Abstract
Background
Physical therapies have been recommended as crucial components in Parkinson’s disease (PD) rehabilitation.
Objective
The study aims to examine the effectiveness of a new dance-physiotherapy combined intervention, called DArT method, in mild PD patients.
Methods
A prospective, randomized, single-blind, controlled pilot trial was conducted on 38 mild PD patients under dopaminergic therapy. The intervention consisted in an add-on protocol: the control group received 1 h of conventional physiotherapy followed by 1 h of conventional physiotherapy each day, 3 times a week, for 5 weeks. The experimental group received 1 h of conventional physiotherapy followed by 1 h of dance class each day, 3 times a week, for 5 weeks. The week before and after the training period, patients were assessed for motor, cognitive, emotional, and sensory components of PD, with MDS-UPDRS-III as primary outcome measure.
Results
DArT method was associated with a 2.72-point reduction in the post-treatment MDS-UPDRS-III total score compared to control group (95% CI − 5.28, − 0.16, p = 0.038, d = 0.71), and with a 2.16-point reduction in the post-treatment MDS-UPDRS-III upper body subscore (95% CI − 3.56, − 0.76, p = 0.003, d = 1.02). Conversely, conventional physiotherapy program was associated with a 2.95-point reduction in the post-treatment trait anxiety compared to the experimental group (95% CI 0.19, 5.71, p = 0.037, d = 0.70). Withdrawal and fall rates were equal to 0% in both groups.
Conclusion
DArT method showed to be safe, well accepted, and more effective than an intensive program of conventional physiotherapy in improving motor impairment in mild PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a multisystem disorder characterized by a core of motor symptoms (tremor, rigidity, and bradykinesia) and several non-motor symptoms (NMS), including neuropsychiatric problems and cognitive impairment [1].
Exercise and physical therapy have been recommended as crucial components in PD rehabilitation, complementing pharmacotherapy and functional surgery [2], and have symptomatic benefits on motor and NMS of PD [3]. In particular, mind-body exercises, including dance, yoga, and tai chi, have been reported to be the most common complementary strategies adopted by patients with PD to enhance their entire wellbeing [4], with several meta-analyses in the most recent years reaching favorable conclusions on dance-based intervention [5, 6].
In line with these evidences, our multidisciplinary group developed a dance-physiotherapy combined intervention called DArT method (DAnce Therapy) and addressed to mild PD patients. This pilot trial aimed to investigate the effectiveness and safety of DArT method compared with an intensive program of conventional physiotherapy.
Methods
Study design and population
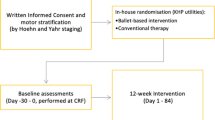
A prospective, randomized, single-blind, controlled pilot trial, using an add-on design, was conducted on 38 patients diagnosed with idiopathic PD according to the Movement Disorder Society (MDS) Clinical Diagnostic Criteria for PD [1], and recruited through both the regional Reference Center of Movement Disorders (Turin, Italy) and its Reference Rehabilitation Hospital (Presidio Sanitario San Camillo, Turin, Italy) by their treating physicians. Participants were eligible for inclusion in the study if they have been classified as mild PD patients, defined as a Hoehn and Yahr (H&Y) score of 1–2 and a MDS-sponsored revision of the Unified Parkinson’s Disease rating scale Part III (MDS-UPDRS-III) score of 1–32 [7], and were on stable dopaminergic therapy for at least 4 weeks. Patients were ineligible if they had cognitive impairments, had severe orthopedic comorbidities, used walking aids, and could not guarantee their presence for the whole study period. About the screening for cognitive impairment, MoCA scale was adopted as more sensitive than MMSE in detecting early cognitive changes in PD [8].
All the experimental procedures were approved by the local Ethics Committee (CS2/472) and conducted in agreement with the Declaration of Helsinki Ethical Principles. Written informed consent was obtained from all participants prior to enrollment. In particular, participants agreed to attend 5 weeks of rehabilitation activities during which the conventional physiotherapy could have been associated to other therapeutic activities.
