Abstract
Treatment of Parkinson’s disease with levodopa/carbidopa/entacapone (LCE) has been studied for a long time. However, the efficacy and safety of LCE in the treatment of early Parkinson’s disease (PD) still need to be assessed. Our objective was to do a meta-analysis of relevant randomized controlled trials (RCTs) to evaluate the efficacy and safety of LCE for early PD. PubMed, Embase, the Cochrane Library, and the Web of Science were searched for RCTs with “levodopa/carbidopa/entacapone” and “Parkinson’s disease” as keywords. The search period was from inception to October 2018. The quality of included studies was strictly evaluated. We evaluated the quality of included studies strictly and six studies met all inclusion criteria. The results showed that LCE could improve activities of daily living and motor function in PD patients. However, LCE therapy was associated with higher risks of total AEs and single AEs compared with traditional therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a neurological disorder with complex evolving layers. It has long been characterized by the classical motor features of Parkinsonism with Lewy bodies (LBs) and loss of dopaminergic neurons in the substantia nigra [1, 2]. LBs is a unique intracytoplasmic inclusion body containing a variety of cellular proteins. α-synaptic nucleoprotein (α-syn) is a major component of LBs. In recent years, the research focus of PD is mainly on the mechanism of α-syn. α-syn monomer is composed of the amino terminus, the carboxyl terminus, and the NAC domain (the substrate of transglutaminase). It normally exists in the body as a disordered monomer. However, in the brains of Parkinson’s patients, these monomers are abnormally expressed and aggregated to form synaptic nucleocapsin aggregates, which along with other proteins form LBs, causing mitochondrial dysfunction and apoptosis, which ultimately leads to brain cell death [3]. PD is characterized by bradykinesia, muscular rigidity, and rest tremor, as well as postural and gait impairment. In addition to motor symptoms, various non-motor features, such as olfactory dysfunction, cognitive impairment, psychiatric symptoms, and sleep disorders, may occur at different stages throughout the disease. As well as treating motor symptoms, these non-motor symptoms must also be addressed, but are often more difficult to treat [2, 4, 5].
PD has a high prevalence and it is associated with social and economic consequences. PD affects about 10–18 out of every 100,000 people every year [2]. The prevalence of PD increases with age and the global burden of care for the condition is likely to increase markedly over the next 25 years since life expectancy is rising worldwide [6].
A major goal of Parkinson’s disease research is to develop disease-modifying drugs that slow or halt the underlying neurodegenerative process [2]. In the 1980s, levodopa was the only medication available for PD (except anticholinergics and amantadine). Levodopa act to enhance synaptic dopamine transmission using the dopamine precursor L-3,4-ihydroxyphenylalanine. However, this drug has side effects such as dyskinesia and motor fluctuations [5]. Most of these dyskinesias occur when levodopa or other dopamine receptor agonists have a concentration in the brain that is sufficient to overactivate dopamine receptors in the putamen [7]. Considering the limitations of levodopa monotherapy, the adjuvant therapy with adding other anti-Parkinson’s drugs has been used in PD treatment.
Levodopa is partly metabolized by catechol O-methyltransferase (COMT). COMT inhibitors increase the elimination half-life of levodopa and boost the effect of each tablet by about 30% [5, 8]. Entacapone is a selective, reversible, peripheral inhibitor of the COMT, which mediates LD metabolized to 3-O-methyldopa (3-OMD) and prolongs LD half-life in vivo [9]. So, using one tablet that combines levodopa, carbidopa, and entacapone should have similar pharmacokinetic and efficacy profile and that can lead to increasing the daily “on” time, decreasing the daily “off” time, and the daily levodopa dose [10,11,12]. However, the plasma half-life of levodopa in rapidopa/levodopa (CD/LD) rapidrelease agents is only about 1.5 h, making it difficult to maintain therapeutic drug concentrations and leading to fluctuations in motor symptoms and a long-term risk of movement disorders. In order to quickly achieve the therapeutic concentration of levodopa and maintain the longer efficacy, CD/LD sustained release agents were developed [13,14,15].
Although a large number of studies have investigated the treatment of motor and non-motor symptoms in PD, there are still many controversies about the diagnosis and treatment of early PD patients (early PD patients are newly diagnosed patients in Hoehn-Yahr (H&Y) stage 1 and up to stage 2 [16]). So, we make this meta-analysis to review the evidence for efficacy and safety of LCE in the treatment of early PD patients.
