Abstract
The aims of this study were to evaluate the epidemiology of nosocomial candidemia in a large teaching hospital in Brescia, Italy, and the in vitro antifungal susceptibility of isolates. We analyzed 196 isolates causing fungemia in patients admitted in our hospital, between January 2009 and December 2015. Strains were identified by VITEK 2 and MALDI-TOF MS. MICs were determined by Sensititre Yeast OneTM. The resistance was defined by using the revised CLSI breakpoints/epidemiological cutoff values to assign susceptibility or wild type to systemic antifungal agents. Most infections were caused by Candida albicans (60%), Candida parapsilosis (15%), Candida glabrata (12%) and Candida tropicalis (6%). The susceptibility rate for fluconazole was 96.5%. Non-Candida species isolates exhibited full susceptibilities to echinocandins according to CLSI breakpoints. Amphotericin B demonstrated excellent activity against all Candida species. Local epidemiological and antifungal susceptibility studies are necessary in order to improve empirical treatment guidelines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Invasive candidiasis (IC) is an important cause of nosocomial infections, related to different risk factors such as previous antimicrobial therapy, immunodeficiency, parenteral nutrition, presence of catheters and intensive care unit permanence. [1, 2]. Although Candida albicans is still the main pathogen, non-albicans Candida species are increasing in IC patients [3]. This change has been attributed to the more frequent use of azole antifungals and invasive procedures [3]. In addition, there are geographical differences in the epidemiology of Candida infection [4]. In fact, Candida glabrata is the species more frequent after C. albicans in North America [5]. On the contrary, Candida parapsilosis or Candida tropicalis is relatively more common in Europe, Australia, Latin America and Asia [6,7,8]. In India, C. tropicalis causes are seen in more cases of candidemia than C. albicans [9]. Furthermore, different species such as Candida guilliermondii and Candida rugosa are diffusing [10, 11].
Compared to other bloodstream infections, IC appears to be associated with a particularly high rate of mortality, due to mainly, in the form of a delay in diagnosis or even failure of the antifungal therapy.
Different papers have shown that the rate of resistance to fluconazole ranges from 2.5 to 9% in Candida spp. isolated from blood [12, 13]. Most Candida species are considered good targets for the three echinocandins (anidulafungin, caspofungin and micafungin) that are used as first-line agents for the treatment of fungemia; however, what has been found is the increasing use of these drugs determines the emergence of resistance in Candida and non-Candida species [14].
An effective infection control is required due to higher incidence of IC, increased mortality and the growing prevalence of resistance to fluconazole.
The aim of this study was to conduct a seven-year retrospective analysis in order to analyze the incidence and species distribution in patients with fungemia in a large hospital located in Brescia, Italy. The rates of antifungal resistance were determined according to the newly revised CLSI clinical breakpoints (CBPs) or, in the absence of CBPs, according to epidemiological cutoff values (ECVs) for nine antifungal agents [15].
Materials and Methods
Collection of Isolates
We conducted this study in a large university hospital, Spedali Civili, in Brescia, North of Italy. We collected isolates from patients with candidemia from January 2009 to December 2015. An episode of candidemia was defined as Candida infection involving at least one blood culture. Only the first episode of fungemia was reported per patient with recurrent or subsequent episodes of infection. Patients whose cultures grew >1 documented species of Candida were excluded from the analysis. The study did not require the approval by the Institutional ethics committee due to the descriptive nature.
Yeast Identification
Yeasts were isolated from patient blood cultures collected during normal routine and processed by the BACTEC (BD Diagnostic Systems, Sparks, MD) system. Identification of all species was performed using VITEK 2 YST cards from bioMérieux (Marcy, l’ Etoile, France) or since 2012, by matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF).
Antifungal Susceptibility Testing
The in vitro susceptibility to antifungal drugs was performed by using Sensititre Yeast One (YO-08 from 2009 to 2012 and YO-10 from 2013 to 2015) in accordance with the manufacturer’s instructions. Because the ranges of amphotericin B, flucytosine, fluconazole and itraconazole were different from those of the previous version (YO-08), MIC values of 0.008–0.12 μg/mL for amphotericin B and of 0.03–0.12 µg/mL for fluconazole were reported as ≤0.12 g/mL; MIC values of 0.03–0.06 μg/mL for flucytosine were reported as ≤0.06 g/mL; and MIC values of 0.008–0.015 μg/mL for itraconazole were reported as ≤0.015 μg/mL.
