Abstract
In 2006, Finnish nationwide surveillance showed an increase of invasive group A streptococcal (iGAS) disease and clinicians were alarmed by severe disease manifestations, prompting the investigation of recent trends and outcome for iGAS. A case of iGAS was defined as Streptococcus pyogenes isolated from blood or cerebrospinal fluid. Cases during 1998–2007 and isolates during 2004–2007 were included. Case-patients’ 7-day outcome was available for 2004–2007. Isolates were emm typed. A total of 1,318 cases of iGAS were identified. The average annual incidence was 2.5/100,000 population. The rate was higher in males than females in persons aged 45–64 years, but lower in persons aged 25–34 years. The annual incidence was highest in 2007 (3.9/100,000). Occasional peaks occurred during midwinter and midsummer. The most common emm types were 28 (21%), 1 (16%), 84 (10%), 75 (7%) and 89 (6%). During 2004–2007, emm1 replaced emm28 as the most predominant type. The overall case fatality was 8%. Cases with emm1 were associated with high case fatality (14% vs. 8% in other types; p < 0.02); that of emm28 infections was 2% (p < 0.01). Changes in emm type prevalence influenced incidence and case fatality. Differences in age- and sex-specific incidence and seasonal patterns suggest variations in predisposing factors and underlying conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus pyogenes (group A streptococcus, GAS) causes a wide array of infections, ranging in severity from mild pharyngitis and skin/soft tissue infections to severe invasive infections. In the late 1980s, a change in the epidemiology of severe GAS infections, with an increase in the incidence and severity of disease, was documented [1]. The role and increased pathogenic potential of type M1 in epidemics were of specific interest [2, 3]. The incidence of severe GAS disease shows variations over time and geographic region, possibly reflecting the population’s susceptibility to particular strains with diverse virulence properties [4–6]. Dynamic changes in the type distribution of GAS may also influence the case fatality of these infections.
In Finland, the incidence rate of invasive group A streptococcal (iGAS) disease has also shown fluctuations during this and the previous decade, and an increasing trend has been noticed [7]. In 2006, clinicians contacted the National Institute for Health and Welfare and described suddenly encountering more severe disease manifestations with a poor outcome. These events prompted us to investigate the recent trend and outcome of iGAS infections and emm type distribution in Finland in more detail.
Methods
Surveillance
The national healthcare system of Finland (population 5.3 million) is organised into 20 healthcare districts (with catchment populations ranging from 58,000 to 1.5 million), forming five tertiary care districts. Since 1995, all clinical microbiology laboratories have mandatorily notified all isolations of S. pyogenes from blood or cerebrospinal fluid (CSF) to the National Infectious Disease Register (NIDR). With each notification, the following information is transmitted (generally electronically) to the NIDR: date and type of specimen, unique national identity code of the patient (since 2004), date of birth, sex and place of treatment. Within an interval of three months, notifications concerning the same patient are merged into a single case. The corresponding S. pyogenes isolates are submitted to the national reference laboratory.
Case definition and outcome
A case of iGAS was defined as S. pyogenes isolated from blood or CSF. In this study, iGAS cases from January 1998 to December 2007 and isolates from January 2004 to December 2007 were included. Case-patients’ vital status at 7 days of positive blood or CSF culture was obtained for 2004–2007 from the Population Information System through the use of the national identity codes.
Identification and characterisation of isolates
Isolates were tested for sensitivity to bacitracin and the Lancefield group A antigen was identified using Streptex latex agglutination (Remel Europe Ltd., UK). emm typing was performed for all isolates according to guidelines by the Centers for Disease Control and Prevention (http://www.cdc.gov/ncidod/biotech/strep/protocol_emm-type.htm) as previously described [8]. Susceptibility to erythromycin, clindamycin and tetracycline was determined with the agar dilution method for all isolates. Susceptibility was assigned according to interpretative criteria for the minimum inhibitory concentration (MIC) as recommended by the Clinical and Laboratory Standards Institute [9].
Data analysis and statistics
Data on the cases of iGAS, their outcome and emm typing and antimicrobial susceptibility data (where available) were linked using the national identity code of the case-patient and the date of the specimen. Where the national identity code was unavailable, other available information was used for linkage. Average annual incidence rates were calculated for 1998–2007 using the population census at the end of the previous year, as obtained from Statistics Finland. Age- and sex-specific incidence rates and male-to-female ratios with 95% confidence intervals (CIs) according to the Poisson distribution were calculated. Seven-day case fatality was calculated for 2004–2007 and for each emm type separately. χ2 and Kruskal–Wallis tests were applied where appropriate to test for statistical significance between subgroups and distributions. Differences were considered to be significant when p < 0.05. Data were analysed with Intercooled Stata™ 9.1 software for Windows (StataCorp, College Station, TX, USA) and R statistical software version 2.9.0 (R Development Core Team, Vienna, Austria).