Methods
Literature search strategy
We searched four databases of PubMed, Embase, Web of science, and the Cochrane Library. The publication period for the search is from inception to October 2018 for all the English language studies that used LCE in the treatment of early PD. The search was conducted using a combination of keywords including “Parkinson Disease,” “Entacapone,” and free words including “Idiopathic Parkinson’s Disease,” “Lewy Body Parkinson Disease,” etc. We used the Boolean logic “AND” to combine the keywords and “OR” for the free words. Reference lists from the resulting publications and reviews were used to identify further relevant publications.
Inclusion and exclusion criteria
The studies to be included had to satisfy the following criteria:
-
1.
Study design: randomized controlled clinical trials (RCTs). The research had to include a comparative treatment of LCE or Stalevo with other Parkinson’s drugs like levodopa/carbidopa (LC) or placebo.
-
2.
Subjects included patients with early PD. The early PD was defined as idiopathic PD, H&Y stage 3 or less, no motor complications history, no treatment, or limited (generally less than 6 months) use of anti-Parkinson’s drugs.
-
3.
Experimental parameters, such as the dose of LCE or Stalevo, and medication time.
-
4.
The scales scores associated with PD, such as PDQ-8, UPDRS II, or III scores, and duration of dyskinesia.
Prespecified exclusion criteria were (1) case reports, abstracts, comments, reviews, and editorials; (2) duplicate publication; (3) uncorrelated experiment; and (4) no treatment-related outcomes were reported.
Data extraction
Data for each study were extracted independently by two researchers (Wu and Liu). For all the studies we searched, we used the title summary screening in the first phase and the full-text reading screening in the second phase to obtain the studies we needed to include in the end. For each study, information was carefully extracted from all the eligible studies, including (1) the first author, year of publication, number of subjects, sex ratio (male/female), mean age of subjects, and inclusion criteria of PD patients; (2) study design, PD scale used, duration of study, and visits of all stages; and (3) intervention characteristics of the trial groups and control groups. We resolved our differences through discussion and consulted a third investigator (Xia) if necessary.
Quality assessment
The two researchers evaluated the methodological quality of the included studies by the Cochrane risk-of-bias assessment tool independently. The tool classifies the studies as having low, moderate, or high risk of bias across six domains: Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), other sources of bias (other bias). Disagreements were resolved through consensus or discussed with a third investigator.
Statistical analysis
The results of each identified trial were combined by using meta-analytic methods to evaluate the overall effect for experimental group (use LCE) versus control (use others). For continuous data (such as UPDRS, PDQ-8), we calculated mean differences (MDs), standard differences (SDs), and their 95% confidence intervals (CIs). For categorical outcomes (such as dyskinesia, risk of AEs), we calculated relative risk (RRs). We used the recommended method from the Cochrane handbook to estimate SDs if they were not available in the included studies. The random effects was used rather than a fixed effects model because this takes into account the heterogeneity between multi-studies. For the assessment of heterogeneity, the I2 statistic was used. When outcome measurements in all studies are made on the same scale, it can be used as a summary statistic in meta-analysis. Publication bias was examined by funnel plot. All analyses performed with RevMan 5.3. p values ≤ 0.05 were considered significant.
Result
Study inclusion
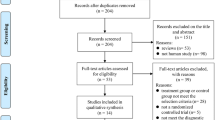
Our literature search yielded 2185 articles, of which 2033 literatures were excluded through title and abstract screening. Through screening the full-text of the remaining 152 articles, 78 studies were excluded because of non-RCT experimental literature, 5 studies were excluded because of the short follow-up time, 32 studies were excluded because of the unclear outcomes, and 31 studies were excluded because the intervention drugs did not included LCE. Ultimately, we included six articles [6, 10, 17,18,19,20] which satisfied the inclusion criteria. The flowchart of studies included and excluded is presented in Fig. 1.
