Abstract
Background
Patients with Parkinson’s Disease (PD) undergo motor injuries, which decrease their quality of life (QL). Dance, added to drug therapy, can help treating these patients
Aims
To conduct a systematic review with meta-analysis with the aim to analyze the effects of dance classes in comparison to other interventions or to the absence of intervention, in randomized clinical trials (RCTs), on functional mobility, motor symptoms and QL of PD patients
Methods
The search was conducted in MEDLINE, LILACS, SciELO, Cochrane and PsycINFO (last searched in August 2017). RCTs analyzing dance effects in comparison to other physical training types or to no intervention, on functional mobility, motor symptoms and QL of PD patients were selected. The outcomes assessed were motor symptoms with Unified PD Rating Scale III (UPDRSIII), functional mobility with Timed Up and Go Test (TUG), endurance with 6 min walking test (6MWT), freezing of gait with Freezing of Gait Questionnaire (FOG_Q), walking velocity with GAITRite and QL with PD Questionnaire (PDQ39). Two reviewers independently extracted methodological quality and studies data. Results are presented as weighted mean differences.
Results
Five RCTs were included, totaling 159 patients. Dance promoted significant improvements on UPDRSIII, and a decrease in TUG time when compared to other types of exercise. In comparison to the absence of intervention, dance practice also showed significant improvements in motor scores.
Conclusion
Dance can improve motor parameters of the disease and patients’ functional mobility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alterations in dopaminergic cells activity of the substantia nigra promote motor and non-motor disturbances in patients with Parkinson’s Disease (PD) [1]. Rigidity, bradykinesia, tremor, postural instability, and alterations in gait patterns are highlighted as motor manifestations, considered as cardinal symptoms of the disease [2]. Among non-motor symptoms are autonomic dysfunctions; and sleep, cognitive and neuropsychiatric disorders, which cause social isolation to the patients, and contribute to the appearance of depressive symptoms [3, 4]. Such commitments impair functional autonomy and quality of life (QL) of this population.
Recently, many researches have been demonstrating that regular physical exercise practice, predominantly aerobic, seems to have a neuroprotective effect in delaying PD harmful symptoms, especially on balance, gait functional mobility and QL of the patients [1, 3, 5]. In addition, dance practice associated to musical stimuli seems to increase the reward system, once there is dopamine release via ventral tegmental area [2]. This implies in improvements in mood state and cognition of the patients, and consequently an improvement in QL [6, 7]. However, it is not clear in which way dance can influence on gait aspects, depressive symptoms and on QL of patients with PD.
Thereafter, understanding the dance potential as recreational activity which promotes visual, auditory and kinesthetic stimuli, in addition to socialization, it is believed that dance can be an important tool for the improvement of gait and QL of this population [8]. In this way, the purpose of this study was to conduct a systematic review with meta-analysis in the aim to analyze the effects of dance classes when compared to other interventions or to the absence of intervention, in randomized clinical trials (RCTs), on functional mobility, motor symptoms and QL of patients with PD.
Methods
The present study is characterized as a Systematic Review with Meta-analysis and is registered in the International prospective register of systematic reviews (PROSPERO) with CRD number 42015025118.
Eligibility criteria
Randomized clinical trials (RCTs) that compared an intervention group undergoing any type of dance for at least 3 weeks of practice, with PD patients at any stage of the disease, of both sexes and at any age, which analyzed functional and biomechanical parameters of the gait and/or quality of life of the participants were included. RCTs should present a control group exposed to any type of intervention, except dance—including other exercises, such as rehabilitation exercises, supervised exercise training—or without intervention. Duplicate articles and substudies were only included once.
