Abstract
Introduction
Autoimmune encephalitis (AE) is caused by the antibodies that target receptors and intracellular or surface proteins. To achieve the appropriate therapeutic results, early and proper diagnosis is still the most important issue. In this review, we provide an overview of FDG-PET imaging findings in AE patients and possible relation to different subtypes and clinical features.
Methods
PubMed, Web of Science, and Scopus were searched in August 2021 using a predefined search strategy.
Results
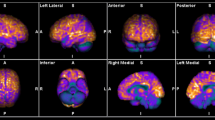
After two-step reviewing, 22 studies with a total of 332 participants were entered into our qualitative synthesis. In anti-NMDAR encephalitis, decreased activity in the occipital lobe was present, in addition, to an increase in frontal, parietal, and specifically medial temporal activity. Anti-VGKC patients showed altered metabolism in cortical and subcortical regions such as striata and cerebellum. Abnormal metabolism in patients with anti-LGI1 has been reported in diverse areas of the brain including medial temporal, hippocampus, cerebellum, and basal ganglia all of which had hypermetabolism. Hypometabolism in parietal, frontal, occipital lobes, temporal, frontal, and hippocampus was observed in AE patients with anti-GAD antibodies.
Conclusion
Our results indicate huge diversity in metabolic patterns among different AE subtypes and it is hard to draw a firm conclusion. Moreover, the timing of imaging, seizures, and acute treatments can alter the PET patterns strongly. Further prospective investigations with specific inclusion and exclusion criteria should be carried out to identify the metabolic defect in different AE subtypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Encephalitis is defined as inflammation of the brain and although there are many causes, the main etiologies can be categorized into two main categories: autoimmune and infectious [1]. Autoimmune encephalitis (AE) is caused by the antibodies that target receptors and intracellular or surface proteins [1]. AE commonly refers to a group of similarly related disease processes which share overlapping neuroimaging findings and clinical features but are differentiated by specific antibody subtypes that initiate the underlying immune attack on different structures of the central nervous system [2, 3].
AE associated antibodies can be subclassified into two groups: antibodies against synaptic proteins and neuronal cell-surface, like the leucine-rich, glioma-inactivated glycoprotein (LGI1), the gamma-aminobutyric acid (GABA) receptor, and the N-methyl-D-aspartate (NMDA) receptor and antibodies against intracellular antigens, like anti-glutamic acid decarboxylase 65 (GAD65), CV2/collapsin response mediator protein 5 (CRMP5), Ma2/Ta, and Hu/antineuronal nuclear antigen type 1 (ANNA-1) [4, 5].
Some clinical symptoms pointing to AE are sleep disturbance, psychiatric symptoms, and subacute memory loss [6]. AE is a challenging clinical diagnosis because of the similarities of the clinical, laboratory, and imaging findings with other types of central nervous system infections [7]. Neuroimaging findings play an important role in the evaluation of cases with suspected encephalitis [8]. It can confirm the diagnosis of encephalitis, indicate specific etiologies, or identify other conditions that mimic encephalitis [8]. Positron emission tomography (PET) is a noninvasive imaging technique that has a wide range of clinical and research applications in the pathophysiology of a variety of brain disorders, including brain tumors, psychiatric disorders, seizures, epilepsy, infection, and neurodegenerative disorders, as well as the study of the normal brain [9]. 18F-fluorodeoxyglucose [18F](FDG) was developed in 1976 to study the glucose metabolism of the brain [10]. Currently, FDG-PET is frequently utilized in nuclear medicine with the growing indication in neurology, cardiology, and oncology [9]. Based on the literature review, in all encephalitis cases, magnetic resonance imaging (MRI) is preferred over computed tomography (CT) because of more sensitiveness and specificity. Advanced brain imaging with SPECT or PET has shown promising results in detecting specific metabolism patterns in patients with Caspr2, LGI1, NMDAR, or other autoantibodies encephalitis [8, 11, 12].
To achieve the appropriate therapeutic results, early and proper diagnosis is still the most important issue [13]. In this review, we provide an overview of FDG-PET imaging findings in AE patients and possible relation to different types and clinical features. Furthermore, our results may help clinicians to better diagnose and also predict future clinical symptoms of subjects with AE.
