Abstract
The aim of this study was to review fungal bloodstream infections at a large tertiary care hospital to evaluate the incidence of fungemia and the distribution of causative species during the period 2001–2005. Another aim was to assess the extent of antifungal resistance. A review of all episodes of fungemia at the University Hospitals of Leuven (Belgium) was conducted between January 2001 and December 2005. For the first yeast isolate collected from each non-mould fungemic episode during a 1-year period (June 2004–June 2005), susceptibility to seven antifungal agents was determined using Sensititre YeastOne plates (Trek Diagnostic Systems, East Grinstead, UK), and the antifungal therapy was reviewed. The annual incidence of fungemia ranged between 1.30 and 1.68 episodes per 10,000 patient-days (on a total of 2,680,932 patient-days), with a decreasing trend observed over the 5-year study period. The most common species were Candida albicans (59%), Candida glabrata (22%), Candida parapsilosis (10%), and Candida tropicalis (4%). Overall, fluconazole resistance was rare (1.6%) and was detected only in C. glabrata and C. krusei. Voriconazole and caspofungin inhibited 100% of the isolates at a concentration of ≤1 μg/ml. Fluconazole was used to treat 75% of fungemic patients. Caspofungin was the second most commonly used antifungal agent (used to treat 11.7% of patients). The incidence of fungemia was higher than usually reported in other European countries. The low proportion of resistance supports the use of fluconazole as the treatment of first choice for candidemia in patients not previously exposed to this drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past three decades, Candida has emerged worldwide as an important cause of bloodstream infections [1, 2]. During the 1980s, an increased rate of candidemia in the USA was reported [3]. Currently, the incidence trend of bloodstream infections (BSIs) due to Candida species seems to be stabilizing or even decreasing [4, 5]. Most of our knowledge of the epidemiology of fungemia originates from studies done in North America. Recently, a review of European surveys on Candida bloodstream infections was published [6]. Incidence rates of candidemia from multi-institutional surveys ranged from 0.17 to 3.8 per 1,000 hospital admissions, from 0.26 to 0.73 per 10,000 patient-days, and from 1.9 to 11.0 per 100,000 inhabitants. Candida albicans was responsible for more than one-half of the cases in all patient populations except patients with haematological malignancies. Rates of antifungal resistance remained low in these European studies. From 1997 to 1999, the European Confederation of Medical Mycology (ECMM) conducted a hospital-based survey of candidemia with the participation of seven nations: France, Germany, Austria, Italy, Spain, Sweden, and the UK [7]. Similar rates of candidemia were reported in the different countries, ranging from 0.20 to 0.38 per 1,000 admissions and from 0.3 to 0.44 per 10,000 patient-days. C. albicans was identified in 56% of the cases. With increasing age, an increasing incidence of Candida glabrata fungemia was seen. The 30-day mortality rate was 37.9%. A 1-year multi-institutional Belgian survey on the epidemiology of candidemia was conducted in 2002 by Swinne et al. [8]. A total of 211 episodes of candidemia were evaluated in this survey. The study provides data on factors predisposing for candidemia and on the susceptibility of the isolates, but it was not designed to estimate the incidence of candidemia.
The first aim of our study was to provide data on the incidence of fungemia and on the distribution of the different causative species from a large (1,900-bed) university hospital in Belgium. The second aim of this study was to determine the susceptibility to seven antifungal agents of all yeasts isolated from blood cultures in our hospital during a 1-year period and to review the antifungal therapy administered to fungemic patients.
Materials and methods
Retrospective analysis
A review of all episodes of fungemia at the University Hospitals of Leuven (Belgium) was conducted between January 2001 and December 2005. Episodes of fungemia were defined as the isolation of a fungal isolate from at least one blood culture. Episodes of fungemia in a single patient were regarded as distinct if they were separated by at least a 30-day period during which no blood cultures were positive for fungi.