Search strategy and study selection
The search was conducted in November 2015 and further updated in August 2017 in MEDLINE, LILACS, SciELO, Cochrane and PsycINFO databases, using MeSH terms and entry terms of the words “Parkinson’s Disease” and “Dancing” and a specific filter for randomized clinical trials. The complete search strategy is presented on ESM Appendix 1. The searches were limited to articles in English, Spanish or Portuguese. Studies selection was performed by two reviewers (M.S.D. and I.G.K.) in an independent and blinded manner, in two phases. Firstly, titles and abstracts were read by the reviewers and the studies that were not RCTs or that did not present dance as intervention for PD patients were excluded. Later, the articles included in the first phase were fully read by the reviewers and only studies that were in accordance to the eligibility criteria were included. In the last phase of the selection, every time that there was discordance between the two reviewers the evaluation was made by a third reviewer (A.N.H.).
Assessment of studies characteristics and risk of bias
A standardized data extraction was conducted by two reviewers (M.S.D. and I.G.K.) in an independent and blinded way, in which methodological characteristics of the studies were included, such as: titles, authors and date, purposes, design, type of intervention, frequency and duration of the classes, sample size, gender, age and stage in Hoehn and Yahr (H&Y) of the participants; and outcomes assessed as: motor symptoms—Unified Parkinson’s Disease Rating Scale III (UPDRS III), functional mobility—Timed Up and Go Test (TUG); endurance—6 min walking test (6MWT), freezing of gait—Freezing of Gait Questionnaire (FOG_Q), velocity of Forward walking and Backward walking (GAITRite) and quality of life—Parkinson Disease Questionnaire (PDQ39).
Studies were assessed regarding risk of bias by two reviewers (M.S.D. and I.G.K.), in an independent and blinded manner, through the items proposed by Cochrane [9]: randomization methods, allocation concealment, blinding of patients and therapist, blinding of outcomes evaluators, incomplete outcomes, selective reporting of outcomes and other possible bias sources. The items were defined as high risk of bias, low risk of bias or unclear risk of bias. Once again, in case of discordance the third reviewer (A.N.H.) was asked for analysis and assessment. The publication bias was not assessed due to the limited number of RCTs for each subgroup analysis.
Synthesis and analysis of data
Due to the differences in the control groups characteristics, meta-analysis were separately conducted for each one of the continuous outcomes presented in RCTs that compared dance to other exercise [2, 7, 10] and for those that compared dance to groups which were not exposed to any intervention [11, 12].
Pooled effect estimates were computed from changing scores between baseline and end of intervention, their standard deviations (SD) and the number of participants. Data from intention-to-treat analyses were included whenever available in the included RCTs. RCTs’ authors were contacted through email for unreported data. Missing SDs of changing values, not provided through email, were imputed based on Cochrane’s Handbook recommendations [13].
Results are presented as weighted mean differences and calculations were performed using fixed effects models, except when heterogeneity was greater than 50%, in which random effect models were adopted. Statistical heterogeneity of treatment effects among studies was evaluated by Cochran’s Q test and I 2 inconsistency test; it was considered that values over 50% indicated high heterogeneity [13]. In addition, a sensitivity analysis was conducted when heterogeneity was significant, in accordance to the following criteria: type of dance applied to the intervention group and total duration of the intervention. Forest plots were generated to present pooled effect and weighted difference (WMD) with 95% confidence interval (CI). Values of α ≤ 0.05 were considered statistically significant, and all analyses were performed using Review Manager version 5.3 (Cochrane Collaboration).
Results
Identification of studies
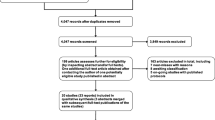
From the 134 articles identified in MEDLINE, LILACS, SciELO, Cochrane and PsycINFO databases, after removal of duplicates, 110 studies remained. Of these, after the reading of titles and abstracts, 89 articles were excluded for not having PD patients as participants or because they were not RCTs. The 21 RCTs selected on phase 1 were fully read, and then, 16 studies were excluded for not meeting the eligibility criteria. Five RCTs were included, which were in accordance to the eligibility criteria of this research [2, 7, 10,11,12]. Figure 1 shows the flowchart of the studies included in this review.