Methods and materials
The present systematic review study was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14].
Search strategy
The PubMed, Web of Science, and Scopus were searched in August 2021 using search strategy consisting of (autoimmune encephalitis OR Limbic encephalitis OR anti-NMDAR encephalitis OR anti-AMPAR encephalitis OR anti-GABA-AR encephalitis OR anti-GABA-BR encephalitis OR anti-LGI1 encephalitis OR anti-CASPR2 encephalitis OR anti-GAD encephalitis OR anti-GlyR encephalitis OR anti-DPPX encephalitis OR anti-mGluR encephalitis OR Hashimoto’s encephalitis OR and steroid-responsive encephalopathy associated with autoimmune thyroiditis) AND (positron emission tomography OR PET scan).
Eligibility criteria
We included publications that reported PET features and clinical status of AE patients. We excluded studies with other types of encephalitis, unconfirmed cases of AE, and case report studies.
Study selection
Two reviewers (A.S and A.S) independently screened the title and abstracts according to our eligibility criteria. Next, the same reviewers retrieved and screened the full text of the remaining studies for final selection. Any disagreements were resolved by the third investigator (F.N) at the end of each step.
Data extraction
The following data were extracted from entered studies by the two reviewers (A.S and A.S): author, year of publication, region, study design, sample size, mean age, number of males, type of AE, PET imaging findings, clinical symptoms, and treatments.
Quality assessments
The quality of included studies was assessed using the Newcastle–Ottawa scale (NOS). Risk of bias of case–control studies assessed in the several domains such as selection, comparability, and exposure, while in cohort studies risk of bias in selection, comparability, and outcome which the highest possible score was 8.
Data synthesis and analysis
The data of entered studies were qualitatively compared and summarized.
Results
Study screen
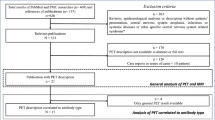
Our literature search and manual addition yielded 630 papers after duplicate removal. At the first step of screening, 489 studies were excluded and the remaining papers were further reviewed accurately and finally, 22 studies with a total of 332 participants were entered into our qualitative synthesis (Fig. 1).
Study characteristics and a qualitative summary
Included studies were conducted in China (n = 7), USA (n = 4), Germany (n = 4), South Korea (n = 2), India (n = 1), Spain (n = 1), France (n = 1), Mexico (n = 1), and Denmark (n = 1). Among included cases, there were anti-LGI1 (n = 144), anti-NMDAR (n = 103), anti-GAD (n = 22), anti-GABA (n = 14), anti-VKGC (n = 23), anti-Hu (n = 9), anti-CASPR2 (n = 4), and anti-Ma2 (n = 2) (Table 1). There was no study of acute disseminated encephalomyelitis (ADEM) which met our eligibility criteria. The included studies were published between 2005 and 2021.
Moreover, all included studies were identified as high quality with a mean score of 7.36 according to NOS criteria (Table 2).
PET findings in anti-LGI1 encephalitis
The four of 22 studies that examined PET imaging in anti-LGI1 encephalitis patients found hypermetabolism in the hippocampus which was along with memory deficits, pyramidal signs, mood disorders, and seizures [15,16,17,18]. Furthermore, six investigations reported hypermetabolism in basal ganglia in patients with psychiatric symptoms [15, 19], hyponatremia [15, 20], cognitive deficits [20, 21], and focal seizures [19, 22, 23]. The metabolic alteration was also observed in cortical regions such as the temporal lobe. In four studies, there was affected glucose uptake in the medial temporal lobe in AE patients with clinical symptoms including behavioral changes [24, 25], memory deficits [22], confusion [24, 25], sleep disorders [24], hyponatremia [20], psychiatric symptoms [19], and seizures [23, 26]. Also, other cortical regions such as frontal, parietal, occipital, cingulate gyrus, and paracentral lobule were described with abnormal metabolism [19,20,21, 23, 26]. These patients mainly displayed memory loss, cognitive impairment, seizure, and psychiatric symptoms. Moreover, two studies found hypermetabolism in the cerebellum in patients with loss of consciousness [19, 27]. Also, there were other reports of amygdala and thalamus which were observed in patients with memory deficits and cognitive decline [21, 24] (Tables 3 and 4).