Results
Incidence rates
From January 1998 to December 2007, 1,318 cases of iGAS (range by year, 100–207) were identified (Table 1). The median age of case-patients was 52 years (range, 0–95 years); 55% were males. The average annual incidence rate of iGAS was 2.5 cases per 100,000 population. The rate was higher in males than in females and among males of adult age groups, it increased with age (Table 2). The highest rates were observed among the elderly patients. Males had a significantly higher rate of infection than females, especially among persons aged 45–64 years, whereas females had a higher rate than males among persons aged 25–34 years.
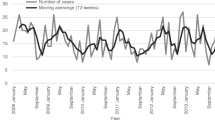
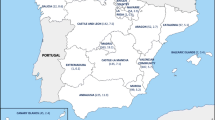
The annual incidence rate fluctuated (range by year, 1.9–3.9 cases per 100,000 population) but showed an increasing trend during the study period. Peaks occurred in 2002 (2.9 cases per 100,000) and in 2006–2007 (3.1–3.9 cases per 100,000). The average annual incidence rate in the five tertiary care districts varied between 1.8–3.1 cases per 100,000. Of all cases, 41% were localised in the tertiary care district around Helsinki, where the annual incidence rate varied within the range of 2.2–4.4 cases per 100,000. Variation in the seasonal activity of iGAS disease could be identified, with occasional peaks of cases occurring during the midwinter (for both sexes) and midsummer (with male cases dominating; Fig. 1a, b).
emm sequence types
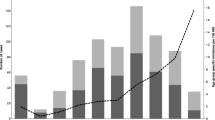
For 602 out of 609 cases during 2004–2007, a corresponding isolate was received. A total of 46 emm types were encountered (range by year, 23–28 types). The annual proportion of non-typable isolates varied within 1–5%. The five most common emm types were 28, 1, 84, 75 and 89, together accounting for 60% of isolates (Table 3). Type emm28 was the most predominant type in 2004, but its proportion declined each year, while that of emm1 increased, and by 2007, emm1 became the most common type. Type emm1 isolates were mainly of subtypes 1.0 and 1.10, but sporadic cases of other subtypes were also encountered. A new and uncommon type, emm84, emerged in 2005 and became the second most common type by 2007.
Type emm28 infections were over-represented in females compared to males (55% vs. 45%; p < 0.01) and the same was true for emm4 infections (73% vs. 27%, respectively; p < 0.05). Specifically, females of child-bearing age (15–44 years) had a significantly larger proportion of infections by emm28 than males of the same age (48% vs. 21% of emm28 infections, respectively; p < 0.01).
Outcome
During 2004–2007, 48/609 case-patients died within 7 days of the positive blood or CSF culture (overall case fatality, 8%). The case fatality did not significantly differ between males and females (9% vs. 6%; p = 0.172). The case fatality was the lowest in 2004 (3%), increasing to its highest in 2005 (12%), and then declined by 2006 (10%) and 2007 (7%). The case-patients with fatal outcome were older than those who survived (median age, 60 vs. 54 years; p < 0.01). No fatalities occurred in patients aged <1 and 15–24 years.
Overall, infections by emm1 were significantly associated with higher case fatality (14% vs. 8% with all other types; p < 0.02, Table 3). Cases by emm75 and 79 were also associated with higher than average case fatality (14% for both). In contrast, emm28 was associated with lower case fatality (2% vs. all other types; p < 0.01) along with type 81 (0%, respectively).
Antimicrobial susceptibility
Of the iGAS isolates tested, 1.5% (9/602) were resistant to erythromycin, 0.5% (3/602) to clindamycin and 16% (97/602) to tetracycline. The tetracycline-resistant strains were mainly of emm types 81 (20 resistant isolates in total; 87% of this type resistant to tetracycline), 27G (19; 100%), 77 (12; 100%) and 85 (9; 100%).
Discussion
This study presents a comprehensive description of the recent epidemiology and outcome of invasive group A streptococcal (S. pyogenes) disease with information on emm type prevalence in a well-defined population. The main strength of the study is the nationwide population-based surveillance and the good coverage of isolate collection. We describe how the incidence and case fatality of iGAS infections were influenced by changes in the emm type prevalence. Age- and sex-specific differences in the incidence, uncharacteristic seasonal patterns of infections and aspects of vaccine coverage are also discussed.
