Abstract
Purpose
The aim of the present study was to analyze candidaemia’s epidemiology (incidence, species distribution, and susceptibility rates) and antifungal consumption during a 9-year period.
Methods
All candidaemias recorded at The University General Hospital of Patras, Greece, between 2009 and 2017 were included. Candida isolates were identified using the germ tube test, API 20C AUX System, and/or Vitek-2 YST card. Antifungal susceptibility was determined by the gradient method according to CLSI.
Results
During the study period, 505 episodes of candidaemia were observed with an overall incidence of 1.5 episodes per 1000 hospital admissions (1.1 episodes in 2009 to 1.9 in 2017: P 0.038, r 0.694). C. albicans was the leading cause (200 cases; 39.6%), followed by C. parapsilosis (185; 36.6%), C. glabrata (56; 11.1%), C. tropicalis (50; 9.9%), C. krusei (8; 0.2%), C. lusitaniae (5; < 0.1%), and C. guilliermondii (1; < 0.1%). Overall resistance to fluconazole, voriconazole, anidulafungin, caspofungin, and micafungin (according to CLSI) were 11.6%, 4.1%, 2.0%, 6.0%, and 0.8%, respectively. The overall consumption of antifungal drugs was stable, with a significant reduction of fluconazole’s use in favor of echinocandins.
Conclusions
An increase in the incidence of candidaemia and a predominance of Candida non-albicans due to decreasing use of fluconazole in favor of more potent antifungals, such as echinocandins, are reported in this study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Candida spp. remain an important cause of hospital acquired bloodstream infection (BSI) as shown by a point prevalence study in European hospitals during 2011–2012, where Candida spp. accounted for the 7.4% of such infections being the sixth most common pathogen [1]. During the last 2 decades, an increase in candidaemias’ incidence has been constantly reported with a parallel change in Candida spp. epidemiology worldwide. Specifically, a decrease of C. albicans in favor of Candida non-albicans (CNA) is reported by many centers [2,3,4,5]. Distribution of CNA depends on the geographical region with C. glabrata being the most common species in Central and North Europe, as well as, in North America, while C. parapsilosis in South Europe and South America [6, 7].
The previous exposure to certain antifungal classes affects species distribution. For example, fluconazole administration had led to selection of Candida spp. with reduced susceptibility to fluconazole such as C. krusei and C. glabrata, while echinocandin administration was a predisposing factor for C. parapsilosis’ candidaemia [3, 8, 9].
Epidemiologic data on candidaemia during the last decade in Greece are limited with most studies focusing on a special population, such as patients with haematological malignancies or those hospitalized in adult Intensive-Care Units (ICU) or neonatal ones (NICU) [9,10,11,12,13]. Aforementioned studies show an important shift towards CNA candidaemias, as compared to studies before 2008 where C. albicans predominated [14, 15].
A previous 11-year study (1998–2008) of candidaemias was performed in our University Hospital in southwestern Greece, which reported 255 candidaemias with C. albicans being the leading cause (163 cases; 64%) [14]. The present study aims to analyze the trends in the incidence of candidaemia, in association with susceptibility rates and antifungal consumption, in the same hospital during the last 9 years (2009–2017).
Materials and methods
This is a retrospective study, carried out at the University General Hospital of Patras (UGHP), Greece, during a 9-year period (2009–2017). The UGHP is a 770 bed tertiary care hospital with approximately 36,700 admissions per year. The adult ICU comprises of 13 beds and hospitalizes 338 patients per year. Data (age, sex, and 14- and 28-day mortality) of ICU patients with candidaemia were collected.
Identification and antifungal susceptibility testing
Positive blood cultures (Bact/Alert 3D, bioMerieux, Marcy l’Etoile, France) were inoculated onto Sabouraud dextrose agar. Plates were incubated at 37 °C for 96 h before assessed as negative. Yeast positive cultures were evaluated and a germ tube test was undertaken to quickly differentiate C. albicans from CAN. All yeasts were identified to species level using the API 20C AUX System (bioMerieux) or Vitek 2 YST card (bioMerieux). Antifungal susceptibility was determined by Etest (AB Biodisk, Solna, Sweden) on RPMI-2% glucose agar, and minimum inhibitory concentrations (MIC) of amphotericin B, fluconazole, voriconazole, posaconazole, anidulafungin, caspofungin, and micafungin were evaluated according to Clinical & Laboratory Standards Institute (CLSI) criteria [16].