Interventions
DArT method has been developed according to the key recommendations for Physical Therapy in PD [9]. As extensively show in the Online supplemental materials S1 and S2, our method consists in an intensive and progressive training that combines the conventional physiotherapy with a contemporary dance style incorporating some elements of classical ballet and avoiding the aid of music, so that the effectiveness of motor rehabilitation component alone can be assessed. Each class of conventional physiotherapy and dance programs is structured as follows, respectively: a first part devoted to the therapist-patient relationship (5 min long for both programs), a warm up session with supports (5 exercises for a total of 20 min versus 4 exercises for 30 min), and a center session without supports (6 exercises for a total of 35 min versus 7 exercises for 25 min). Auditory cues are administered to all participants in form of hand clapping, breaths, and footfalls. In addition, visual, cognitive, and, in some cases, tactile cues are administered as well.
The choice to use an add-on design was due to ethical reasons (all patients were guaranteed to receive the best validated treatment, i.e., conventional physiotherapy) and because it was a novel approach in the field of physical therapy for PD [5, 6, 10]. In particular, the control group received 1 h of conventional physiotherapy followed (after 30-min break) by 1 h of conventional physiotherapy each day, 3 times a week, for 5 weeks. The experimental group received 1 h of conventional physiotherapy followed (after 30-min break) by 1 h of dance class each day, 3 times a week, for 5 weeks. The weekly training intensity was superior to the one suggested by the European Physiotherapy Guideline for PD for conventional physiotherapy (3 times a week for 45 min) and dance (3 times a week for 60 min) [11].
Each day of the training period, a physiotherapist conducted the first hour of the conventional physiotherapy program (up to 13 patients for class), whereas a different physiotherapist conducted the second hour (up to 7 patients). A dance therapist with strong background in neuroscience and work experiences with PD conducted the dance program (up to 7 patients for class).
Outcome measurements
Three cycles of rehabilitation activities were conducted. The week preceding and the week following the 5-week training period patients were assessed for motor, cognitive, emotional, and sensory components of PD. Both rehabilitation activities and patients’ assessments took place at the San Camillo Hospital. In order to reduce patients’ discomfort, all tests were administered over the ON medications hours. In particular, the “On time” was defined in agreement with patient’s perception, who said that “he/she was in his/her best on-time.”
The selected primary outcome, representative of the motor component, was the MDS-UPDRS-III total score [7, 12]. The upper, lower, and axial body subscores of the MDS-UPDRS-III were calculated as well (see S3 in Online supplemental materials).
Secondary outcomes were (1) Six-Minute Walking Test (6MWT) [11], Time Up and Go (TUG) [11, 13], Mini-Balance Evaluation Systems Test (Mini-BESTest) [11], and New Freezing of Gait Questionnaire (NFOG-Q) [14], as for the motor component; (2) Montreal Cognitive Assessment (MoCA) [8] and TUG with dual task (TUG-DT) [15], as for the cognitive component; (3) 39-item Parkinson’s Disease Questionnaire Summary Index (PDQ-39-SI) [16], Beck Depression Inventory (BDI) [17], State-Trait Anxiety Inventory (STAY) Y 1 and 2 [18], and Falls Efficacy Scale-International (FES-I) [19], as for the emotional component; and (4) King’s PD pain scale [20] and Parkinson Fatigue Scale-16 (PFS-16) [21], as for the sensory component. As regard the FES-I, the previously published cut-points were adopted to differentiate participants based on their degree of concern [19]. Medications were logged during the first assessment visit, and the L-dopa equivalent daily dose (LEDD) was calculated for each participant [22].
Randomization and blinding
The study coordinator, not directly involved in patients’ care and recruitment, performed a simple randomization using computer-generated random numbers (1:1 ratio between control and experimental groups), and concealed the code until the first day of rehabilitation program. The performance bias due to the inability to blind participants and therapists to the interventions was addressed blinding participants to study hypothesis. All assessors were blinded for group allocation: one neurologist and one psychologist, the “first assessors,” administered the array of tests and filmed patients during examinations; a “second assessor” (neurologist) evaluated the video off-line. To avoid being able to make direct comparisons, one month had to pass between viewing the pre- and post-training videos of the same patient. The potential bias of order effect due to the fact that the “second assessor” was not blind to pre- and post-condition was equally present in both control and experimental groups.