Description of included studies
Among the included studies, three RCTs compared a dance intervention group to a comparator group that performed other type of exercise [2, 7, 10], and two RCTs compared a dance intervention group to a comparator group that was not exposed to any type of intervention [11, 12]. A total of 159 PD patients participated in the included RCTs, with 79 in the intervention groups with dance and 80 in the comparator groups.
Presented in the first subgroup: Rios Romenets et al. [2] compared a group composed by 18 participants exposed to Tango classes, with frequency of 2 times per week and duration of 1 h during 12 weeks to a group of 15 PD patients who practiced self-directed exercises during the same period; Volpe et al. [7] analyzed 12 patients with PD exposed to weekly classes of Irish dance, with duration of 1.5 h and duration of 6 months compared to 12 parkinsonian patients who performed physiotherapy exercises during the intervention period; and Hackney et al. [10] evaluated 9 participants exposed to 20 Tango classes, with frequency of two times per week and duration of 1 h in comparison to 10 patients who performed traditional exercise during the same period.
In the second subgroup, two studies compared PD patients exposed to Tango classes with duration of 1 h and frequency of two times per week to PD patients that did not accomplish any intervention. The studies of Duncan and Earhart [11] analyzed interventions of 12 months with 26 patients in each group, whereas the study of Hackney and Earhart [12] conducted the comparisons after 10 weeks of intervention with 14 participants in the Tango group and 17 subjects in the comparator group.
Tables 1 and 2 summarize the characteristics of these five RCTs divided in their subgroups that, besides presenting frequency, intervention period, class duration and type of intervention, also reported the mean age of the participants and stage of the disease through H&Y scale.
Risk of bias and methodological quality
Methodological quality of the included studies was evaluated by two reviewers and of the five RCTs inserted in this review, four studies [2, 7, 11, 12] presented adequate generation of randomized sequence and reported allocation concealment, four studies [7, 10,11,12] accomplished blinding of outcomes evaluators; only one study [7] presented incomplete outcomes and selective reporting of outcomes. None of the studies performed the blinding of the patients and therapists. All the studies [2, 7, 10,11,12] presented unclear risk in other bias sources.
Outcomes analyzed for dance versus other exercise
In the studies that compared dance classes to other interventions [2, 7, 10], meta-analyses were conducted for motor symptoms, freezing of gait, functional mobility and QL outcomes. Meta-analyses graphics can be observed in Fig. 2.
To measure motor symptoms, the three RCTs [2, 7, 10] used UPDRS III scale, with a total number of 76 patients. This scale indicates the patients’ motor difficulties, the higher the score of the patient, the greater the motor injury. The results show significant improvements in favor of the group exposed to dance classes, decreasing the punctuation in the scale after the dance intervention (− 2.52 points, CI − 4.59 to − 0.45 points, p = 0.02), with heterogeneity of 39% (p = 0.19) (Fig. 2a).
To measure functional mobility, 52 participants of two studies [2, 10] were included, which used TUG test. The results showed significant improvements in favor of the dance intervention group, in which the time required for the accomplishment of the test tasks was reduced, demonstrating an increase of functional mobility (−1.15 s, CI − 2.03 to − 0.27 s, p = 0.01), with 0% of heterogeneity (p = 0.87), (Fig. 2b).
To measure patients’ freezing of gait, the three studies [2, 7, 10] applied FOG_Q, with a total number of 76 patients. The questionnaire indicates the presence and the intensity of freezing of gait in the patients, the higher the score, the greater the freezing. The results of the comparison were favorable to dance, however, the reduction of the scores was not statistically significant (−1.41, CI − 5.53 to 2.71, p = 0.50). As the studies heterogeneity was evaluated as high and significant, 89% (p < 0.001), the random effect type was adopted for this meta-analysis. (Fig. 2c).