Identification of organisms
Blood specimens were processed by an automated blood culture system (BacT/Alert; bioMérieux, Durham, NC, USA), and fungi were subcultured on Sabouraud dextrose agar. BacT/Alert FA (aerobic), FN (anaerobic), and PF (paediatric) FAN media blood culture bottles (bioMérieux) were used. Species identification was based on germ tube production and colony colour on CHROMagar Candida (CHROMagar, Paris, France) supplemented with API ID32C (bioMérieux) for identification of all non-albicans Candida isolates. From June 2004 on, the first yeast isolate from each fungemic episode was stored at −70°C in glycerol, 10% v/v.
Moulds were identified on the basis of the results of macroscopical and microscopical examination of the cultures. The isolates were subcultured on Takashio medium (containing 0.2% glucose) to stimulate conidia formation. Moulds were stored on Sabouraud agar at −70°C.
Antifungal susceptibility testing
Susceptibility testing was performed on the first yeast isolate from each fungemic episode collected during a 1-year period (June 2004–June 2005). No susceptibility testing was done for mould isolates.
Prior to testing, each isolate was passed on Sabouraud dextrose agar. Susceptibility testing was performed using a Sensititre YeastOne panel (Trek Diagnostic Systems, East Grinstead, UK) according the instructions of the manufacturer. The MICs were read visually after 24 h of incubation at 36°C. Antifungal drugs tested were fluconazole, amphotericin B, voriconazole, caspofungin, flucytosine, ketoconazole, and itraconazole. Quality control was ensured by testing the Clinical Laboratory Standards Institute (CLSI)-recommended strains C. krusei ATCC 6258 and Candida parapsilosis ATCC 22019. Interpretative criteria for fluconazole, itraconazole, flucytosine, and voriconazole were those of the CLSI: fluconazole, susceptible (S) MIC ≤8 μg/ml, resistant (R) MIC ≥64 μg/m; voriconazole, S MIC ≤1 μg/ml, R MIC ≥4 μg/ml, itraconazole, S MIC ≤0.125 μg/ml, R MIC ≥1 μg/ml; flucytosine, S MIC ≤4 μg/ml, R MIC ≥32 μg/ml [9].
Use of antifungal agents
Data on antifungal agents prescribed for each patient with fungemia during a 1-year period (June 2004–June 2005) were obtained from the hospital pharmacy.
Hospital epidemiology data
Epidemiological data were extracted from the Hospital Information System.
Statistical analysis
Linear trends over time were analysed by nonparametric Spearman rank order correlation analysis (Analyse-it software, Leeds, UK).
Results
Incidence rates and species distribution
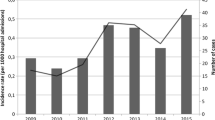
Over the 5-year period, 411 episodes of fungemia were detected in 408 patients on a total of 2,680,932 patient-days. Three patients had two episodes of fungemia. The incidence of fungemia per 10,000 patient-days showed a decreasing trend over the 5-year period (Fig. 1, r s = −0.90, p = 0.04) and was highest in 2001 (1.68 episodes/10,000 patient-days) and lowest in 2004 (1.30 episodes/10,000 patient-days). The incidence of candidemia was very similar, since few episodes were due to non-Candida species, and ranged between 1.65 and 1.26 per 10,000 patient-days (r s = −0.87, p = 0.05).
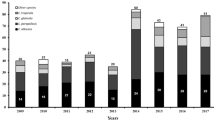
The species distribution over the 5-year period is shown in Table 1. There were no real shifts in the species distribution during this period. C. albicans remained the predominant species, representing between 51% and 73% of all isolates recovered, and C. glabrata was the second most common species. In patients less than 15 years of age (n = 32), C. parapsilosis was the second most prevalent species after C. albicans, followed by Candida lusitaniae. No C. glabrata isolates were cultured from patients in this age group. The distribution of the isolates by age group is shown in Table 2.
Fifteen patients (3.7% of all patients) harboured two different Candida species during the episode of fungemia; in 10 of these patients, the two species were C. albicans and C. glabrata.