Statistical analysis
A sample size calculation for the primary outcome measure was performed and, assuming a minimal effects size of 0.7, it showed that a whole sample size of 64 participants was needed to provide a statistical power at the recommended 0.80 level. Normal distribution of baseline characteristics of patients was verified by Shapiro-Wilk test. In order to rule out possible differences, a between-group analysis was performed using the independent sample Student’s t-test if the normality assumption was satisfied, or the Mann–Whitney U test if it was not. Chi-square test was used for dichotomous (sex) and ordinal (H&Y stage, FES-I cutoff points, patients affected or not by freezing) variables.
The efficacy analysis was performed by ANCOVA-POST model, using post-treatment scores as dependent variable, baseline scores as a covariate, and treatment group as independent variable. If the assumption of normality was violated, the nonparametric ANCOVA was used. Previously published values of minimal detectable change (MDC), minimal clinically important difference (MCID), or smallest detectable difference (SDD) were used as optimal cutoff points to calculate the percentage of patients who exceeded these thresholds [23,24,25,26,27,28,29,30,31].
A within-group analysis was also conducted using the paired sample Student’s t-test if the normality assumption was satisfied, or the Wilcoxon test if it was not. Due to the exploratory nature of the study, no multiplicity adjustment was applied. Since it provides additional information than any statistical test, Cohen’s d effect size was calculated. In case of non-normal data R statistic was computed and then converted to Cohen’s d. All the analyses were performed with SPSS, version 25.0 (IBM Corp., Armonk, NY) using two-tailed p values with a level of significance of 0.05.
Results
The recruitment period started on 26 February 2018 and ended on 28 February 2019. One-hundred and twenty PD patients were screened and 38 were eligible for the pilot trial; 19 were assigned to the experimental group and 19 to the control group. Fall and withdrawal rates were equal to 0% for both groups. All 38 patients were included in the statistical analysis and were analyzed according to the group they were originally assigned (see S4 for the CONSORT flow diagram and S5 for CONSORT checklist in Online supplemental materials). No significant differences were found in the baseline characteristics between control and experimental groups (Table 1).
Primary outcome
As shown in Fig. 1 and Table 2, post-treatment MDS-UPDRS-III total score was significantly lower in the experimental group (i.e., DArT method, adjusted mean difference − 2.72 (95% CI − 5.28, − 0.16), p = 0.038) after adjustment of post-treatment score for baseline score, with a moderate effect size (d = 0.71). Moreover, post-treatment MDS-UPDRS-III upper body subscore was significantly lower in the experimental group (− 2.16 (95% CI − 3.56, − 0.76), p = 0.003), with a large effect size (d = 1.02). Clinically significant changes in the MDS-UPDRS-III total score were found in 68% of experimental group patients and 63% of control group patients, even if these percentages did not differ between groups (p = 0.732; Table 3). Significant within-group improvements in the MDS-UPDRS-III total score were found in both the experimental (p < 0.0001, d = 1.14) and control (p = 0.001, d = 1.00) groups, as well as in both the experimental (upper p = 0.0002 and d = 1.48, lower p = 0.007 and d = 0.61, axial p = 0.021 and d = 0.81) and control (upper p = 0.004 and d = 0.76, lower p = 0.003 and d = 1.10, axial p < 0.0001 and d = 0.96) subgroups.