Even using random effect type for the comparison of FOG_Q values in this subgroup, heterogeneity remained high and significant, 89% (p = 0.0001). In this way, sensitivity analyses were done and the study of Volpe et al. [7] was excluded of the meta-analysis, due to the difference in the time of intervention and the proposed type of dance be different from the other studies [2, 10]. The results of the meta-analysis with FOG_Q, Romenets et al. [2] and Hackney et al. [10] studies, with 52 participants, which showed heterogeneity of 0% (p = 0.65; χ 2 = 0.21; df = 1), were not statistically significant (0.56, CI − 0.81–1.93, p = 0.42, Z = 0.80) (Fig. 2c).
For the QL analysis, two studies [2, 7] with 57 participants used PDQ-39. In this questionnaire, a smaller final score means higher levels of QL. There was a more pronounced improvement of the results for dance in comparison to the practice of other exercise, however, this difference between interventions, which showed 0% of heterogeneity, was not significant (−2.03, CI − 8.33–4.26, p = 0.53) (Fig. 2d).
Outcomes analyzed for dance versus no intervention
In the studies comparing dance practice to the absence of intervention [11, 12], meta-analyses were made for motor symptoms, endurance, freezing of gait and velocities of forward walking and backward walking. In this group, all the analyses showed 0% of heterogeneity. The graphics of these meta-analyses can be observed in Fig. 3.
To measure motor symptoms, the two RCTs [11, 12] used UPDRS III scale, totaling 83 patients. The results indicated significant improvements in favor of the group exposed to dance classes, which showed a smaller punctuation in the scale, that means a reduction of the disease motor injuries (−8.35 points, CI − 13.79– 2.91 points, p = 0.003) (Fig. 3a).
To evaluate the endurance of the 83 patients, the studies [11, 12] used 6MWT. In this test, the distance performed is measured in meters over 6 min, thus, the greater the distance, the greater the endurance of the subject. Although the results were favorable to dance, they did not show significant differences between the groups (36.24 m, CI − 6.72 to 79.19 m, p = 0.10) (Fig. 3b).
Both RCTs [11, 12] used FOG_Q to measure freezing of gait of the 83 subjects. Again, the results favored dance, with a reduction of the punctuation, indicating a decrease of the freezing of gait in relation to the comparator group, however, the reduction of the scores was not statistically significant for the intervention (−2.33, CI − 4.95 to 0.29, p = 0.08) (Fig. 3c).
The analysis of the velocity of forward and backward walking was performed by the two RCTs [11, 12]. In the two forms of walking, the group exposed to dance showed better results, with greater velocities, however this difference was not significant between the interventions (Forward walking 0.09 m/s, CI − 0.33 to 0.20, p = 0.15; Backward Walking 0.07 m/s, CI − 0.09 to 0.24, p = 0.38) (Fig. 3d, e).
Discussion
The purpose of this systematic review was to conduct a systematic review of the literature, with meta-analysis, aiming to analyze the effects of dance in comparison to other interventions or to the absence of intervention in RCTs, on functional mobility, motor symptoms and QL of patients with PD. After the selection of the five RCTs [2, 7, 10,11,12] which met the eligibility criteria, the studies were separated into two subgroups for the accomplishment of the meta-analysis: those that presented another type of exercise as comparator [2, 7, 10], and those that had control groups not exposed to other intervention [11, 12].
The results found in the studies with comparisons between dance and other exercises indicate that dance practice induces better responses in motor symptoms and in functional mobility in individuals with PD than an intervention with other type of physical activity like physiotherapy, self-directed or traditional exercises, presented in the analyzed studies. These findings have a big relevance, since that a better functional mobility and a decrease of the motor symptoms of the disease can promote benefits in independence and in functional autonomy of the patients [1], as well as can also help preventing falls [4, 10], which are very usual in this population.
In this first group analyzed, regarding freezing of gait and QL, dance did not obtain significantly better results than the control groups. This can be explained by the fact that other exercises practice can also be efficient for these outcomes.