Thirty-five percent of all fungemic episodes occurred in patients hospitalised in the (medical and surgical) intensive care units (ICUs). The emergency unit ranked second (7%) in the recovery of fungi from blood cultures. Relatively few fungemic episodes were detected in the neonatal ICU (n = 8) or the haematology units (n = 17). The average annual incidence rates for these different hospitalisation units were as follows: neonatal ICUs, 0.30 episodes per 10,000 patient-days; haematology units, 0.46 episodes per 10,000 patient-days; emergency units 3.04 episodes per 10,000 patient-days; and ICUs, 1.74 episodes per 10,000 patient-days. The species distribution according to the hospitalisation unit is shown in Table 3.
Antifungal susceptibility testing and antifungal therapy
A total of 62 yeast isolates from 60 patients were collected from June 2004 until June 2005. The MIC ranges and the MIC50 (μg/ml) and MIC90 (μg/ml) values for the seven antifungal agents are shown in Table 4. The susceptibility of the isolates to the antifungal agents for which CLSI breakpoints are available (fluconazole, voriconazole, itraconazole, and flucytosine) are shown in Table 5.
All C. albicans isolates were fully susceptible to fluconazole. Eighty-two percent of the C. glabrata isolates showed decreased susceptibility (MIC >8 μg/ml) to fluconazole, including one resistant isolate (MIC = 64 μg/ml). Voriconazole and caspofungin inhibited 100% of the isolates at a concentration of ≤1 μg/ml. The two isolates for which voriconazole MICs were highest (1 μg/ml) were two C. glabrata isolates for which fluconazole MICs were high (32 and 64 μg/ml). The MIC of caspofungin for these two isolates was low (≤0.06 μg/ml). The highest MICs of caspofungin (0.5 μg/ml) were found for C. parapsilosis isolates.
Seventy-five percent of fungemic patients between June 2004 and June 2005 received fluconazole therapy. Caspofungin was the second most commonly used antifungal agent (10%). Four of the six patients treated with caspofungin had C. glabrata candidemia (fluconazole susceptible-dose-dependent [SDD] isolates). For the two other patients, caspofungin was chosen because the patient received fluconazole prophylaxis or the patient experienced a second candidemic episode. In these two patients, a fluconazole-susceptible C. albicans isolate was cultured from blood. In five patients, therapy was switched from fluconazole to caspofungin. In four patients, therapy was switched because a non-albicans Candida isolate was cultured: C. glabrata (fluconazole SDD and resistant isolate) in two patients and C. parapsilosis in two other patients. In one patient, therapy was switched to caspofungin because of persistent isolation of C. parapsilosis during fluconazole therapy. Two patients were treated with conventional amphotericin B: one patient with a fluconazole SDD C. glabrata isolate, and the other with a fluconazole-susceptible C. albicans isolate cultured from the blood. The latter patient received fluconazole during the 6 days preceding the isolation of the C. albicans from the blood. No antifungal therapy was administered to three patients who died before or on the day the yeasts were cultured from the blood.
In the period preceding the fungemic episode, five patients received fluconazole for treatment of a Candida infection or as prophylaxis. Four of these patients developed candidemia caused by a fluconazole-susceptible isolate of C. albicans or C. parapsilosis. A fluconazole-resistant isolate (C. krusei) was cultured from one patient. This patient, who had undergone allogeneic stem cell transplantation and had graft-versus-host disease, was on fluconazole (400 mg/day) for 2 months.
Discussion
The average annual incidence of fungemia (candidemia) in this retrospective study was 1.53 (1.51) per 10,000 patient-days, with a decreasing trend observed between 2001 and 2005. C. albicans caused more than 50% of the fungemic episodes, followed by C. glabrata. No clear shift in the species distribution occurred during this study period. Overall, fluconazole resistance was rare (1.6%) and was detected only in isolates of C. glabrata and C. krusei.