Forest plot of the adjusted mean differences and their 95% confidence intervals (CIs) related to post-treatment MDS-UPDRS-III total score, and subscores, assessed in both experimental and control groups. Each adjusted mean difference was obtained by calculating the difference between post-treatment mean score adjusted for baseline mean score in the experimental group and post-treatment mean score adjusted for baseline mean score in the control group. Horizontal bars not crossing the 0 vertical line are statistically significant (marked by an open white diamond). Adjusted mean differences < 0 favor experimental (DArT method) versus control group, while adjusted mean differences > 0 favor control versus experimental group
Secondary outcomes
Significant between-group differences were not found, except for STAI-Y2 assessments (Table 2), where post-treatment score was significantly lower in the control group (adjusted mean difference 2.95 (95% CI 0.19, 5.71), p = 0.037) after adjustment of post-treatment score for baseline score, with a moderate effect size (d = 0.70). Significant within-group improvement for STAI-Y2 were only found in the control group (p = 0.002 and d = 0.37 vs p = 0.753 in the experimental group).
Both experimental and control groups exceeded the optimal cutoff point specific for 6MWT (21% vs 11%, respectively), Mini-BESTest (5% vs 5%, respectively), and PDQ-39 (42% vs 37%, respectively), but no significant between-group difference was observed (p = 0.374, p = 1.000, p = 0.740, respectively), as shown in Table 3.
As for the within-group analysis, significant improvements in both experimental and control groups were found for 6MWT (p = 0.002 and d = 0.73 vs p = 0.001 and d = 0.47), TUG (p = 0.010 and d = 0.58 vs p = 0.023 and d = 0.38), and PDQ-39 (p = 0.010 and d = 0.92 vs p = 0.001 and d = 1.36). Significant improvements in cognitive domain were observed in the experimental group only (MoCA p = 0.033 and d = 0.38 vs p = 0.175; TUG-DT p = 0.021 and d = 0.81 vs p = 0.799). Conversely, significant improvements in freezing (NFOG-Q p = 0.005 and d = 1.02 vs p = 0.075), BDI (p = 0.009 and d = 0.94 vs p = 0.118), King’s PD pain scale (p = 0.034 and d = 0.73 vs p = 0.088), and fatigue (PFS-16 p = 0.030 and d = 0.34 vs 0.181) were only found in the control group. No significant improvements occurred for Mini-BESTest, FES-I, and STAI-Y1.
Discussion
This pilot trial investigated the effectiveness and safety of DArT method (i.e., the experimental group) as rehabilitation add-on intervention for 38 individuals affected by mild PD. First, our findings indicate that DArT method was more effective in reducing the MDS-UPDRS-III total score than the intensive program of conventional physiotherapy administered to the control group, with a moderate effect size. Second, it was more effective in reducing the MDS-UPDRS-III upper body subscore, with a large effect size. Third, the intensive conventional physiotherapy program was more effective than DArT method in reducing post-treatment trait anxiety, as assessed by means of STAI-Y2, with a moderate effect size. Fourth, the DArT method showed significant within-group improvements (i.e., post-training compared to baseline assessments) in motor impairment (MDS-UPDRS-III total score and subscores), cognitive domain (MoCA and TUG-DT), postural instability and functional mobility (TUG), endurance (6MWT), and quality of life (PDQ-39). Fifth, only the DArT method showed significant within-group improvements in cognitive domain (MoCA and TUG-DT). Sixth, it demonstrated to be safe and very well accepted by mild PD patients, confirmed by fall and withdrawal rates of 0%.
A formal comparison among the rehabilitation interventions investigated up to now is difficult to perform considering the wide variety of physical therapy interventions and the outcomes assessed [10]. Nevertheless, restricting the focus on recent meta-analyses about dance-based interventions, the DArT method effectiveness in improving motor, cognitive, and emotional components after the training period compared to baseline is consistent with previous findings [5, 6]. Interestingly, to our knowledge the present study is the first showing that a dance-based intervention addressed to mild PD patients is able to elicit a marked improvement in motor impairment affecting the upper body, including facial expression, rigidity in neck and arms, upper body bradykinesia, postural/kinetic tremor, and rest tremor (see S3 in Online supplemental materials). This selective improvement may be explained by the daily longer warm up session delivered to the experimental group compared to the control group (20 + 30 min versus 20 + 20 min), and by the exercises characterizing this specific session of the dance program, such as the visualizations and improvisations exercises sustained by externally given visual and auditory cues. Indeed, these specific exercises have been previously shown to help PD patients in compensating the slowness of thought, in movement planning, in increasing both reaction time and bradykinesia [32], and therefore they may have also contributed to the within-group improvements observed in the cognitive domain (MoCA and TUG-DT). Moreover, the therapist-patient relationship session characterizing the first 5 min of each dance class and aimed at enhancing patients’ positive expectations and both testing and pushing physical and mental limits, may have specifically contributed to improving bradykinesia in the experimental group. Indeed, this cardinal symptom of PD has been previously shown to be very sensitive to placebo effects [33, 34].