Data Analysis
MIC values determined by the Sensititre Yeast One YO-08 and YO-10 were interpreted according to current CLSI species specific CBPs [15]; if no CBPs were defined, ECVs were used.
The CLSI resistance breakpoint for fluconazole was defined as an MIC of >4 g/mL against C. albicans, C. parapsilosis and C. tropicalis, and of >32 μg/mL against C. glabrata; for voriconazole, as an MIC of >0.5 μg/mL against C. albicans, C. parapsilosis, and C. tropicalis, and of >1 μg/mL against C. krusei. The CLSI resistance breakpoint for echinocandins was indicated as an MIC of >0.5 μg/mL against C. albicans and C. tropicalis and of 4 g/mL against C. parapsilosis; for both anidulafungin and caspofungin, an MIC of >0.25 μg/mL was defined against C. glabrata and an MIC of >0.12 μg/mL for micafungin. The ECV of >0.5 μg/mL was used to identify non-WT isolates of C. glabrata to voriconazole; ECVs of >0.06, >0.25, >0.12, and >2 μg/mL were used to identify non-WT isolates of C. albicans, C. parapsilosis, C. tropicalis and C. glabrata, respectively, to posaconazole [16]. ECVs were also used to identify non-WT isolates of C. albicans, C. parapsilosis, C. tropicalis and C. glabrata to amphotericin B (>2 μg/mL for all) and flucytosine (>0.5 μg/mL for all) [16].
Results and Discussion
A total of 196 distinct episodes of candidemia (192 adults and 4 adolescents <18 years of age) were identified during the study period. Of these, 113 cases (58%) comprised of males and 83 (42%) where females. The median age of patients was 81 years (ranging from 17 to 96 years).
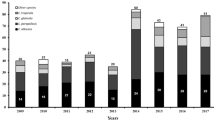
The average incidence of candidemia was 0.365 per 1000 admissions, which is comparable to that reported for centers in Denmark (0.41 case per 1000 admissions) [17], but lower than that of Israel [18], China [19], Brazil [20], Portugal [21] and even from that of different Italian regions [22, 23]. These differences in candidemia rates may depend on various factors such as differences in demographic characteristics, variations in health care practice, long antibacterial therapies and on the local resistance epidemiology. During the years analyzed, this incidence rate varied, increasing from 0.23 cases per 1000 admissions in the year 2009 to 0.55 cases per 1000 admissions in 2015 (Fig. 1). This might be due to an increased exposure to different risk factors such as prolonged antibiotic therapy, the use of urinary catheters, parenteral nutrition and central venous catheters.
The four most prevalent species were Candida albicans (60%), Candida parapsilosis (15%), Candida glabrata (12%), and Candida tropicalis (6%), while other species were relatively rare: Candida famata (1.5%), Candida lusitaniae (1.5%), Candida lipolytica (1%), Candida ciferrii (0.5%), Candida dubliniensis (0.5%) and Candida guilliermondii (0.5%). The most affected age group was 61-80 years and these where mostly isolated from intensive care units, medical and surgical wards (Table 1). This result is not surprising, due to the fact that most of the patients admitted to these wards are in immunosuppressive conditions, and are often submitted to antibiotic therapies, predisposing them to an increasing rate of fungal infections.
Tables 2 and 3 show all the data for susceptibility testing using CLSI guidelines.
All isolates studied showed a WT phenotype to amphotericin B. The MIC50 and MIC90 values of amphotericin B for all the species were either 0.25 or 0.5 mg/L (Table 2). All isolates were inhibited at drug concentrations of 0.5 mg/L.
This full susceptibility to amphotericin B is important because, although this agent is not a first-line drug for the treatment of invasive candidiasis and candidemia in most clinical approaches [14], it may be used as alternative therapeutic option or where the isolates exhibit a resistance against azoles or echinocandins.
When we looked at the susceptibility to flucytosine, 10 isolates across C. albicans (2/116 isolates; 1.7%), C. parapsilosis (3/28 isolates; 10.7%), C. glabrata (2/22 isolates; 9%) and C. tropicalis (2/12 isolates; 25%) were found to be not WT to flucytosine.
The most frequently used antifungals systematically and locally are the azoles. Of the azoles used systematically, fluconazole is the most frequently used one in the yeasts.