This study provides the first data on the incidence of fungemia in Belgium. Although this study is a single-hospital study, a large sample size (more than 2.5 million patient-days) was attained. The incidence of candidemia in our centre is similar to the rates reported from centres in the USA [10]. Generally, the incidences published from surveys conducted in European countries are lower [6, 11–13]. Incidence rates above 1.5 per 10,000 patient-days have been reported only in Italy and Spain [7, 14]. In Italy, the high incidence was due partly to a large outbreak of Candida bloodstream infections during the study period. We speculate that the high incidence of fungemia in our hospital is likely due to the large patient population at risk for these invasive infections. Patients from all over Belgium are referred to this 1,900-bed hospital. The University Hospitals of Leuven have an important transplant programme, with approximately 100 kidney, 5 pancreas, 50 liver, 50 lung, and 25 heart transplantations each year. Analysis of the data of the National Nosocomial Infection Surveillance (NNIS) system in the USA showed a clear-cut association between the incidence of candidemia and the number of hospital beds and/or the academic affiliation [3]. The ECMM survey also showed that the rate of candidemia increased with the number of hospital beds [7]. The incidence of candidemia at our neonatal ICU was low (0.30 episodes/10,000 patient-days). Due to this low incidence of candidemia, no fluconazole prophylaxis is given in our neonatal ICU.
The distribution of the different species was similar to that of the 1-year survey conducted in Belgium in 2002 (C. albicans 55%, C. glabrata 22%, and C. parapsilosis 13%) [8]. C. albicans causes more than 50% of the cases in European studies except in patients with haematological malignancies. C. glabrata is the second most common species recovered, except in Italy and Spain, where it ranked third and fourth, respectively [7, 13, 14].
The prophylactic use of azoles has an impact on the Candida species encountered. In haematology patients, non-albicans species, mainly C. glabrata, accounted for two-thirds of the episodes in the study of Tortorano et al. [6]. In our study, about half of the fungemic episodes in this patient group were caused by C. glabrata. C. krusei and C. tropicalis were isolated only once in our haematological population, whereas these two species were present in up to 18% (C. tropicalis) and 13% (C. krusei) in this patient group in the ECMM survey and in the surveillance study performed by the European Organization for Research and Treatment of Cancer (EORTC) [15]. Patients receiving chemotherapy for acute leukaemia or myelodysplastic syndrome are given fluconazole prophylaxis in our hospital. Patients undergoing allogeneic haematopoietic stem cell transplantation receive prophylaxis with itraconazole.
C. krusei is a rare species in our hospital: the five patients with C. krusei fungemia were hospitalised in five different units.
The fact that two different Candida species were cultured concomitantly in nearly 4% of fungemic patients supports the use of a chromogenic medium to subculture blood cultures in which fungi were detected microscopically. The presence of C. glabrata in a mixture with C. albicans can be missed with the germ tube test, and this may have therapeutic consequences.
As expected, few filamentous fungi were cultured from blood. Penicillium species, with the exception of Penicillium marneffei, are generally considered to be contaminants. In this study, Penicillium species were cultured from six blood culture bottles, taken on two different days, from a haematopoietic stem cell transplant recipient. The blood was taken from a long-term central venous catheter. This catheter was considered to be colonised by Penicillium species and was replaced. No Penicillium species were cultured after the catheter was changed.
The results of the susceptibility testing were similar to those reported by Swinne et al. [8] and to those of other European studies [6, 16–18], with the exception of a Danish study [19]. Resistance to fluconazole was rare (1.6%) and was detected only in C. glabrata and C. krusei. No isolates for which the MIC of amphotericin B exceeded 1 μg/ml were found. Voriconazole and caspofungin were active against all isolates tested. In Denmark, fluconazole resistance was detected in a broader range of species.