Conversely, the shorter daily center session delivered to the experimental group compared to the control group (35 + 25 versus 35 + 35 min) could explain the tendency toward a less effectiveness of DArT method on the lower body. Moreover, the reduced aerobic training experienced by the experimental group during the center session could explain the significant lower improvements obtained in trait anxiety (STAI-Y2). Indeed, recent findings show that prolonged physical activity increases serum concentrations of endocannabinoids producing both central and peripheral effects among which analgesia, anxiolysis, and a sense of wellbeing [35].
As regard the scalability and generalizability of DArT method, the obtained preliminary results could be exported also outside of the healthcare setting. The ludic phase of this new rehabilitation training, represented by the dance program, would create a bridge of interest and complicity with the patient, where caregivers could also be involved whenever possible. According to this perspective, new figures of therapists, who are both trained dancers and persons knowing the PD condition, would become of crucial importance.
Our findings should be tempered by important limitations. Eighty-two PD patients were excluded from the trial because showing the symptoms of moderate PD (H&Y of 3 and MDS-UPDRS-III of 33–58). This selection process had in fact underpowered the study, hindering the generalizability of its results, even if the minimal effects size of 0.7 for the MDS-UPDRS-III total score was confirmed by our results. The MDS-UPDRS-III upper body subscore was analyzed as a whole, without investigating the single scores. As for the Mini-BESTest, our participants obtained high scores at baseline already, making us unable to detect significant between- and within-group improvements in this specific measure of balance. Moreover, all outcomes were determined over the ON medications hours, so the confounding effects of variable responses to dopaminergic medication could not be ruled out. As regard the role of music, the pioneering choice to exclude it from our dance program may have reduced the effectiveness of DArT method [5, 6, 36, 37]. Video assessments were not truly unbiased since all pre-intervention videos were analyzed prior to the post-intervention videos. All pre- and post-intervention videos will be randomized in future studies and their analysis will be undertaken by multiple raters, allowing inter-rater reliability to be assessed as well. Since rigorous follow-up examinations were not performed after the completion of the study, no information about long-term effects of DArT method is available. Those improvements in freezing phenomenon observed during dance classes were generally not reproduced during post-training assessments as already documented in literature [38], thus suggesting the utility of video-recording patients during both dance and physiotherapy classes.
In accordance to present findings and limitations, further studies are underway to reach the optimal sample size and investigate additional motor PD symptoms, like bradykinesia, and NMS, like heart rate variability analysis and sleep quality. Finally, the extension of DArT method to a wider PD population, ranging from mild to moderate, represents an additional possibility that we will explore from a safety and efficacy perspective in future research.
Data Availability
The data that support the findings of this study are available on request from the corresponding author (Frisaldi Elisa).
Code availability (software application or custom code)
Not applicable.