Antifungal consumption
Overall antifungal consumption of liposomal amphotericin B, azoles (fluconazole, voriconazole, and posaconazole), and echinocandins (anidulafungin, caspofungin, and micafungin) was calculated using the defined daily dose (DDD) per 100-patient-days, as described by the WHO ATC/DDD [17]. Consumption data were collected from the Pharmacology Department.
Statistic analysis
Statistic analysis was performed with SPSS version 23.0 (SPSS, Chicago, IL, USA). Categorical variables were analyzed using the Fisher’s exact test. The MIC trends and the antibiotic consumption over the 5 years were assessed using Spearman’s correlation analysis. A P value < 0.05 was considered significant.
Results
Epidemiology
During the study period, 505 candidaemias were observed among 478 patients. Twenty-seven patients developed two episodes of candidaemia, either by the same species in more than 60 days apart or by different one. Overall incidence rate was 1.5 episodes per 1000 hospital admissions ranging from 1.1 episodes in 2009 to 1.9 in 2017 (P 0.038, r 0.694). The higher incidence was observed in the ICU (37.6 episodes per 1000 ICU admissions). The rate also showed a steady increase from 19.7 in 2009 up to 66.4 in 2017 (P 0.010, r 0.800). Fourteen- and 28-day mortality of ICU patients with candidaemia was 21.6% and 37.8%, respectively. Mortality was associated with older patients (55.9 years ± 18.7 vs 64.8 years ± 13.7; P 0.041), while candidaemia due to C. parapsilosis was associated with a better survival as compared to other species (25.0% vs 50.6%; P 0.036).
Species distribution
C. albicans was the leading cause (200 cases; 39.6%), followed by C. parapsilosis (185; 36.6%), C. glabrata (56; 11.1%), C. tropicalis (50; 9.9%), C. krusei (eight; 0.2%), C. lusitaniae (five; < 0.1%), and C. guilliermondii (one; < 0.1%).
Department distribution
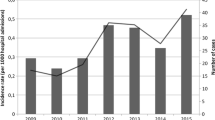
Most candidaemias occurred to patients hospitalized in medical wards (238 cases; 47.1%), followed by surgical wards (117; 23.2%), adult ICU (111; 22.0%), and NICU (39; 7.7%). Table 1 depicts the species and department distribution of candidaemias. Adult and neonatal ICU showed higher rate (P < 0.001) of C. parapsilosis (45.5% and 64.1%, respectively) as compared to medical and surgical wards (32.4% and 28.8%, respectively). During the study period, an increase of candidaemias was observed (P 0.015, r 0.773). This is due to an increase of C. albicans (P 0.004, r 0.845), C. parapsilosis (P 0.030, r 0.717), and C. tropicalis (P 0.023, r 0.740). Figure 1 depicts the annual episodes of candidaemia.
From June to December 2014, two C. parapsilosis BSI outbreaks were observed at the same time period in adult ICU (11 cases; 22.0% of all C. parapsilosis BSI in ICU) and in NICU (nine cases; 36.0% of all C. parapsilosis BSI in NICU). Both epidemics were successfully controlled by reinforcing hand hygiene policies, as well as refreshing courses to the staff of both units for compliance with infection control practices and handling of central venous catheters.
Antifungal susceptibility
MIC to antifungals and rates of susceptibility according to CLSI are depicted at Table 2. Overall resistance to fluconazole, voriconazole, anidulafungin, caspofungin, and micafungin were 11.6%, 4.1%, 2.0%, 6.0%, and 0.8%, respectively. Only one isolate (C. lusitaniae) had an MIC of 2 mg/L to amphotericin B. During the study period, an increase of MIC to amphotericin (P 0.013, r 0.110), fluconazole (P < 0.001, r 0.359), voriconazole (P < 0.001, r 0.405), and posaconazole (P < 0.001, r 0.359) was observed, while no trend concerning echinocandins was detected.