The observed incidence rate of 3.9 cases per 100,000 population in 2007 is the highest in Finland since 1995. This study together with our earlier research shows that the incidence of iGAS disease has fluctuated since the 1990s but continued its increasing trend [7, 10]. During 2003–2004, the UK, Sweden, Denmark and Finland had the highest rates of infection (range 2.5–3.3 per 100,000) in a European study [4]. Since then, indications of an increase in the incidence are seen in Sweden to 4.5 cases per 100,000 by 2007 (http://www.smittskyddsinstitutet.se/in-english/statistics/beta-hemolytic-group-a-streptococci-gas-invasiv-infection/) and very recently in the UK [11]. In contrast, the incidence of iGAS disease in the USA has remained relatively stable, at an average of 3.5 cases per 100,000 during 1995–2004, with a moderate increase to 3.8 in 2007 (http://www.cdc.gov/abcs/survreports.htm) [5].
The incidence of iGAS disease in Finland generally increased with age as expected, but the rate in the elderly (≥65 years of age) still remained at a lower level than has been reported in Sweden, Denmark, the UK or the USA [5, 12–14]. Males had a higher incidence of iGAS disease compared to females, which has also been noted by some other studies [4, 5]. Differences could also be identified in the age- and sex-specific rates, with males having significantly more infections than females in the age range 45–64 years. In contrast, females had more infections in the age range 25–34 years than males. A similar clear peak in the incidence of females around the same age has been noticed in earlier studies from Denmark and the UK [13, 14].
The characteristic fluctuation in the incidence of iGAS disease is presumably a reflection of changes in the emm type distribution and the population’s susceptibility towards particular strains, especially of emerging types [5, 12, 15]. The emm type distribution in Finland underwent major changes during 2004–2007 when emm1 replaced emm28 as the most predominant type. The incidence of iGAS disease increased simultaneously with the increasing prevalence of type emm1, as has also been observed by others [3]. However, changes in the prevalence of other types, such as the sudden emergence of emm84, also contributed to the increase [10].
The type distribution of iGAS isolates in Finland has common features with that of other countries but also some unique characteristics. emm types 1, 28 and 89 are common globally, but 75 and 84 are rarer and have not featured among the five most common emm types elsewhere [5, 6, 12, 14, 16, 17]. The low prevalence of cases by emm3 is of interest, as it is a common type in many countries, and is often associated with high case fatality [5, 6, 14, 17]. The emm types included in the putative 26-valent recombinant vaccine would have covered approximately half of the Finnish isolates in 2004–2007, a notably smaller proportion than has been estimated for the USA (79%) and Japan (82%) [5, 17, 18].
Within the last 20 years, a periodic fluctuation of peaks and troughs of the two most common genotypes, emm1 and emm28, has occurred in Finland. Peaks in numbers of M/emm1 have been observed earlier in 1988–1990 and 1997–1998, and in this study in 2006–2007 [7, 19, 20]. Similarly, the prevalence of type emm28 has peaked in 1993–1995 and 2002–2004 [7, 20]. Such a pattern of alternating type prevalence may be a reflection of changes in the general immunity of the population.
Types emm28 and, more rarely, also emm4 have been known to associate with postpartum infections and puerperal sepsis [6, 21, 22]. Also in this study, emm28 infections were found to be specifically concentrated in females of child-bearing age. However, estimations of how many of these cases were pregnancy-related infections are beyond the scope of this study.
The overall erythromycin resistance during 2004–2007 was expectedly low, as the rate has declined during this decade in Finland (http://www.ktl.fi/portal/english/projects/fire/finres/finres__antimicrobial_resistance_statistics_/streptococcus_pyogenes/). The Finnish iGAS strains remain susceptible to clindamycin. Similar low resistance rates have been reported, for example, in Denmark and the USA [14, 23]. Tetracycline resistance was associated with certain clones, as has also been found previously, and the resistant strains were mainly of uncommon emm types [24].
The overall case fatality of iGAS disease in Finland was relatively low at 8%, compared to those reported by some other European countries and the USA [5, 12, 13, 16]. A sudden four-fold increase in the case fatality occurred from 3% in 2004 to 12% in 2005. During the study, the proportion of infections by types emm1 and emm75 increased, these types being associated with a high case fatality. In parallel, the proportion of emm28, associated with a low case fatality, decreased. Having an infection by emm types 1 or 3 has been identified as an independent factor associated with death due to iGAS disease [5]. Also in our study, the case fatality by emm1 was higher than average; however, we did not adjust it for age.