References
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Abbruzzese G, Marchese R, Avanzino L, Pelosin E (2016) Rehabilitation for Parkinson’s disease: current outlook and future challenges. Parkinsonism Relat Disord 22:S60–S64. https://doi.org/10.1016/j.parkreldis.2015.09.005
Sacheli MA, Murray DK, Vafai N, Cherkasova MV, Dinelle K, Shahinfard E, Neilson N, McKenzie J, Schulzer M, Appel-Cresswell S, McKeown MJ, Sossi V, Stoessl JA (2018) Habitual exercisers versus sedentary subjects with Parkinson’s disease: multimodal PET and fMRI study. Mov Disord 33:1945–1950. https://doi.org/10.1002/mds.27498
Kwok JYY, Choi KC, Chan HYL (2016) Effects of mind–body exercises on the physiological and psychosocial well-being of individuals with Parkinson’s disease: a systematic review and meta-analysis. Complementary Ther Med 29:121–131. https://doi.org/10.1016/j.ctim.2016.09.016
Kalyani HHN, Sullivan K, Moyle G, Brauer S, Jeffrey ER, Roeder L, Berndt S, Kerr G (2019) Effects of dance on gait, cognition, and dual-tasking in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis 9:335–349. https://doi.org/10.3233/JPD-181516
dos Santos Delabary M, Komeroski IG, Monteiro EP, Costa RR, Haas AN (2018) Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res 30:727–735. https://doi.org/10.1007/s40520-017-0836-2
Martinez-Martin P, Chaudhuri KR (2018) Comprehensive grading of Parkinson’s disease using motor and non-motor assessments: addressing a key unmet need. Expert Rev Neurother 18:41–50. https://doi.org/10.1080/14737175.2018.1400383
Biundo R, Weis L, Bostantjopoulou S, Stefanova E, Falup-Pecurariu C, Kramberger MG, Geurtsen GJ, Antonini A, Weintraub D, Aarsland D (2016) MMSE and MoCA in Parkinson’s disease and dementia with Lewy bodies: a multicenter 1-year follow-up study. J Neural Transm 123:431–438. https://doi.org/10.1007/s00702-016-1517-6
Keus SHJ, Bloem BR, Hendriks EJM, Bredero-Cohen AB, Munneke M, Practice Recommendations Development Group (2007) Evidence-based analysis of physical therapy in Parkinson’s disease with recommendations for practice and research. Mov Disord 22:451–460. https://doi.org/10.1002/mds.21244
Tomlinson CL, Herd CP, Clarke CE, Meek C, Patel S, Stowe R, Deane KHO, Shah L, Sackley CM, Wheatley K, Ives N (2014) Physiotherapy for Parkinson’s disease: a comparison of techniques. Cochrane Database Systematic Rev CD002815. https://doi.org/10.1002/14651858.CD002815.pub2
Keus S, Munneke M, Graziano M, Paltamaa J, Pelosin E, Domingos J, Brühlmann S, Ramaswamy B, Prins J, Struiksma C, Rochester L, Nieuwboer A, Bloem B (2014) European physiotherapy guideline for Parkinson’s disease. https://www.parkinsonnet.com/discipline/physiotherapy. Accessed 2 Mar 2021
Goetz CG, Fahn S, Martinez-Martin P, Poewe W, Sampaio C, Stebbins GT, Stern MB, Tilley BC, Dodel R, Dubois B, Holloway R, Jankovic J, Kulisevsky J, Lang AE, Lees A, Leurgans S, LeWitt PA, Nyenhuis D, Olanow CW, Rascol O, Schrag A, Teresi JA, Van Hilten JJ, LaPelle N (2007) Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan. Mov Disord 22:41–47. https://doi.org/10.1002/mds.21198
Bouça-Machado R, Maetzler W, Ferreira JJ (2018) What is functional mobility applied to Parkinson’s disease? J Parkinsons Dis 8:121–130. https://doi.org/10.3233/JPD-171233
Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30:459–463. https://doi.org/10.1016/j.gaitpost.2009.07.108
Christofoletti G, Andrade LP, Beinotti F, Borges G (2014) Cognition and dual-task performance in older adults with Parkinson’s and Alzheimer’s disease. Int J Gen Med 7:383–388. https://doi.org/10.2147/IJGM.S65803
Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res 4:241–248. https://doi.org/10.1007/BF02260863
Visser M, Leentjens AFG, Marinus J, Stiggelbout AM, van Hilten JJ (2006) Reliability and validity of the Beck Depression Inventory in patients with Parkinson’s disease. Mov Disord 21:668–672. https://doi.org/10.1002/mds.20792