Antifungal consumption
The overall consumption of antifungal drugs was stable during the study period with a mean of 1451.6 DDD per 10,000 patient-days. The consumption of fluconazole diminished significantly from 591.2 in 2009 to 186.7 DDD per 10,000 patient-days (P 0.007, r − 0.821), whereas consumption of all echinocandins increased significantly from 62.4 in 2009 to 311.1 DDD per 10,000 patient-days (anidulafungin: P < 0.001, r 0.959; caspofungin: P < 0.001, r 0.880; micafungin: P < 0.001, r 0.981). Consumption of amphotericin B, voriconazole, and posaconazole did not change significantly during the study period. Figure 2 shows the annual consumption of each antifungal category.
Discussion
Our data show an increase of overall incidence of candidaemia during the study period, which is in accordance to the previous studies from different geographical areas [2, 3, 5, 18,19,20]. The incidence reported in the present study is comparable to that from Italy and Taiwan (1.1–2.2 episodes per 1000 hospital admissions) [3, 4, 20], while it is significantly higher as compared to that reported from Norway, Denmark, Italy, and Spain (0.2–0.9 episodes per 1000 hospital admissions, respectively) [5, 18, 19, 21]. Moreover, the incidence determined in the present study was higher than that reported in a previous publication from our institution (0.5 episodes per 1000 hospital admissions) during an 11-year period (1998–2008) [14]. This difference is mainly due to an abrupt and ongoing increase of CNA between 2008 and 2009 [14]. In the previous studies from the adult ICU of the UGHP, KPC-producing K. pneumoniae infection was an independent factor for the development of candidaemia by non-albicans species [9]. KPC-producing K. pneumoniae was isolated in our hospital in 2008. From 2009, it became endemic with increasing yearly incidence, which can explain this abrupt shift of candidaemias’ epidemiology and its increasing annual incidence [22].
Candida albicans remains the most common cause of candidaemia in our hospital and worldwide. However, there is high discrepancy among studies from different geographical areas, with rates surpassing 50% in North and Central Europe or studies conducted before 2010, whereas rates below 50% were reported in more recent ones (after 2010) from south Europe [6, 7]. In accordance to the literature, C. albicans’ incidence is declining over time in favor of CNA species, especially C. parapsilosis [2,3,4]. In the present study, C. parapsilosis was the predominant species among CNA and the cause of 36.6% of all candidaemias, as compared to only 13.3% in our institution during 1998–2008 [14]. In the literature, C. parapsilosis and C. glabrata represent the second or third most common species, depending on the area or the patient population [6, 7]. C. parapsilosis is predominant in neonates, in South Europe and Asia, and has a high propensity to cause catheter-associated BSI and to provoke outbreaks, while the elderly and those with abdominal surgery are more prone to infection by C. glabrata [6, 7, 23].
An outbreak provoked by C. parapsilosis was observed simultaneously during the second half of 2014 in the adult and neonatal ICU of our hospital. As reported, C. parapsilosis has the ability to provoke outbreaks in such units with most reports coming from NICUs [24, 25]. Molecular studies from the previous outbreaks showed that genotypes of infecting isolates were commonly isolated from health care workers hands [24, 25]. Since these exogenous isolates can been transferred from health care workers to patients’ skin or to indwelling devices (central venous catheter, arterial catheters, etc) during manipulation, reinforcing infection control practices including hand hygiene and handling of central venous catheters are imperative. These practices were successful in controlling the epidemic which was abated 6 months later in the aforementioned departments.
In accordance to the literature, 28-day mortality of ICU candidaemias was 37.8% [3, 10, 20, 21]. As shown in the previous studies, C. parapsilosis BSI was associated with lower mortality (25.0% vs 50.6%; P 0.036) as compared to all other species [3]. Pfaller et al. analyzed 3648 patients with candidaemia from North America showed that C. parapsilosis had the lowest 30-day mortality (22.1%) as compared to each other species (> 30%) [26]. This may be partially explained by the fact that since most infections caused by C. parapsilosis are catheter-related, the source of infection can be more easily identified and eliminated by removing the infected catheter [21].