For the comparison between the groups exposed to dance classes and controls without intervention, once again a significant improvement of dance compared to control groups in UPDRS III scores was noticed, demonstrating that dance is able to reduce clinical motor symptoms of the disease, a hypothesis that had already been reported in the literature [6, 8, 10]. For the other outcomes analyzed in this subgroup, a greater improvement in favor of dance was noticed, however, without significant differences between the groups.
Although all outcomes analyzed showed favorable results for dance, only the three cited ones, UPDRS III in both subgroups and TUG test in the comparison to other exercises demonstrated statistically significant results for dance in comparison to control groups. It is highlighted the importance that, although the other outcomes have not presented significant results favoring dance, the findings of these analyses are very relevant for the population and the field of study, since UPDRS III motor scale is considered as gold standard in the evaluation of PD cardinal symptoms [1, 5], and TUG test is an important tool to measure functional mobility, associated to autonomy and independence of the patients [1].
Aiming to relieve disease injuries and to administrate its progression, the patients are daily exposed to a big quantity of drugs [14]. Pharmacological treatment provides a momentary relief, however, besides causing many side effects, it leads to a big oscillation of the symptoms, therefore, the aid of other therapeutic practices is required in combination to the treatment, in order to soften certain injuries caused by PD [4, 14, 15].
Inside this field of knowledge, dance is able to assist in motor parameters, in the decrease of depressive symptoms [15], increasing socialization, providing a greater motivation to body practice, a better motor performance and an increase of QL of this population [8]. Presenting possibilities of physical, social and psychological rehabilitations, dance can be considered as an efficient therapeutic agent due to its recreational, pleasant and engaging characteristics [16], able to achieve a considerable adherence of the patients to programs of this nature. Body practice is only beneficial when practiced in a regular way; therefore, the acceptance and participation of the patients in the interventions become crucial [6].
In this way, it is believed that a dance program, in order to have an efficient role in the rehabilitation of this population, must include specific activities, like visual and auditory cues [3, 10, 14], rhythm tasks, recreational activities that motivate socialization of the participants and mainly assistance for participants to reach the heart rate levels able to promote the beneficial effects of neuroprotection [1, 5, 17].
Study limitations
Although this systematic review with meta-analysis was performed with the maximum methodological rigor possible, some limitations should be highlighted.
In relation to the studies included in this research, it is noticed that the interventions protocols in which the participants were exposed to were little described. It is not possible to clearly understand what was performed in each dance class in the RCTs. Only basic characteristics as frequency, duration and type of intervention are described. In general, the studies that report dance practice effects in the parkinsonian population are recent and do not usually specify and describe in details the intensities of the classes and activities proposed. In addition, as it is a recent field of study, the number of published articles adopting dance as complementary rehabilitation method for patients with PD is very limited, what might have contributed for the results in which significant differences were not observed. Moreover, the included studies conducted dance interventions with different characteristics between them, like the type of dance proposed (tango and Irish dance). Still, these studies only recruited elderly people, which makes it impossible to extrapolate the results of the present meta-analysis to other age groups of this population.
Conclusion
Dance proved to be able to assist in motor parameter of the disease and in functional mobility. These gains are very relevant for the population, due to the impact that motor injuries cause in their daily life. Perceiving the importance of the periodization in rehabilitation programs and the lack of quantitative information about the volume of these body interventions, it is suggested that new researches be conducted, with well defined protocols, periodization of the intensity of the activities in accordance to the musical progress, the heart rate, and the complexity of the addressed tasks, to have studies with greater and better scientific accuracy in the study area.