Basic characteristic of studies
Six RCTs met the inclusion criteria, of which 1983 participants were included. The levodopa/carbidopa/entacapone group contained 983 participants and the control group contained 1000 participants. One study adopted levodopa/DDCI+ entacapone (L/D/E) and five studies adopted levodopa/carbidopa (LC) in the control group. The average age of the included participants was about 60 to 70, the proportion of male was slightly higher than female, the average duration of PD was about 5.3 years, and all participants’ Hoehn and Yahr staging was no more than three. In the six studies, treatment duration ranged from 6 to 134 weeks, and the number of participants ranged from 95 to 745. In terms of experimental outcomes, UPDRS as the outcome measure was observed in four studies, CGI as the outcome measure was reported in two studies, and PDQ-39 as the outcome measure was observed in four studies. All studies reported adverse events (AEs), included total adverse events and single adverse events (nausea, diarrhea, dyskinesia, dizziness, and so on). The basic characteristics of the six studies were summarized in Table 1.
Risk of bias
We used the Cochrane Collaboration’s tool for assessing risk of bias. Six studies described the sequence generation and provided complete outcome data. Five studies have complete allocation sequence concealment. Four studies described blinding of participants, personnel, and outcome assessors. Therefore, all studies, included finally, were considered to have low risk of bias, of which average bias risk score is 5. Table 2 summarized the risks of bias of each study.
UPDRS II
Three of included studies reported the results of UPDRS II. In these studies, the intervention of control group was levodopa/carbidopa (LC). These studies contained 702 participants. It showed that the effect of LCE was more obvious than LC for reducing UPDRS II scores and the heterogeneity was not significant (WMD = − 0.98, 95% CI − 1.48 to − 0.48, p = 0.0001; heterogeneity, Chi2 = 3.07, p = 0.22, I2 = 35%; Fig. 2a).
UPDRS III
Similarly, UPDRS III data was available from four studies, of which the intervention of control group was LC. These studies contained 1061 participants. We found the significant effect of LCE compared with LC, and we used the random effects models for pool analysis because of the heterogeneity (WMD = − 1.93, 95% CI − 4.25 to 0.39, p = 0.10; heterogeneity, Chi2 = 4.69, p = 0.0001, I2 = 85%; Fig. 2b). However, we did not make further analysis to explore the sources of heterogeneity because of the limitations.
CGI
Two studies that reported clinical global impression (CGI) scores were included in the efficacy analysis. The CGI scores were evaluated by investigators. The control group of one article was LC and the other was levodopa/DDCI+ entacapone (L/D/E). The results showed that there was no significant difference in overall efficacy between the LCE group and the control group (RR = 1.01, 95% CI 0.93 to 1.10, p = 0.80; heterogeneity, Tau2 = 0.00, Chi2 = 0.25, p = 0.61, I2 = 0%; Fig. 3, (1)). However, the LCE group, reported “very much improved” had a larger probability (RR = 1.49, 95% CI 0.54 to 4.11, p = 0.44, Fig. 3, (2)) and reported “much improved” had smaller a probability compared with the control group (RR = 0.90, 95% CI 0.64 to 1.27, p = 0.55, Fig. 3, (3)). These results had no significantly statistical difference, of which p values ranged from 0.44 to 0.84. We should interpreted the result cautiously because of the small number of included studies.
PDQ-39
The Parkinson’s disease Questionnaire (PDQ-39) data was available from four studies with 1622 participants included in this pool analysis. The control groups of four studies were LC. The effect of LCE group was slightly lower than that of LC group on improving quality of daily life (WMD = 0.62, 95% CI − 0.71 to 1.96, p = 0.36; heterogeneity, Chi2 = 4.59, p = 0.20, I2 = 35%; Fig. 4). This pool analysis did not have an obvious publication bias because the funnel plot was roughly symmetric for the PDQ-39 score (Fig. 6a).
Adverse events
We analyzed the adverse events (AEs) in detail. All studies with 1983 participants reported the total number of participants with AEs. The incidence of AEs was 80.4% in the LCE group and 66.8% in the control group. The result showed that the LCE group had a higher probability of AEs than the control group (RR = 1.26, 95%CI 1.02 to 1.57, p = 0.03; heterogeneity, Tau2 = 0.06, Chi2 = 58.00, p < 0.00001, I2 = 91%; Fig. 5a).