Among the group of azoles, C. parapsilosis species was susceptible to all four azoles tested. With regard to C. albicans, 3 isolates were resistant to fluconazole and posaconazole (2.8 and 2.6%, respectively). Three isolates (2.5%) were resistant to voriconazole, while two isolates (1.7%) were resistant to itraconazole. With regard to C. glabrata, one isolate (4.5%) was resistant to fluconazole, 2 isolates (8.3%) were resistant to itraconazole, and four isolates (16.6%) were resistant to voriconazole. All isolates were of the WT phenotype for posaconazole. With regard to C. tropicalis, 1 isolate (10%) was resistant to fluconazole and 2 isolates (18%) were resistant to posaconazole. All isolates were of the WT phenotype for itraconazole.
Overall, the frequency of fluconazole resistance in our isolates was 3.5%. The MIC values for fluconazole were between 0.25 and 32 mg/L for the 175 Candida strains analyzed.
Our findings show a low resistance to fluconazole by C. glabrata isolates (4.5%), a percentage comparable to that reported in other Italian studies [23, 24]. Furthermore, we found some isolates of C. glabrata susceptible to fluconazole and resistant to voriconazole, a phenotype already described [25].
Echinocandins are in general active against various Candida and Aspergillus spp. and this explains their large use in clinical practice. In fact, recent European guidelines are recommending echinocandins as the first-line treatment in severe or neutropenic patients, or when there is prior use of azoles or suspected resistance to azoles [26].
In our study, all Candida–non-albicans species were full susceptible to echinocandins. Echinocandin resistance in Candida albicans was low (1/54, 1.8%) for all the three echinocandins and was similar to that reported in Spain [27], however, different from other countries such as the USA where echinocandin resistance is emerging [28].
Table 4 shows the trend in the resistance rate over the period of the study. We observed a stable trend for azole resistance during the study period with the exception of the year 2014 where there was a decline in the rate of resistance.
This study has several limitations, i.e., the data associated with underlying diseases, risk factors, mortality and previous antifungal therapy were unknown, and therefore, this information could not be analyzed. It is also important to highlight the relatively small size of our samples and underline that more isolates (by extending the period of study) would give more reliable and substantial importance to our results.
Finally, since we included only isolates from a single institution, we may not able to extrapolate them to other hospitals.
However, the rates of fluconazole and echinocandin resistance that we have recorded were similar to those reported from other countries [25,26,27,28,29,30].
Antifungal susceptibility studies performed locally should be a priority in order to monitor continuously the emergence of Candida or non-Candida species with intrinsically reduced susceptibility or resistance.
References
Viudes A, Peman J, Canton E, Ubeda P, Lopez-Ribot L, Gobernado M. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur J Clin Microbiol Infect Dis. 2002;21:767–74.
Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63.
Abi-Said D, Anaissie E, Uzun O, Raad I, Pinzcowski H, Vartivarian S. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997;24:1122e8.
Guo F, Yang Y, Kang B, Cui W, Qin B, Qin F, Fang Q, Qin T, Jiang D, Li W, et al. Invasive candidiasis in intensive care units in China: a multicenter prospective observational study. J Antimicrob Chemother. 2013;68:1660–8.
Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53.
Nucci M, Queiroz-Telles F, Tobon AM, Restrepo A, Colombo AL. Epidemiology of opportunistic fungal infections in Latin America. Clin Infect Dis. 2010;51:561–70.
Wang H, Xiao M, Chen SC, Kong F, Sun ZY, Liao K, Lu J, Shao HF, Yan Y, Fan H, et al. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2012;50:3952–9.
Chen S, Slavin M, Nguyen Q, Marriott D, Playford EG, Ellis D, Sorrell T. Australian candidaemia study. Active surveillance for candidemia, Australia. Emerg Infect Dis. 2006;12:1508–16.
Chander J, Singla N, Sidhu SK, Gombar S. Epidemiology of Candida bloodstream infections: experience of a tertiary care centre in North India. J Infect Dev Ctries. 2013;7:670–5.
Chen SC, Marriott D, Playford EG, Nguyen Q, Ellis D, Meyer W, Sorrell TC, Slavin M. Australian candidaemia study. Candidaemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009;15:662–9.
Pfaller MA, Diekema DJ, Colombo AL, Kibbler C, Ng KP, Gibbs DL, Newell VA. Candida rugosa, an emerging fungal pathogen with resistance to azoles: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J Clin Microbiol. 2006;44:3578–82.