References
Monteiro EP, Franzoni LT, Cubillos DM et al (2017) Effects of Nordic walking training on functional parameters in Parkinson’s disease: a randomized controlled clinical trial. Scand J Med Sci Sports 27:351–358. doi:10.1111/sms.12652
Romenets SR, Anang J, Fereshtehnejad SM et al (2015) Tango for treatment of motor and non-motor manifestations in Parkinson’s Disease: a randomized control study. Complement Ther Med 23:175–184. doi:10.1016/j.ctim.2015.01.015
Gallo PM, McIsaac TL, Garber CE (2014) Walking economy during cued versus non-cued self-selected treadmill walking in persons with Parkinson’s disease. J Parkinsons Dis 4:705–716. doi:10.3233/JPD-140445
Marinho MS, Chaves PM, Tarabal TO (2014) Dupla-tarefa na doença de Parkinson: uma revisão sistemática de ensaios clínicos aleatorizados. Rev Bras Geriatr Gerontol 17:191–199
Alberts JL, Linder SM, Penko AL et al (2011) It is not about the bike, it is about the pedaling: forced exercise and Parkinson’s disease. Exerc Sport Sci Rev 39:177–186. doi:10.1097/JES.0b013e31822cc71a
Sharp K, Hewitt J (2014) Dance as an intervention for people with Parkinson’s disease: a systematic review and meta-analysis. Neurosci Biobehav Rev 47:445–456. doi:10.1016/j.neubiorev.2014.09.009
Volpe D, Signorini M, Marchetto A et al (2013) A comparison of Irish set dancing and exercises for people with Parkinson’s disease: a phase II feasibility study. BMC Geriatr 13:1–6. doi:10.1186/1471-2318-13-54
Shanahan J, Morris ME, Bhriain ON et al (2015) Dance for people with Parkinson disease: what is the evidence telling us? Arch Phys Med Rehabil 96:141–153. doi:10.1016/j.apmr.2014.08.017
Deeks J, Higgins J, Altman D (eds) (2011) Analysing data and undertaking meta-analysis. In: Higgins J, Green S (eds) Cochrane handbook for systematic reviews of interventions, version 5.1.0. The cochrane collaboration. Accessed Sep 2015
Hackney ME, Kantorovich S, Levin R et al (2007) Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neuro Phys Ther 31:173–179. doi:10.1097/NPT.0b013e31815ce78b
Duncan R, Earhart G (2012) Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabilit Neural Repair 26:132–143. doi:10.1177/1545968311421614
Hackney ME, Earhart G (2009) Effects of dance on movement control in Parkinson Disease: a comparison of Argentine Tango and American ballrrom. J Rehabil Med 41:475–481. doi:10.2340/16501977-0362
Higgins J (2011) Analysing data and undertaking metaanalysis. In: Higgins J, Green S (eds) Cochrane handbook for systematic reviews of interventions. Version 5.1.0. http://www.cochrane-handbook.org. Accessed Dec 2015
Gonçalves GB, Leite MAA, Pereira JS (2011) Influência das distintas modalidades de reabilitação sobre as disfunções motoras decorrentes da Doença de Parkinson. Rev Bras Neurol 47:22–30
Tuon T, Valvassori SS, Dal Pont GC et al (2014) Physical training prevents depressive symptoms and a decrease in brain-derived neurotrophic factor in Parkinson’s disease. Brain Res Bull 108:106–112. doi:10.1016/j.brainresbull.2014.09.006
Hackney ME, Bennett C (2014) Dance therapy for individuals with Parkinson’s disease: improving quality of life. J Parkinsonism Restless Legs Syndr 4:17–25
Frazzitta G, Balbi P, Maestri R et al (2013) The Beneficial Role of Intensive Exercise on Parkinson Disease Progression. Am J Phys Med Rehabilit 92:1–10. doi:10.1097/PHM.0b013e31828cd254
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This study was funded by the authors.
Conflict of interest
The authors state that there was no conflict of interests with any financial or personal relationships or organizations that could influence the research results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos Delabary, M., Komeroski, I.G., Monteiro, E.P. et al. Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson’s disease: a systematic review with meta-analysis. Aging Clin Exp Res 30, 727–735 (2018). https://doi.org/10.1007/s40520-017-0836-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-017-0836-2