The number of discontinuation due to AEs was 102 in the LCE group with 983 participants, and 76 in the control group with 1000 participants. In the subgroup analysis for the discontinuation due to AEs, the proportion of the LCE group was superior to the control group (RR = 1.36, 95%CI 1.02 to 1.80, p = 0.03; heterogeneity, Chi2 = 2.19, p = 0.82, I2 = 0%; Fig. 5b). The funnel plot was not completely symmetrical for the number of discontinuation due to AEs, so it suggested some certain publication bias (Fig. 6b).
We did further analysis about single adverse events. The risk of nausea, diarrhea, dyskinesia, dizziness, and urine abnormality was 23.0%, 12.9%, 5.9%, 10.6%, and 13.6% in LCE group compared with 13.0%, 6.7%, 4.5%, 8.0%, and 3.0% in control group. The results showed that the risk of those single AEs in the LCE group was greater than that in the control group (Fig. 5c). Among them, there is significant statistical difference in the results of risk analysis of nausea and diarrhea (p < 0.0001 and p = 0.0009), and risk analysis of nausea, diarrhea, and dizziness revealed good homogeneity (I2 = 0%, 20%, and 0%). The result reported that the LCE group was more likely to have nausea, diarrhea, dyskinesia, dizziness, and urine abnormality than the control group.
Discussion
Summary of evidence
Our meta-analysis found that LCE therapy improve the UPDRS I and UPDRS II score compared with traditional drug therapy in early PD patients. However, there was no obvious difference in CGI scores. What’s more, the result showed that LCE therapy was not as effective as LC therapy with PDQ-39 as the outcome measure. LCE therapy also increased the risk of total AEs, nausea, diarrhea, dyskinesia, dizziness, urine abnormality, and discontinuation risk when compared with traditional therapy. Most of our results are in line with clinical observations in the published paper [21,22,23,24]. The results demonstrated that patients who received LCE therapy could evidently show improvement on activities of daily living and motor symptoms. This part of our result is the same with a pooled analysis of phase III studies with entacapone [25]. According to the pool analysis of CGI, this study has shown that LCE provided equivalent benefits to those obtained with separately administered levodopa/DDCI and entacapone tablets or levodopa/carbidopa tablets. Improvement in motor scores may have driven the changes observed in the PDQ-39 and may have been temporized by the presence of AEs [18]. The AEs that produced by stalevo tablets should be of more concern. Specially, the risk of urine abnormal in LCE group was observed to almost three times as likely as that in control group (p = 0.08).
Interpretation of the results
LCE provided the greatest symptomatic benefit for PD than LC [23]. One meta-analysis suggested that LCE could improve activities of daily living and motor symptoms in PD patients [26]. We found LCE could significantly improve the UPDRS II and UPDRS III scores compared with LC in early Parkinson patients, consistent with previous results. In our meta-analysis, LCE did not show improvement in CGI scores, which may be explained by a small amount of included studies. The statistical power of this analysis is low. We look forward to further experimental studies to refine our results. One probable reason why LCE participants had a slightly worsening in PDQ-39 scores was that using PDQ-39 scores could be relative insensitivity to change of this measure, as has been suggested in early Parkinson patients [18]. The other probable reason was that the high-frequency presence of AEs caused a lower PDQ-39 scores compared with traditional therapy. The incidence of AEs was 80.4% in the LCE group and 66.8% in the control group. Discontinuation risk was 10.4% in the LCE group and 7.9% in the control group. The stalevo treatment with a more common in AEs was not unexpected, as it occurred in previous studies with entacapone. The increase in AEs was a result of enhanced dopaminergic activity [21, 22, 24]. Urine abnormality was the most common AE in the LCE group, but it was a benign event associated with the color of the metabolites of entacapone excreted in urine.