Overall resistance to fluconazole is high (11.6%) and may partially explain the annual decrease in its consumption in favor of echinocandins. The other explanation is that echinocandins are associated with better outcome than fluconazole. Thus, they remain the first-line treatment of these infections with the exception C. parapsilosis where the use of fluconazole might be preferable [27]. This increase in echinocandins’ utilization during the last decade was reported from other countries [28, 29]. Echinocandins resistance was low (anidulafungin: 2.0%, caspofungin: 6.0%, and micafungin: 0.8%) in accordance to the previous studies [3, 4], even though their use was increasing annually. In the contrary, a study from a French ICU showed that the increase in caspofungin utilization from 2004 to 2013 led to an increase of caspofungin’s MICs among Candida spp. [28]. An eventual consequence of the increasing utilization of echinocandins might be the predominance of CAN and especially C. parapsilosis, since it exhibits reduced susceptibility to such antifungals [8, 10]. Fluconazole’s resistance rate of the present study was higher to those previously reported from our Institution or from other European countries [3, 5, 14, 19]. Caspofungin resistance is higher as compared to other echinocandins. However, there is major concern in interpreting such results, since high rates of false caspofungin resistance have been reported [30].
Limitations of the present study include the absence of clinical data to identify potential risk factors for the development and predictors of mortality of these infections. Antifungal consumption reflects treatment of all types of infection, not only candidaemia, as well as prophylactic administration. Another limitation was the absence of molecular studies of Candida spp. isolates, especially those recovered during the ICU and NICU outbreak.
In conclusion, this study is providing useful data on the shifting epidemiology of candidaemia in southwestern Greece with an increase in its incidence and a predominance of CAN, especially C. parapsilosis over C. albicans. The predominance of CNA can explain the increasing MIC to azoles during the study period and the decreasing use of fluconazole in favor of more potent antifungals such as echinocandins, which also exhibit low resistance rates. Local antifungal susceptibility studies are important to monitor the epidemiology of candidaemia and also the susceptibility rates of Candida spp. to guide empirical treatment.
References
European Centre for Disease Prevention and Control (ECDC). Point prevalence survey of healthcare associated infections and antimicrobial use in European acute care hospitals. Stockholm: ECDC; 2013.
Goemaere B, Becker P, Van Wijngaerden E, et al. Increasing candidaemia incidence from 2004 to 2015 with a shift in epidemiology in patients preexposed to antifungals. Mycoses. 2018;61:127–33.
Barchiesi F, Orsetti E, Gesuita R, et al. Epidemiology, clinical characteristics, and outcome of candidemia in a tertiary referral center in Italy from 2010 to 2014. Infection. 2016;44:205–13.
Hii IM, Chang HL, Lin LC, et al. Changing epidemiology of candidemia in a medical center in middle Taiwan. J Microbiol Immunol Infect. 2015;48:306–15.
De Francesco MA, Piccinelli G, Gelmi M, et al. Invasive candidiasis in Brescia, Italy: analysis of species distribution and antifungal susceptibilities during seven years. Mycopathologia. 2017;182:897–905.
Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954-66.
Antinori S, Milazzo L, Sollima S, et al. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med. 2016;34:21–8.
Farmakiotis D, Kontoyiannis DP. Epidemiology of antifungal resistance in human pathogenic yeasts: current viewpoint and practical recommendations for management. Int J Antimicrob Agents. 2017;50:318–24.
Papadimitriou-Olivgeris M, Spiliopoulou A, Fligou F, et al. Risk factors and predictors of mortality of candidaemia among critically ill patients: role of antifungal prophylaxis in its development and in selection of non-albicans species. Infection. 2017;45:651–7.
Kofteridis DP, Valachis A, Dimopoulou D, et al. Factors influencing non-albicans candidemia: a case-case-control study. Mycopathologia. 2017;182:665–72.
Vogiatzi L, Ilia S, Sideri G, et al. Invasive candidiasis in pediatric intensive care in Greece: a nationwide study. Intensive Care Med. 2013;39:2188–95.
Gamaletsou MN, Walsh TJ, Zaoutis T, et al. A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20:O50-7.