Several commercial methods are available for routine testing of antifungal susceptibility of yeasts in the clinical laboratory. The E test has been shown to be a reliable method for the susceptibility testing of Candida species to amphotericin B, flucytosine, fluconazole, voriconazole, and caspofungin by different investigators [20, 21]. Furthermore, the Sensititre YeastOne assay was shown to be an excellent alternative procedure to both E test and the CLSI reference procedure for routine antifungal susceptibility testing of Candida species [22, 23]. Canton et al. [24] demonstrated that Sensititre YeastOne detects strains with reduced caspofungin susceptibility. Recently, it was shown that the Neo-Sensitabs tablet diffusion assay (Rosco Diagnostica, Taastrup, Denmark) may be an alternative method in the clinical laboratory to determine the susceptibility of yeasts to caspofungin, fluconazole, itraconazole, and voriconazole [25]. Further studies are required to establish reference quality-control zone diameters and interpretive zone diameters for caspofungin and itraconazole to better evaluate the suitability of this method. This method was unable to identify some well-documented amphotericin-B-resistant isolates.
It is known that MICs of amphotericin B for isolates of Candida species are tightly clustered between 0.25 and 1 μg/ml when broth dilution methods are used and that these methods do not consistently permit detection of resistant isolates. For surveillance studies, the E test is preferred over broth dilution techniques for amphotericin B susceptibility testing [17]. The E test produces the widest distribution of MICs. However, none of the test formats studied up to now for amphotericin B susceptibility testing generate results that significantly correlate with therapeutic success or failure [26].
A limitation of our retrospective analysis of fungemia is its design as single-centre study. We recently extended the susceptibility testing to Candida isolates from patients with candidemia hospitalised in seven Belgian hospitals [27]. The results were consistent with the findings of the University Hospitals of Leuven. Susceptibility data are available only for a 1-year period at the end of the study. Consequently, no conclusions about the trends in antifungal susceptibility over the whole study period can be drawn.
In conclusion, the low proportion of antifungal resistance detected in our hospital is reassuring and supports the use of fluconazole as the drug of choice for empirical treatment of candidemia in patients who have not received azole prophylaxis and are not known to be colonised with resistant species. The results also support the restricted use of antifungal susceptibility testing in our hospital.
References
Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C (2005) The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis 41:1232–1239
Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, Ellis D, Sorrell T (2006) Active surveillance for candidemia, Australia. Emerg Infect Dis 12:1508–1516
Banerjee SN, Emori TG, Culver DH, Gaynes RP, Jarvis WR, Horan T et al (1991) Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med 91:86S–89S
Sobel JD (2006) The emergence of non-albicans Candida species as causes of invasive candidiasis and candidemia. Curr Infect Dis Rep 8:427–433
Trick WE, Fridkin SK, Edwards JR, Hajjeh RA, Gaynes RP (2002) Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin Infect Dis 35:627–630
Tortorano AM, Kibbler C, Peman J, Bernhardt H, Klingspor L, Grillot R (2006) Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents 27:359–366
Tortorano AM, Peman J, Bernhardt H, Klingspor L, Kibbler CC, Faure O et al (2004) Epidemiology of candidaemia in Europe: results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur J Clin Microbiol Infect Dis 23:317–322
Swinne D, Watelle M, Suetens C, Mertens K, Fonteyne PA, Nolard N (2004) A one-year survey of candidemia in Belgium in 2002. Epidemiol Infect 132:1175–1180
Clinical and Laboratory Standards Institute (2006) Quality control minimal inhibitory concentration (MIC) limits for broth microdilution and MIC interpretive breakpoints. Supplement M44-S1. CLSI, Wayne, PA
Hajjeh RA, Sofair AN, Harrison LH, Lyon GM, Arthington-Skaggs BA, Mirza SA et al (2004) Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol 42:1519–1527
Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J et al (2004) Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis 38:311–320