PET findings in anti-NMDAR encephalitis
All studies which described PET findings in anti-NMDAR encephalitis revealed identified metabolic alteration in the temporal lobe. Other cortical regions were also detected with abnormal glucose uptake including such as frontal lobe in subjects with psychiatric symptoms, seizures, memory deficits, and behavioral disorders [19, 20, 25,26,27,28,29,30]. In addition, parietal, occipital, cingulate gyrus is altered to be altered in anti-NMDAR encephalitis patients [19,20,21, 26, 27, 29, 30]. A study by Tripathi et al. reported increased metabolism in basal ganglia, thalamus, caudate, and hippocampus, and hypometabolism in the cerebellum in patients with clinical symptoms consisting of seizures, behavioral changes, and cognitive decline [21]. On the other hand, another study found hypermetabolism in the cerebellum [19]. Baumgartner et al. reported AE patients with anti-NMDAR positive which had hypometabolism in temporal lobes, hemispheric cortex, thalamus, and crossed cerebellar diaschisis and hypermetabolism in striata which experienced brainstem syndrome and ataxia (Tables 3 and 4).
PET findings in anti-GAD encephalitis
A study that examined PET imaging as a diagnostic factor for AE found hypometabolism in parietal, frontal, and occipital lobes [26]. Another investigation found the same result in temporal, frontal, and left hippocampus in patients with epilepsy, stiff-person syndrome, cerebellar ataxia, and cognitive impairment [31]. Furthermore, increased activity in medial temporal and basal ganglia, and also decreased metabolism in the parietal lobe were reported in anti-GAD encephalitis patients suffering from behavioral disturbance, cognitive decline, and gait ataxia [21]. Another investigation reported medial temporal metabolic alterations along with nausea and hallucinations [32] (Tables 3 and 4).
PET findings in anti-VGKC encephalitis
Four studies reported PET findings in anti-VGKC encephalitis patients. Medial temporal lobe hypermetabolism and mild diffuse cortical dysfunction were observed in a study that enrolled patients with paraneoplastic anti-VGKC encephalitis who experienced a seizure, memory disturbances, and hypersomnia [33]. The anti-VGKC encephalitis patients in this study experienced memory disturbances, confusion, hyponatremia, and hypersomnia. Another study found bilateral temporal lobe hypermetabolism in these patients along with altered mental status, hyponatremia, and seizures [34]. Also, medial temporal metabolic alterations, hypometabolism in temporoparietooccipital, and hypermetabolism in striata and cerebellum were reported in anti-VGKC encephalitis patients who suffered from gait disturbance, hypomania, somnolence, hallucinations, cognitive deficits, disorientation, attention, and mania [32] (Tables 3 and 4).
Other types of AE
A study reported affected activity in temporal, frontal, occipital, and parietal lobes in patients with anti-CASPR2 encephalitis diagnosed with cognitive impairment and autonomic seizures [20]. Another PET study also described cases with hallucinations and hypometabolism in temporal and occipital cortices [17].
There were few cases of anti-Hu encephalitis. Based on included studies, patients with anti-Hu encephalitis had both hypo- and hypermetabolism in the medial temporal lobe and hypometabolism in cortical regions such as frontal, occipital, and parietal lobes while suffering from motor weakness, distal paresthesia, ataxia, cognitive deficits, memory deficits, altered mental status, seizure, and brainstem syndrome [26, 32, 33].
There were only two cases with anti-Ma2 encephalitis which had hypometabolism in temporal, frontal, occipital, and parietal lobes with cognitive impairment, seizure, and neurological deficits [26] (Tables 3 and 4).
Discussion
This study systematically reviewed the literature on PET studies in patients with AE. We aimed to find possible associations between clinical symptoms and metabolic patterns presented in PET imaging of patients. Our results indicate that there is huge diversity in metabolic patterns among different AE subtypes. Altered glucose uptake in cortical regions was observed in all most subtypes. However, there were some unspecific metabolic changes such as hypometabolism in the cerebellum in anti-NMDAR and metabolic alteration of the amygdala and thalamus in anti-LGI1. A reason for these findings may be the larger sample size and higher number of studies that investigated the FDG-PET in anti-NMDAR and anti-LGI1. Our findings also showed a similar metabolic pattern for anti-CASPR2, anti-Hu, anti-Ma2, and anti-alpha 3ACHR. These results should be interpreted carefully due to the small sample size and the low number of studies.