Mondolo F, Jahanshahi M, Granà A, Biasutti E, Cacciatori E, Di Benedetto P (2007) Evaluation of anxiety in Parkinson’s disease with some commonly used rating scales. Neurol Sci 28:270–275. https://doi.org/10.1007/s10072-007-0834-9
Delbaere K, Close JCT, Mikolaizak AS, Sachdev PS, Brodaty H, Lord SR (2010) The Falls Efficacy Scale International (FES-I). A comprehensive longitudinal validation study. Age Ageing 39:210–216. https://doi.org/10.1093/ageing/afp225
Chaudhuri KR, Rizos A, Trenkwalder C, Rascol O, Pal S, Martino D, Carroll C, Paviour D, Falup-Pecurariu C, Kessel B, Silverdale M, Todorova A, Sauerbier A, Odin P, Antonini A, Martinez-Martin P, EUROPAR and the IPMDS Non Motor PD Study Group (2015) King’s Parkinson’s disease pain scale, the first scale for pain in PD: an international validation. Mov Disord 30:1623–1631. https://doi.org/10.1002/mds.26270
Nilsson MH, Bladh S, Hagell P (2013) Fatigue in Parkinson’s disease: measurement properties of a generic and a condition-specific rating scale. J Pain Symptom Manag 46:737–746. https://doi.org/10.1016/j.jpainsymman.2012.11.004
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653. https://doi.org/10.1002/mds.23429
Horváth K, Aschermann Z, Ács P, Deli G, Janszky J, Komoly S, Balázs E, Takács K, Karádi K, Kovács N (2015) Minimal clinically important difference on the motor examination part of MDS-UPDRS. Parkinsonism Relat Disord 21:1421–1426. https://doi.org/10.1016/j.parkreldis.2015.10.006
Steffen T, Seney M (2008) Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther 88:733–746. https://doi.org/10.2522/ptj.20070214
Hulzinga F, Nieuwboer A, Dijkstra BW, Mancini M, Strouwen C, Bloem BR, Ginis P (2020) The New Freezing of Gait Questionnaire: unsuitable as an outcome in clinical trials? Mov Disord Clin Pract 7:199–205. https://doi.org/10.1002/mdc3.12893
Godi M, Franchignoni F, Caligari M, Giordano A, Turcato AM, Nardone A (2013) Comparison of reliability, validity, and responsiveness of the Mini-BESTest and Berg Balance Scale in patients with balance disorders. Phys Ther 93:158–167. https://doi.org/10.2522/ptj.20120171
Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS (2011) Minimal detectable change of the Timed “Up & Go” Test and the Dynamic Gait Index in people with Parkinson disease. Phys Ther 91:114–121. https://doi.org/10.2522/ptj.20090126
Huang LS, Hsieh CL, Wu RM, Lu WS (2017) Test-retest reliability and minimal detectable change of the Beck Depression Inventory and the Taiwan Geriatric Depression Scale in patients with Parkinson’s disease. PLoS One 12:e0184823. https://doi.org/10.1371/journal.pone.0184823
Jonasson SB, Nilsson MH, Lexell J (2014) Psychometric properties of four fear of falling rating scales in people with Parkinson’s disease. BMC Geriatr 14:66. https://doi.org/10.1186/1471-2318-14-66
Horváth K, Aschermann Z, Kovács M, Makkos A, Harmat M, Janszky J, Komoly S, Karádi K, Kovács N (2017) Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology 48:1–8. https://doi.org/10.1159/000455863
Siciliano M, Chiorri C, De Micco R, Russo A, Tedeschi G, Trojano L, Tessitore A (2019) Fatigue in Parkinson’s disease: Italian validation of the Parkinson Fatigue T Scale and the Fatigue Severity Scale using a Rasch analysis approach. Parkinsonism Relat Disord 65:105–110. https://doi.org/10.1016/j.parkreldis.2019.05.028
Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146. https://doi.org/10.1093/brain/124.11.2131
Benedetti F, Carlino E, Frisaldi E, Piedimonte A, Zibetti M, Lanotte M, Lopiano L (2020) Placebo and nocebo responses in Parkinson’s disease. In: Martin CR, Preedy VR (eds) The neuroscience of Parkinson’s disease. Elsevier, San Diego ISBN-13: 9780128159583