Richet H, Roux P, Des CC, Esnault Y, Andremont A (2002) Candidemia in French hospitals: incidence rates and characteristics. Clin Microbiol Infect 8:405–412
Bedini A, Venturelli C, Mussini C, Guaraldi G, Codeluppi M, Borghi V et al (2006) Epidemiology of candidaemia and antifungal susceptibility patterns in an Italian tertiary-care hospital. Clin Microbiol Infect 12:75–80
Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M et al (2005) Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43:1829–1835
Viscoli C, Girmenia C, Marinus A, Collette L, Martino P, Vandercam B et al (1999) Candidemia in cancer patients: a prospective, multicenter surveillance study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin Infect Dis 28:1071–1079
Cuenca-Estrella M, Rodriguez D, Almirante B, Morgan J, Planes AM, Almela M et al (2005) In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002–2003. J Antimicrob Chemother 55:194–199
Pfaller MA, Messer SA, Boyken L, Tendolkar S, Hollis RJ, Diekema DJ (2004) Geographic variation in the susceptibilities of invasive isolates of Candida glabrata to seven systemically active antifungal agents: a global assessment from the ARTEMIS Antifungal Surveillance Program conducted in 2001 and 2002. J Clin Microbiol 42:3142–3146
Swinne D, Watelle M, Van der Flaes FM, Nolard N (2004) In vitro activities of voriconazole (UK-109,496), fluconazole, itraconazole and amphotericin B against 132 non-albicans bloodstream yeast isolates (CANARI Study). Mycoses 47:177–183
Arendrup MC, Fuursted K, Gahrn-Hansen B, Jensen IM, Knudsen JD, Lundgren B et al (2005) Seminational surveillance of fungemia in Denmark: notably high rates of fungemia and numbers of isolates with reduced azole susceptibility. J Clin Microbiol 43:4434–4440
Fleck R, Dietz A, Hof H (2007) In vitro susceptibility of Candida species to five antifungal agents in a German university hospital assessed by the reference broth microdilution method and E test. J Antimicrob Chemother 59:767–771
Pfaller MA, Diekema DJ, Messer SA, Boyken L, Hollis RJ (2003) Activities of fluconazole and voriconazole against 1,586 recent clinical isolates of Candida species determined by broth microdilution, disk diffusion, and E test methods: report from the ARTEMIS Global Antifungal Susceptibility Program, 2001. J Clin Microbiol 41:1440–1446
Morace G, Amato G, Bistoni F, Fadda G, Marcone P, Montagna MT et al (2002) Multicenter comparative evaluation of six commercial systems and the National Committee for Clinical Laboratory Standards M27-A broth microdilution method for fluconazole susceptibility testing of Candida species. J Clin Microbiol 40:2953–2958
Davey KG, Szekely A, Johnson EM, Warnock DW (1998) Comparison of a new commercial colorimetric microdilution method with a standard method for in-vitro susceptibility testing of Candida spp. and Cryptococcus neoformans. J Antimicrob Chemother 42:439–444
Canton E, Peman J, Gobernado M, Alvarez E, Baquero F, Cisterna R et al (2005) Sensititre YeastOne caspofungin susceptibility testing of Candida clinical isolates: correlation with results of NCCLS M27-A2 multicenter study. Antimicrob Agents Chemother 49:1604–1607
Espinel-Ingroff A, Canton E, Gibbs D, Wang A (2007) Correlation of Neo-Sensitabs tablet diffusion assay on three media, with CLSI broth microdilution M27-A2 and disk diffusion M44-A methods for susceptibility testing of Candida spp. and Cryptococcus neoformans, with amphotericin B, caspofungin, fluconazole, itraconazole, and voriconazole. J Clin Microbiol 45:858–864
Park BJ, Arthington-Skaggs BA, Hajjeh RA, Iqbal N, Ciblak MA, Lee-Yang W et al (2006) Evaluation of amphotericin B interpretive breakpoints for Candida bloodstream isolates by correlation with therapeutic outcome. Antimicrob Agents Chemother 50:1287–1292
Boel A, Cartuyvels R, De Beenhouwer H, Frans J, Oris E, Vandecandelaere P et al (2006) Susceptibility of yeasts isolated from hemocultures in 7 Belgian hospitals. Program and abstracts of the 16th Congress of the International Society for Human and Animal Mycology, Paris, 2006, Abstract no. P-0454
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lagrou, K., Verhaegen, J., Peetermans, W.E. et al. Fungemia at a tertiary care hospital: incidence, therapy, and distribution and antifungal susceptibility of causative species. Eur J Clin Microbiol Infect Dis 26, 541–547 (2007). https://doi.org/10.1007/s10096-007-0339-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0339-8