Anti-NMDAR
In almost all of the studies on anti-NMDAR encephalitis, decreased activity in the occipital lobe was present, in addition, to an increase in frontal and temporal (specifically medial temporal) activity. Some studies reported this pattern as an increased frontal to the occipital gradient in glucose metabolism [29]. Decreased occipital metabolism seems like a reliable result being reported recurrently and its correlation with patients’ visual acuity has been proved [35]. Ge et al. found that this pattern could differ in patients with distant triggers; in anti-NMDAR encephalitis patients with cryptogenic etiology, an asymmetric increase in frontotemporal metabolism was found [28]. It contrasts the paraneoplastic encephalitis with symmetric increased metabolism in these regions. In patients whose viral infection triggered their encephalitis, hypermetabolism in temporal areas correlated with abnormalities in MRI while no specific MRI pattern has been found in anti-NMDAR encephalitis patients and 70% of patients come up with normal MRI results [28]. The reported correlation can be explained by inflammatory reactions and T-cell mediated necrosis in these areas following the viral infection.
In children with anti-NMDAR encephalitis, the same pattern was observed besides that increased activity in basal ganglia was also reported which was correlated with movement disorders in this group [30]. On the other hand, in older patients in this AE subgroup, diffuse cortical hypometabolism was observed, a pattern that resembles what is seen in neurodegenerative diseases. These patients had cognitive problems that cannot be only due to encephalitis [21].
Patients usually suffered from cognitive disorders such as memory dysfunction, psychiatric symptoms, behavioral disorders, and seizure. Given the important role of frontal and temporal lobe networks in cognitive processes and regulation of behavior, these symptoms can be partially explained [36]. Studies have reported reduced functional connectivity between medial frontal and hippocampus and within medial temporal lobe networks. In addition, NMDAR has its highest expression in the frontal lobe and hippocampus and its specific role in learning has been fully investigated [37, 38]. Internalization of NMDAR followed by the increased amount of extra-cellular antibodies and T-cell mediated responses against intra-cellular antibodies were proposed as pathophysiologic reasons of dysfunctions in these regions [37]. Regarding epilepsy, the temporal lobe is the most common origin of epilepsy and the epileptogenic characteristics of patients can be justified by temporal lobe dysfunctions.
Anti-LGI1
LGI1 is a glycoprotein secreted from presynaptic neurons and is related to VGKCs. One in seven patients with anti-VGKC encephalitis has LGI1 antibodies [19]. Abnormal metabolism in patients with LGI1 has been reported in diverse regions of the brain including medial temporal, hippocampus, cerebellum, and basal ganglia all of which had hypermetabolism. However, hypermetabolism in the basal ganglia was the most prominent in patients with faciobrachial dystonic seizures (FBDS). As FBDS is characteristic of encephalitis with VGKC/LGI antibodies [39], movement features of FBDS may be attributed to basal ganglia dysfunction. However, this claim needs robust investigations [40]. Overall, anti-LGI1 induced metabolic changes depend on disease course, diagnosis time point, treatment regimen, and initiation time [19]. The diverse results may be due to a higher number of investigations with different methodologies and heterogenous participants which make it hard to find an association between clinical symptoms and patterns of metabolism changes. Previous studies demonstrated that anti-LGI1 patients commonly have FBDS, hyponatremia, and epileptic seizures [41, 42].
Anti-GAD
Glutamic acid decarboxylase (GAD) is an intracellular synaptic antigen that is exposed to antibodies during vesicle fusion and reuptake [43]. A high level of anti-GAD antibodies is associated with limbic encephalitis and cerebellar ataxia [44]. Based on our review, these patients generally had hypometabolism in cortical regions including parietal, frontal, occipital lobes, temporal, frontal, and hippocampus [16, 21, 32]. Also, they experienced cognitive impairment and behavioral disorders which is logical due to widespread cortical hypometabolism. Furthermore, the metabolic alteration existed in several patients at basal ganglia and cerebellum which resulted in gait impairment and ataxia [21].