Seasonal variation in iGAS disease was observed; in addition to a midwinter peak season, there were also occasional peaks during the midsummer. In the northern hemisphere, peaks of cases are generally observed during the winter and spring [4–6, 12]. There were also sex-specific differences in the peak seasons, with male cases dominating during the summer. These two peak seasons might be associated with different predisposing factors and underlying conditions. However, the unusual pattern and irregularity of the seasonality observed in this study may partly arise from natural variation due to a reasonably small study size.
There are some limitations to this study. Firstly, part of the increase in the incidence may be due to more frequent blood culturing activity [25]. It is also possible but less likely that the incidence would have been influenced by changes in the reporting system, which was established in 1995. Secondly, case definitions of iGAS disease differ between countries and complicate comparisons of incidence rates. Exclusion of non-bacteraemic cases with other sterile site isolations and/or toxic shock by the Finnish case definition may partly explain the observed lower incidence rate. Thirdly, differences in blood culture sampling practices between countries may have an effect on the observed incidence [25]. In addition, the observed differences in fatality may arise from the case definition used, the timing of death considered (7/30-day/in-hospital mortality) and the source of information (e.g. population registry vs. hospital records). In this study, the 7-day mortality information obtained from the Population Information System was used. Finally, the surveillance does not include the collection of clinical data of disease manifestations, possible risk factors and underlying conditions of patients. These data would be needed in order to address reasons behind the observed differences in the age-and sex-specific incidence, and such a study has been undertaken within one healthcare district in Finland [26].
In conclusion, dynamic changes in the emm type prevalence influenced the incidence and case fatality of iGAS disease in Finland. An unusual seasonal pattern with peaks in the midwinter and midsummer was observed. Differences in age- and sex-specific incidence rates and seasonality suggest variations in predisposing factors and underlying conditions.
References
Schwartz B, Facklam RR, Breiman RF (1990) Changing epidemiology of group A streptococcal infection in the USA. Lancet 336:1167–1171
Martin DR, Single LA (1993) Molecular epidemiology of group A streptococcus M type 1 infections. J Infect Dis 167:1112–1117
Strömberg A, Romanus V, Burman LG (1991) Outbreak of group A streptococcal bacteremia in Sweden: an epidemiologic and clinical study. J Infect Dis 164:595–598
Lamagni TL, Darenberg J, Luca-Harari B, Siljander T, Efstratiou A, Henriques-Normark B, Vuopio-Varkila J, Bouvet A, Creti R, Ekelund K, Koliou M, Reinert RR, Stathi A, Strakova L, Ungureanu V, Schalén C, Jasir A (2008) Epidemiology of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 46:2359–2367
O’Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, Farley MM, Barrett NL, Spina NL, Beall B, Harrison LH, Reingold A, Van Beneden C (2007) The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000–2004. Clin Infect Dis 45:853–862
Tyrrell GJ, Lovgren M, Kress B, Grimsrud K (2005) Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J Clin Microbiol 43:1678–1683
Siljander T, Toropainen M, Muotiala A, Hoe NP, Musser JM, Vuopio-Varkila J (2006) emm typing of invasive T28 group A streptococci, 1995–2004, Finland. J Med Microbiol 55:1701–1706
Siljander T, Karppelin M, Vähäkuopus S, Syrjänen J, Toropainen M, Kere J, Vuento R, Jussila T, Vuopio-Varkila J (2008) Acute bacterial, nonnecrotizing cellulitis in Finland: microbiological findings. Clin Infect Dis 46:855–861
Clinical and Laboratory Standards Institute (CLSI) (2007) Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement, M100-S17, vol 27, no 1
Siljander T, Lyytikäinen O, Vähäkuopus S, Säilä P, Jalava J, Vuopio-Varkila J (2009) Rapid emergence of emm84 among invasive Streptococcus pyogenes infections in Finland. J Clin Microbiol 47:477–480
Lamagni TL, Efstratiou A, Dennis J, Nair P, Kearney J, George R (2009) Increase in invasive group A streptococcal infections in England, Wales and Northern Ireland, 2008–9. Euro Surveill 14
Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalén C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH (2007) Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis 45:450–458
