on invasive infections due to Streptococcus pyogenes and Streptococcus dysgalactiae subsp. equisimilis in Argentina. J Clin Microbiol 43(2):802–807. doi: 10.1128/JCM.43.2.802-807.2005
Rantala S, Vähäkuopus S, Vuopio-Varkila J, Vuento R, Syrjänen J (2010) Streptococcus dysgalactiae subsp. equisimilis bacteremia, Finland, 1995–2004. Emerg Infect Dis 16(5):843–846. doi:10.3201/eid1605.080803
Kittang BR, Skrede S, Langeland N, Haanshuus CG, Mylvaganam H (2011) emm gene diversity, superantigen gene profiles and presence of SlaA among clinical isolates of group A, C and G streptococci from western Norway. Eur J Clin Microbiol Infect Dis 30(3):423–433. doi:10.1007/s10096-010-1105-x
McDonald M, Towers RJ, Andrews RM, Carapetis JR, Currie BJ (2007) Epidemiology of Streptococcus dysgalactiae subsp. equisimilis in tropical communities, Northern Australia. Emerg Infect Dis 13(11):1694–1700
Siljander T, Lyytikäinen O, Vähäkuopus S, Säilä P, Jalava J, Vuopio-Varkila J (2009) Rapid emergence of emm84 among invasive Streptococcus pyogenes infections in Finland. J Clin Microbiol 47(2):477–480. doi:10.1128/JCM.01663-08
Sriprakash KS, Hartas J (1996) Lateral genetic transfers between group A and G streptococci for M-like genes are ongoing. Microb Pathog 20(5):275–285. doi:10.1006/mpat.1996.0026
Tanaka D, Isobe J, Watahiki M, Nagai Y, Katsukawa C, Kawahara R, Endoh M, Okuno R, Kumagai N, Matsumoto M, Morikawa Y, Ikebe T, Watanabe H; Working Group for Group A Streptococci in Japan (2008) Genetic features of clinical isolates of Streptococcus dysgalactiae subsp. equisimilis possessing Lancefield’s group A antigen. J Clin Microbiol 46(4):1526–1529. doi:10.1128/JCM.02188-07
Acknowledgements
This work was partly supported by a grant from The Emil Aaltonen Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vähäkuopus, S., Vuento, R., Siljander, T. et al. Distribution of emm types in invasive and non-invasive group A and G streptococci. Eur J Clin Microbiol Infect Dis 31, 1251–1256 (2012). https://doi.org/10.1007/s10096-011-1436-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1436-2