Nowadays, PET scans are considered more than before due to several advantages. First, PET scan metabolic patterns are more correlated with clinical symptoms of patients rather than MRI abnormalities [45]. Second, there is an association between the place of the antigens and PET findings, intracellular antigens are more frequent in mesiotemporal regions and show hypermetabolic patterns while surface antigens are found outside of the limbic lobe and cause diminished activity in those regions [20]. Third, although the volume of literature on the diagnostic accuracy of PET in AE is limited, PET can increase the sensitivity of MRI for the diagnosis of AE. As some subgroups such as anti-NMDAR encephalitis appear normal in MRI or do not present with exclusive findings. Fourth, the activity of NMDAR can be measured and quantified with PET studies, which may give rise to a better understanding of the pathophysiology of this disease and serve as a diagnostic marker [46].
PET is a relatively new imaging technology; therefore, it is not promptly available in clinical practice for all patients. Along with multiple advantages, we have to consider the cost-effectiveness of PET scans which are expensive [47]. Arterial spin labeling (ASL) MR perfusion recently attracted attention as a non-invasive technique that does not require intravenous contrasts. ASL-MRI given results consistent with FDG-PET and it is easily available in clinical practice to early detection of AE [3].
However, there are some challenges in using PET scan in AE. PET results do not have good specificity and altered metabolism can be related to any other condition [46]. More expertise and quantitative measures need to be considered in the analysis of PET results. Using automatic approaches for analysis may be biased based on the normalization site of the brain. In addition, all the results of PET studies should be used with caution, as most of the patients with AE were under treatment and some of these medications such as those used to induce narcosis before PET can decrease the brain metabolism. This effect is based on how long before PET scan the medications have been administered and their pharmacokinetics in the body [19]. Some of the studies had a retrospective design and the delay between symptoms onset and PET scan results differed between patients. A consensus was not reached on the diagnosis of all patients [45]. Small sample size was a drawback of all the studies and given diversity in encephalitis subgroups, conducting larger studies is advisable to substantiate present findings. Moreover, measuring the association of PET findings and clinical symptoms and also the type of AE was not the aim of the included studies.
The use of a wide range of imaging protocols with different resolutions may be one of the reasons for the diverse results of MRI and PET imaging of AE patients [12]. Also, the use of advanced imaging methods is currently limited only to common subtypes of AE which makes it difficult to reach an exact conclusion. Future extension of the imaging methods to all AE variants will pave the way for finding discriminative patterns of different AE subtypes.
Conclusion
In general, as clinical diagnosis of patients with AE may be challenging in some cases, rapid diagnosis and treatment initiation can significantly improve patients’ prognoses. Our results indicate huge diversity in metabolic patterns among different AE subtypes and it is hard to draw a firm conclusion. However, the limited use of such imaging techniques in all AE variants and different settings makes it difficult to find discriminative patterns. Moreover, the timing of imaging, epileptic seizures, and acute treatments can alter the PET patterns strongly which were not addressed in our study due to the lack of sufficient demographical information of included studies. Also, neuroimaging features of each AE subtype can lead to more pathophysiological understanding. Further prospective investigations with specific inclusion and exclusion criteria should be carried out to identify the metabolic defect in different AE subtypes.