Lamagni TL, Neal S, Keshishian C, Alhaddad N, George R, Duckworth G, Vuopio-Varkila J, Efstratiou A (2008) Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg Infect Dis 14:202–209
Luca-Harari B, Ekelund K, van der Linden M, Staum-Kaltoft M, Hammerum AM, Jasir A (2008) Clinical and epidemiological aspects of invasive Streptococcus pyogenes infections in Denmark during 2003 and 2004. J Clin Microbiol 46:79–86
O’Brien KL, Beall B, Barrett NL, Cieslak PR, Reingold A, Farley MM, Danila R, Zell ER, Facklam R, Schwartz B, Schuchat A (2002) Epidemiology of invasive group A streptococcus disease in the United States, 1995–1999. Clin Infect Dis 35:268–276
Ekelund K, Darenberg J, Norrby-Teglund A, Hoffmann S, Bang D, Skinhøj P, Konradsen HB (2005) Variations in emm type among group A streptococcal isolates causing invasive or noninvasive infections in a nationwide study. J Clin Microbiol 43:3101–3109
Ikebe T, Hirasawa K, Suzuki R, Ohya H, Isobe J, Tanaka D, Katsukawa C, Kawahara R, Tomita M, Ogata K, Endoh M, Okuno R, Tada Y, Okabe N, Watanabe H (2007) Distribution of emm genotypes among group A streptococcus isolates from patients with severe invasive streptococcal infections in Japan, 2001–2005. Epidemiol Infect 135:1227–1229
McNeil SA, Halperin SA, Langley JM, Smith B, Warren A, Sharratt GP, Baxendale DM, Reddish MA, Hu MC, Stroop SD, Linden J, Fries LF, Vink PE, Dale JB (2005) Safety and immunogenicity of 26-valent group A Streptococcus vaccine in healthy adult volunteers. Clin Infect Dis 41:1114–1122
Hoe NP, Nakashima K, Lukomski S, Grigsby D, Liu M, Kordari P, Dou SJ, Pan X, Vuopio-Varkila J, Salmelinna S, McGeer A, Low DE, Schwartz B, Schuchat A, Naidich S, De Lorenzo D, Fu YX, Musser JM (1999) Rapid selection of complement-inhibiting protein variants in group A Streptococcus epidemic waves. Nat Med 5:924–929
Muotiala A, Seppälä H, Huovinen P, Vuopio-Varkila J (1997) Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis 175:392–399
Chuang I, Van Beneden C, Beall B, Schuchat A (2002) Population-based surveillance for postpartum invasive group a streptococcus infections, 1995–2000. Clin Infect Dis 35:665–670
Colman G, Tanna A, Efstratiou A, Gaworzewska ET (1993) The serotypes of Streptococcus pyogenes present in Britain during 1980–1990 and their association with disease. J Med Microbiol 39:165–178
Richter SS, Heilmann KP, Beekmann SE, Miller NJ, Miller AL, Rice CL, Doern CD, Reid SD, Doern GV (2005) Macrolide-resistant Streptococcus pyogenes in the United States, 2002–2003. Clin Infect Dis 41:599–608
Kataja J, Huovinen P, Muotiala A, Vuopio-Varkila J, Efstratiou A, Hallas G, Seppälä H (1998) Clonal spread of group A streptococcus with the new type of erythromycin resistance. Finnish Study Group for Antimicrobial Resistance. J Infect Dis 177:786–789
Skogberg K, Lyytikäinen O, Ruutu P, Ollgren J, Nuorti JP (2008) Increase in bloodstream infections in Finland, 1995–2002. Epidemiol Infect 136:108–114
Rantala S, Vuopio-Varkila J, Vuento R, Huhtala H, Syrjänen J (2009) Clinical presentations and epidemiology of beta-haemolytic streptococcal bacteraemia: a population-based study. Clin Microbiol Infect 15:286–288
Acknowledgements
Laboratory technicians Aila Soininen, Saija Perovuo, Suvi Kavenius, Tuula Randell and Minna Lamppu are greatly acknowledged for their excellent technical assistance. Data Manager Joonas Iivonen is acknowledged for the technical expertise in retrieving and combining data from several database sources. Clinical microbiology laboratories are acknowledged for submitting notifications and isolates.
Previous presentations
These results have partly been presented at the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), October 2007, Stockholm, Sweden (poster number B.A.1.28), at the XVII Lancefield International Symposium on Streptococci and Streptococcal Diseases (LISSSD), June 2008, Porto Heli, Greece (abstract number O 4.4), and at the European Scientific Conference on Applied Infectious Disease Epidemiology (ESCAIDE), October 2009, Stockholm, Sweden (abstract number 20090261).
Funding sources
Funding was received from the Ministry of Social Affairs and Health, the Paulo Foundation, the Sigrid Jusélius Foundation, the University of Helsinki, the Finnish Cultural Foundation and the Alfred Kordelin Foundation.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siljander, T., Lyytikäinen, O., Vähäkuopus, S. et al. Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis 29, 1229–1235 (2010). https://doi.org/10.1007/s10096-010-0989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-010-0989-9