Frisaldi E, Carlino E, Zibetti M, Barbiani D, Dematteis F, Lanotte M, Lopiano L, Benedetti F (2017) The placebo effect on bradykinesia in Parkinson’s disease with and without prior drug conditioning. Mov Disord 32:1474–1478. https://doi.org/10.1002/mds.27142
Dietrich A, McDaniel WF (2004) Endocannabinoids and exercise. Br J Sports Med 38:536–541. https://doi.org/10.1136/bjsm.2004.011718
Nombela C, Hughes LE, Owen AM, Grahn JA (2013) Into the groove: can rhythm influence Parkinson’s disease? Neurosci Biobehav Rev 37:2564–2570. https://doi.org/10.1016/j.neubiorev.2013.08.003
De Bartolo D, Morone G, Giordani G, Antonucci G, Russo V, Fusco A, Marinozzi F, Bini F, Spitoni GF, Paolucci S, Iosa M (2020) Effect of different music genres on gait patterns in Parkinson’s disease. Neurol Sci 41:575–582. https://doi.org/10.1007/s10072-019-04127-4
Heiberger L, Maurer C, Amtage F, Mendez-Balbuena I, Schulte-Mönting J, Hepp-Reymond MC, Kristeva R (2011) Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson’s disease. Front Aging Neurosci 3:14. https://doi.org/10.3389/fnagi.2011.00014
Carlino E, Piedimonte A, Frisaldi E (2014) The effects of placebos and nocebos on physical performance. Handb Exp Pharmacol 225:149–157. https://doi.org/10.1007/978-3-662-44519-8_9
Galili DF (2015) Gaga: moving beyond technique with Ohad Naharin in the twenty-first century. Dance Chron 38:360–392. https://doi.org/10.1080/01472526.2015.1085759
Acknowledgements
We would like to acknowledge the Direction, health workers, and staff of the San Camillo Hospital (Turin, Italy) for the expertise, administrative and technical supports, gyms, and equipment made available for the whole study period; all patients for their time, enthusiasm, and commitment to this research; Jan Vollert, from the Imperial College of London, for his assistance in statistical analysis; and Vanessa Maher, Honorary Professor in Cultural Anthropology at the University of Verona and native-English speaker, for valuable editing the description of DArT method.
Funding
This study was supported by grants from Innovative Clinical Trainings, Trials & Healthcare Worldwide Initiative (Italy/Switzerland, grant number KCL-001) to Fabrizio Benedetti; the Ministry of Education, University and Research (MIUR) under the program “Dipartimenti di Eccellenza ex L. 232/2016” to the Department of Surgical Sciences of the University of Turin; and the Opera San Camillo Foundation, Milan, Italy.
Author information
Authors and Affiliations
Contributions
Design: Benedetti, Frisaldi, Lopiano, Zibetti, Massazza, Milano, Bottino, Trucco. Execution and data collection: Frisaldi (dance classes), Bottino (recruitments, reading and signing of the informed consent, reference doctor for the enrolled patients), De Ceglia (second hours of physiotherapy classes), Esposito (first hours of physiotherapy classes), Fabbri (recruitment and first assessor), Zibetti (recruitment and second assessor), Barbiani (first assessor), Camerone (first assessor), Costa (first assessor), Destefanis (recruitment). Statistical analysis: Frisaldi. Manuscript: Frisaldi (first draft); Frisaldi, Trucco, De Ceglia (supplementary material); all co-authors (review and critique).
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the local Ethics Committee (CS2/472) and conducted in agreement with the Declaration of Helsinki Ethical Principles.
Consent to participate
All subjects provided written informed consent.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 300 kb)
Rights and permissions
About this article
Cite this article
Frisaldi, E., Bottino, P., Fabbri, M. et al. Effectiveness of a dance-physiotherapy combined intervention in Parkinson’s disease: a randomized controlled pilot trial. Neurol Sci 42, 5045–5053 (2021). https://doi.org/10.1007/s10072-021-05171-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05171-9