References
Armangue T, Petit-Pedrol M, Dalmau J (2012) Autoimmune encephalitis in children. J Child Neurol 27(11):1460–1469. https://doi.org/10.1177/0883073812448838
Kelley BP, Patel SC, Marin HL, Corrigan JJ, Mitsias PD, Griffith B (2017) Autoimmune encephalitis: pathophysiology and imaging review of an overlooked diagnosis. AJNR Am J Neuroradiol 38(6):1070–1078. https://doi.org/10.3174/ajnr.A5086
Dinoto A, Cheli M, Ajčević M, Dore F, Crisafulli C, Ukmar M, Sartori A, Manganotti P (2021) ASL MRI and 18F-FDG-PET in autoimmune limbic encephalitis: clues from two paradigmatic cases. Neurol Sci 42(8):3423–3425. https://doi.org/10.1007/s10072-021-05207-0
Dalmau J (2016) NMDA receptor encephalitis and other antibody-mediated disorders of the synapse: the 2016 Cotzias lecture. Neurology 87(23):2471–2482. https://doi.org/10.1212/wnl.0000000000003414
Daif A, Lukas RV, Issa NP, Javed A, VanHaerents S, Reder AT, Tao JX, Warnke P, Rose S, Towle VL, Wu S (2018) Antiglutamic acid decarboxylase 65 (GAD65) antibody-associated epilepsy. Epilepsy & behavior : E&B 80:331–336. https://doi.org/10.1016/j.yebeh.2018.01.021
Vallabhaneni D, Naveed MA, Mangla R, Zidan A, Mehta RI (2018) Perfusion imaging in autoimmune encephalitis. Case reports in radiology 2018:3538645. https://doi.org/10.1155/2018/3538645
Lancaster E (2016) The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol 12(1):1–13. https://doi.org/10.3988/jcn.2016.12.1.1
Venkatesan A, Jagdish B (2019) Imaging in encephalitis. Semin neurol 39(3):312–321. https://doi.org/10.1055/s-0039-1687838
Lameka K, Farwell MD, Ichise M (2016) Positron emission tomography. Handb Clin Neurol 135:209–227. https://doi.org/10.1016/b978-0-444-53485-9.00011-8
Wei YC, Tseng JR, Wu CL, Su FC, Weng WC, Hsu CC, Chang KH, Wu CF, Hsiao IT, Lin CP (2020) Different FDG-PET metabolic patterns of anti-AMPAR and anti-NMDAR encephalitis: case report and literature review. Brain and behavior 10(3):e01540. https://doi.org/10.1002/brb3.1540
Steiner I, Budka H, Chaudhuri A, Koskiniemi M, Sainio K, Salonen O, Kennedy PG (2005) Viral encephalitis: a review of diagnostic methods and guidelines for management. Eur J Neurol 12(5):331–343. https://doi.org/10.1111/j.1468-1331.2005.01126.x
Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C (2015) Imaging of autoimmune encephalitis—relevance for clinical practice and hippocampal function. Neuroscience 309:68–83. https://doi.org/10.1016/j.neuroscience.2015.05.037
Tobin WO, Pittock SJ (2017) Autoimmune neurology of the central nervous system. Continuum (Minneapolis, Minn) 23 (3, Neurology of Systemic Disease):627–653. https://doi.org/10.1212/con.0000000000000487
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. https://doi.org/10.1136/bmj.b2700
Jang Y, Lee S-T, Bae J-Y, Kim T-J, Jun J-S, Moon J, Jung K-H, Park K-I, Irani SR, Chu K, Lee SK (2018) LGI1 expression and human brain asymmetry: insights from patients with LGI1-antibody encephalitis. J Neuroinflammation 15(1):279. https://doi.org/10.1186/s12974-018-1314-2
Deuschl C, Rüber T, Ernst L, Fendler WP, Kirchner J, Mönninghoff C, Herrmann K, Quesada CM, Forsting M, Elger CE, Umutlu L (2020) 18F-FDG-PET/MRI in the diagnostic work-up of limbic encephalitis. PLoS ONE 15(1):e0227906–e0227906. https://doi.org/10.1371/journal.pone.0227906
Chen Y, Xing XW, Zhang JT, Wang RX, Zhao W, Tan QC, Liu RZ, Wang XQ, Huang XS, Yu SY (2016) Autoimmune encephalitis mimicking sporadic Creutzfeldt-Jakob disease: a retrospective study. J Neuroimmunol 295–296:1–8. https://doi.org/10.1016/j.jneuroim.2016.03.012
Celicanin M, Blaabjerg M, Maersk-Moller C, Beniczky S, Marner L, Thomsen C, Bach FW, Kondziella D, Andersen H, Somnier F, Illes Z, Pinborg LH (2017) Autoimmune encephalitis associated with voltage-gated potassium channels-complex and leucine-rich glioma-inactivated 1 antibodies - a national cohort study. Eur J Neurol 24(8):999–1005. https://doi.org/10.1111/ene.13324