Peman J, Canton E, Minana JJ, Forez JA, Echeverria J, Ortega DN, Alarcon JM, Fontanals D, Sard BG, Moreno BB, et al. Changes in the epidemiology of fungaemia and fluconazole susceptibility of blood isolates during the last 10 years in Spain: results from the FUNGEMYCA study. Rev. Iberoam Micol. 2011;28:91–9.
Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates report from the SENTRY Antimicrobial Surveillance Program (2008–2009). J Clin Microbiol. 2011;49:396–9.
Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2441 patients. Antimicrob Agents Chemother. 2011;55:532–8.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4. 2012; Clinical and Laboratory Standards Institute, Wayne PA.
Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50(50):2846–56.
Arendrup MC, Bruurn B, Christensen JJ, Fuursted K, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Moller J, Nielsen L. National surveillance of fungemia in Denmark (2004–2009). J Clin Microbiol. 2011;49:325–34.
Rennert G, Rennert H, Pitlik S, Finkelstein R, Kitzes-Cohen R. Epidemiology of candidemia-a nationwide survey in Israel. Infection. 2000;28:26–9.
Li D, Zhang W, Zheng S, Ma Z, Zhang P, Liu Z. Surveillance study of candidemia in cancer patients in North China. Med Mycol. 2013;51:378–84.
Motta AL, Almeida GM, Almeida Junior JN, Burattini MN, Rossi F. Candidemia epidemiology and susceptibility profile in the largest Brazilian teaching hospital complex. Braz J Infect Dis. 2010;14:441–8.
Costa-de-Oliveira S, Pina-Vaz C, Mendonca D, Goncalves Rodrigues A. A first Portuguese epidemiological survey of fungaemia in a university hospital. Eur Clin Microbiol Infect Dis. 2008;27:365–74.
Tortorano AM, Prigitano A, Lazzarini C, Passera M, Deiana ML, Cavinato S, De Luca C, Grancini A, Lo Cascio G, Ossi C, et al. A 1-year prospective survey of candidemia in Italy and changing epidemiology over one decade. Infection. 2013;41:655–62.
Posteraro B, Spanu T, Fiori B, De Maio F, De Carolis E, Giaquinto A, Pete V, De Angelis G, Torelli R, D’Inzeo T, et al. Antifungal susceptibility profiles of bloodstream yeast isolates by Sensititre YeastOne: a nine-year experience at a large Italian teaching hospital. Antimicrob Agents Chemother. 2015;59:3944–55.
Barchiesi F, Orsetti E, Gesuita R, Skrami E, Manso E. The candidemia study group. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection. 2016;44:205–13.
Szweda P, Gucwa K, Romanowska E, Dzierzanowska-Fangrat K, Naumiuk L, Brillowska-Dabrowska A, Wojciechowska-Koszsko I, Milewski S. Mechanisms of azole resistance among clinical isolates of Candida glabrata in Poland. J Med Microbiol. 2015;64:610–9.
Kullberg BJ, Verweij PE, Akova M, Arendrup MC, Bille J, Calandra T, Cuenca-Estrella M, Herbrecht R, JacobsF Kalin M, et al. European expert opinion on the management of invasive candidiasis in adults. Clin Microbiol Infect. 2011;17:1–12.
Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, Pfaller MA. Frequency of fks mutations among Candida glabrata isolstes from a 10-year global collection of bloodstream infection isolates. Antimicrob Agents Chemother. 2014;58:577–80.
Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724–32.
Guinea J, Zaragoza O, Escribano P, Martin-Mazuelos E, Peman J, Sanchez-Reus F, Cueca-Estrella M. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain from 2010 to 2011. Antimicrob Agents Chemother. 2014;58:1529–37.
Shields RK, Nguyen MH, Clancy CJ. Clinical perspectives on echinocandin resistance among Candida species. Curr Opin Infect Dis. 2015;28:514–22.
Acknowledgement
We are sincerely obliged to Dr. Surace Antonella for revising the text.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
De Francesco, M.A., Piccinelli, G., Gelmi, M. et al. Invasive Candidiasis in Brescia, Italy: Analysis of Species Distribution and Antifungal Susceptibilities During Seven Years. Mycopathologia 182, 897–905 (2017). https://doi.org/10.1007/s11046-017-0155-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11046-017-0155-3