Papadimitriou-Olivgeris M, Spiliopoulou A, Fligou F, et al. Association of KPC-producing Klebsiella pneumoniae colonization or infection with Candida isolation and selection of non-albicans species. Diagn Microbiol Infect Dis. 2014;80:227–32.
Spiliopoulou A, Vamvakopoulou S, Bartzavali C, et al. Eleven-year retrospective survey of candidaemia in a university hospital in southwestern Greece. Clin Microbiol Infect. 2010;16:1378–81.
Vardakas KZ, Michalopoulos A, Kiriakidou KG, et al. Candidaemia: incidence, risk factors, characteristics and outcomes in immunocompetent critically ill patients. Clin Microbiol Infect. 2009;15:289–92.
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement, M27-S4. Wayne: CLSI; 2012.
WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment, 2018. Oslo. 2017. https://www.whocc.no/filearchive/publications/guidelines.pdf. Accessed 29 Jun 2018.
Arendrup MC, Dzajic E, Jensen RH, et al. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect. 2013;19:E343–E353.
Hesstvedt L, Gaustad P, Andersen CT, et al. Twenty-two years of candidaemia surveillance: results from a Norwegian national study. Clin Microbiol Infect. 2015;21:938–45.
Tedeschi S, Tumietto F, Giannella M, et al. Epidemiology and outcome of candidemia in internal medicine wards: a regional study in Italy. Eur J Intern Med. 2016;34:39–44.
Puig-Asensio M, Padilla B, Garnacho-Montero J, et al. Epidemiology and predictive factors for early and late mortality in Candida bloodstream infections: a population-based surveillance in Spain. Clin Microbiol Infect. 2014;20:O245-54.
Spyropoulou A, Papadimitriou-Olivgeris M, Bartzavali C, et al. A ten-year surveillance study of carbapenemase-producing Klebsiella pneumoniae in a tertiary care Greek university hospital: predominance of KPC- over VIM- or NDM-producing isolates. J Med Microbiol. 2016;65:240–6.
Spiliopoulou A, Dimitriou G, Jelastopulu E, et al. Neonatal intensive care unit candidemia: epidemiology, risk factors, outcome, and critical review of published case series. Mycopathologia. 2012;173:219–28.
Wang H, Zhang L, Kudinha T, et al. Investigation of an unrecognized large-scale outbreak of Candida parapsilosis sensu stricto fungaemia in a tertiary-care hospital in China. Sci Rep. 2016;6:27099.
Hernandez-Castro R, Arroyo-Escalante S, Carrillo-Casas EM, et al. Outbreak of Candida parapsilosis in a neonatal intensive care unit: a health care workers source. Eur J Pediatr. 2010;169:783–7.
Pfaller M, Neofytos D, Diekema D, et al. Epidemiology and outcomes of candidemia in 3648 patients: data from the prospective antifungal therapy (PATH Alliance(R)) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74:323–31.
Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18:19–37.
Bailly S, Maubon D, Fournier P, et al. Impact of antifungal prescription on relative distribution and susceptibility of Candida spp.—trends over 10 years. J Infect. 2016;72:103–11.
Mencarini J, Mantengoli E, Tofani L, et al. Evaluation of candidemia and antifungal consumption in a large tertiary care Italian hospital over a 12-year period. Infection. 2018;46:469–76
Arendrup MC, Pfaller MA, Danish Fungaemia Study G. Caspofungin Etest susceptibility testing of Candida species: risk of misclassification of susceptible isolates of C. glabrata and C. krusei when adopting the revised CLSI caspofungin breakpoints. Antimicrob Agents Chemother. 2012;56:3965–8.
Funding
This study was supported by funds of the Department of Microbiology, School of Medicine, University of Patras.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the Bioethics’ Committee of the UGHP (no. 3324).
Rights and permissions
About this article
Cite this article
Papadimitriou-Olivgeris, M., Spiliopoulou, A., Kolonitsiou, F. et al. Increasing incidence of candidaemia and shifting epidemiology in favor of Candida non-albicans in a 9-year period (2009–2017) in a university Greek hospital. Infection 47, 209–216 (2019). https://doi.org/10.1007/s15010-018-1217-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-018-1217-2