Wegner F, Wilke F, Raab P, Tayeb SB, Boeck AL, Haense C, Trebst C, Voss E, Schrader C, Logemann F, Ahrens J, Leffler A, Rodriguez-Raecke R, Dengler R, Geworski L, Bengel FM, Berding G, Stangel M, Nabavi E (2014) Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol 14:136. https://doi.org/10.1186/1471-2377-14-136
Moreno-Ajona D, Prieto E, Grisanti F, Esparragosa I, Sánchez Orduz L, Gállego Pérez-Larraya J, Arbizu J, Riverol M (2020) (18)F-FDG-PET imaging patterns in autoimmune encephalitis: impact of image analysis on the results. Diagnostics (Basel, Switzerland) 10 (6). https://doi.org/10.3390/diagnostics10060356
Tripathi M, Tripathi M, Roy SG, Parida GK, Ihtisham K, Dash D, Damle N, Shamim SA, Bal C (2018) Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology 60(2):189–198. https://doi.org/10.1007/s00234-017-1956-2
Liu X, Shan W, Zhao X, Ren J, Ren G, Chen C, Shi W, Lv R, Li Z, Liu Y, Ai L, Wang Q (2020) The clinical value of (18) F-FDG-PET in autoimmune encephalitis associated with LGI1 antibody. Front Neurol 11:418–418. https://doi.org/10.3389/fneur.2020.00418
Li T-R, Zhang Y-D, Wang Q, Shao X-Q, Lv R-J. Recognition of seizure semiology and semiquantitative FDG-PET analysis of anti-LGI1 encephalitis. CNS Neuroscience & Therapeutics n/a (n/a). https://doi.org/10.1111/cns.13707
Chen C, Wang X, Zhang C, Cui T, Shi WX, Guan HZ, Ren HT, Shao XQ (2017) Seizure semiology in leucine-rich glioma-inactivated protein 1 antibody-associated limbic encephalitis. Epilepsy & behavior : E&B 77:90–95. https://doi.org/10.1016/j.yebeh.2017.08.011
Fisher RE, Patel NR, Lai EC, Schulz PE (2012) Two different 18F-FDG brain PET metabolic patterns in autoimmune limbic encephalitis. Clin Nucl Med 37(9):e213-218. https://doi.org/10.1097/RLU.0b013e31824852c7
Solnes LB, Jones KM, Rowe SP, Pattanayak P, Nalluri A, Venkatesan A, Probasco JC, Javadi MS (2017) Diagnostic value of (18)F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 58(8):1307–1313. https://doi.org/10.2967/jnumed.116.184333
Leypoldt F, Buchert R, Kleiter I, Marienhagen J, Gelderblom M, Magnus T, Dalmau J, Gerloff C, Lewerenz J (2012) Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry 83(7):681–686. https://doi.org/10.1136/jnnp-2011-301969
Ge J, Deng B, Guan Y, Bao W, Wu P, Chen X, Zuo C (2021) Distinct cerebral (18)F-FDG PET metabolic patterns in anti-N-methyl-D-aspartate receptor encephalitis patients with different trigger factors. Ther Adv Neurol Disord 14:1756286421995635. https://doi.org/10.1177/1756286421995635
Kerik-Rotenberg N, Diaz-Meneses I, Hernandez-Ramirez R, Muñoz-Casillas R, Reynoso-Mejia CA, Flores-Rivera J, Espinola-Nadurille M, Ramirez-Bermudez J, Aguilar-Palomeque C (2020) A metabolic brain pattern associated with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics 61(1):39–48. https://doi.org/10.1016/j.psym.2019.08.007
Lagarde S, Lepine A, Caietta E, Pelletier F, Boucraut J, Chabrol B, Milh M, Guedj E (2016) Cerebral (18)fluorodeoxy-glucose positron emission tomography in paediatric anti N-methyl-D-aspartate receptor encephalitis: a case series. Brain Develop 38(5):461–470. https://doi.org/10.1016/j.braindev.2015.10.013
Zhu F, Shan W, Lv R, Li Z, Wang Q (2020) Clinical characteristics of GAD 65-associated autoimmune encephalitis. Acta Neurol Scand 142(3):281–293. https://doi.org/10.1111/ane.13281
Baumgartner A, Rauer S, Mader I, Meyer PT (2013) Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol 260(11):2744–2753. https://doi.org/10.1007/s00415-013-7048-2
Masangkay N, Basu S, Moghbel M, Kwee T, Alavi A (2014) Brain 18F-FDG-PET characteristics in patients with paraneoplastic neurological syndrome and its correlation with clinical and MRI findings. Nucl Med Commun 35(10):1038–1046. https://doi.org/10.1097/mnm.0000000000000163
Newey CR, Sarwal A, Hantus S (2016) [(18)F]-Fluoro-deoxy-glucose positron emission tomography scan should be obtained early in cases of autoimmune encephalitis. Autoimmune diseases 2016:9450452. https://doi.org/10.1155/2016/9450452
Probasco JC, Solnes L, Nalluri A, Cohen J, Jones KM, Zan E, Javadi MS, Venkatesan A (2018) Decreased occipital lobe metabolism by FDG-PET/CT: an anti-NMDA receptor encephalitis biomarker. Neurology(R) neuroimmunology & neuroinflammation 5(1):e413. https://doi.org/10.1212/nxi.0000000000000413
Siddiqui SV, Chatterjee U, Kumar D, Siddiqui A, Goyal N (2008) Neuropsychology of prefrontal cortex. Indian J Psychiatry 50(3):202–208. https://doi.org/10.4103/0019-5545.43634
Gibson LL, McKeever A, Coutinho E, Finke C, Pollak TA (2020) Cognitive impact of neuronal antibodies: encephalitis and beyond. Transl Psychiatry 10(1):304. https://doi.org/10.1038/s41398-020-00989-x
Li F, Tsien JZ (2009) Memory and the NMDA receptors. N Engl J Med 361(3):302–303. https://doi.org/10.1056/NEJMcibr0902052
Irani SR, Vincent A (2011) NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep 11(3):298–304. https://doi.org/10.1007/s11910-011-0186-y
Shin YW, Lee ST, Shin JW, Moon J, Lim JA, Byun JI, Kim TJ, Lee KJ, Kim YS, Park KI, Jung KH, Lee SK, Chu K (2013) VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol 265(1–2):75–81. https://doi.org/10.1016/j.jneuroim.2013.10.005
Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A (2010) Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain : a journal of neurology 133(9):2734–2748. https://doi.org/10.1093/brain/awq213
Lancaster E, Martinez-Hernandez E, Dalmau J (2011) Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology 77(2):179–189. https://doi.org/10.1212/WNL.0b013e318224afde
Lancaster E, Dalmau J (2012) Neuronal autoantigens—pathogenesis, associated disorders and antibody testing. Nat Rev Neurol 8(7):380–390. https://doi.org/10.1038/nrneurol.2012.99
Saiz A, Blanco Y, Sabater L, González F, Bataller L, Casamitjana R, Ramió-Torrentà L, Graus F (2008) Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain : a journal of neurology 131(10):2553–2563. https://doi.org/10.1093/brain/awn183
Turpin S, Martineau P, Levasseur MA, Meijer I, Décarie JC, Barsalou J, Renaud C, Decaluwe H, Haddad E, Lambert R (2019) 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imaging 46(6):1309–1324. https://doi.org/10.1007/s00259-019-04302-x
Morbelli S, Arbizu J, Booij J, Chen MK, Chetelat G, Cross DJ, Djekidel M, Drzezga A, Ekmekcioglu O, Garibotto V, Hesse S, Ishii K, Jafari L, Lammertsma AA, Law I, Mathews D, Minoshima S, Mosci K, Pagani M, Pappata S, Silverman DH, Signore A, Van De Giessen E, Villemagne V, Barthel H (2017) Erratum to: the need of standardization and of large clinical studies in an emerging indication of [18 F]FDG PET: the autoimmune encephalitis. Eur J Nucl Med Mol Imaging 44(3):559–560. https://doi.org/10.1007/s00259-016-3598-8
Saif MW, Tzannou I, Makrilia N, Syrigos K (2010) Role and cost effectiveness of PET/CT in management of patients with cancer. Yale J Biol Med 83(2):53–65
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This systematic review has been done in accordance with the rules of the ethical committee of Tehran University of medical sciences.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabizadeh, F., Ramezannezhad, E., Sardaripour, A. et al. [18F]FDG brain PET and clinical symptoms in different autoantibodies of autoimmune encephalitis: a systematic review. Neurol Sci 43, 4701–4718 (2022). https://doi.org/10.1007/s10072-022-06094-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06094-9