Limitations
A number of limitations of this study should be considered. First, meta-analyses combining evidence from several high-quality RCTs generally considered the highest level of evidence for effects of interventions. However, due to the limited number of studies included in this meta-analysis, the risk of overestimation of intervention effects cannot be excluded [27, 28]. Thus, we did not conduct a subgroup analysis of the effect of different doses of LCE on the treatment of motor complications in early PD patients. In addition, most of the studies included in this meta-analysis are about the comparison of LCE and LC; only one is about the difference between LCE and L/D/E. Consequently, further large, well-designed RCTs that evaluate the long-term balance of benefit and harm, comparing LCE with L/D/E, are urgently needed. Second, we compared the p value and I2 value of the results of multiple groups and found heterogeneity in some studies. However, due to the insufficient sample size, we did not conduct further subgroup analysis. This indicated that our results may be biased. Third, all the trials were carried out in Europe, Australia, USA, and so on. There are insufficient data on the Asian and Africa. It would inevitably be valuable to know whether the initial treatment of PD is discrepancy in different regions. Forth, only English-language studies were included. This is another defect that limited the generalization of the findings potentially. In spite of these limitations, our meta-analysis also had possessed some advantages. First, all of the included trials were well designed and were considered to have a low risk of bias and provided promising evidences. Second, we simply analyzed the efficacy of LCE in patients with early PD, while previous meta-analysis was to analyze the efficacy of LCE in the whole stage of PD patients, so this meta-analysis provided more clear opinions for the treatment of early PD patients.
Implications for future research
A major goal of PD research is the development of disease-modifying drugs that slow or stop the underlying neurodegenerative process [2]. Levodopa, as the gold standard for the treatment of PD, is associated with the development of motor complications. So a drug that combined entacapone (as an inhibitor of the COMT) with levodopa and carbidopa has recently emerged to reduce the side effects of using levodopa alone. However, although many studies have been conducted to explore the therapeutic effect of LCE on PD patients, few studies have been conducted on the efficacy and safety of early PD patients, so more RCTs are needed to confirm our results in the future.
Conclusions
This is the first meta-analysis to determine the efficacy and safety of levodopa/carbidopa/entacapone (LCE) in early Parkinson patients with Hoehn-Yahr (H&Y) less than three. Current meta-analysis has proved that LCE is more effective than other Parkinson’s drugs in the treatment of early PD, but it has more adverse reactions than other drugs. However, one factor affecting the validity of findings is that the number of studies included in this meta-analysis is very limited.
References
Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson’s disease. Mov Disord 28(1):24–30. https://doi.org/10.1002/mds.25032
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Chen Y, Shen J, Ke K, Gu X (2020) Clinical potential and current progress of mesenchymal stem cells for Parkinson’s disease: a systematic review. Neurol Sci 1:11. https://doi.org/10.1007/s10072-020-04240-9
Gaenslen A, Berg D (2010) Early diagnosis of Parkinson’s disease. Int Rev Neurobiol 90:81–92. https://doi.org/10.1016/s0074-7742(10)90006-8
Ossig C, Reichmann H (2015) Treatment strategies in early and advanced Parkinson disease. Neurol Clin 33(1):19–37. https://doi.org/10.1016/j.ncl.2014.09.009
Brooks DJ (2008) Optimizing levodopa therapy for Parkinson’s disease with levodopa/carbidopa/entacapone: implications from a clinical and patient perspective. Neuropsychiatr Dis Treat 4(1):39–47
Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of Parkinsonism—chronic treatment with L-dopa. N Engl J Med 280:337–345
Ascherio A, Schwarzschild MA (2016) The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 15(12):1257–1272. https://doi.org/10.1016/s1474-4422(16)30230-7
Gordin A, Kaakkola S, Teravainen H (2004) Clinical advantages of COMT inhibition with entacapone—a review. J Neural Transm (Vienna) 111(10–11):1343–1363. https://doi.org/10.1007/s00702-004-0190-3
Fung VSC, Herawati L, Wan Y, Boyle R, Hughes A, Lueck C et al (2009) Quality of life in early Parkinson’s disease treated with levodopa/carbidopa/entacapone. Mov Disord 24(1):25–31. https://doi.org/10.1002/mds.21878
Poulopoulos M, Waters C (2010) Carbidopa/levodopa/entacapone: the evidence for its place in the treatment of Parkinson’s disease. Core Evid 5:1–10
Tveiten OV, Skeie GO, Haugarvoll K, Muller B, Larsen JP, Tysnes OB (2013) Treatment in early Parkinson’s disease: the Norwegian ParkWest study. Acta Neurol Scand 128(2):107–113. https://doi.org/10.1111/ane.12055
Espay AJ, Pagan FL et al (2017) Optimizing extended-release carbidopa/levodopa in Parkinson disease: consensus on conversion from standard therapy. Neurol Clin Pract 7:86–93
Abbruzzese G (2008) Optimising levodopa therapy. Neurol Sci 29:377–379. https://doi.org/10.1007/s10072-008-1051-x
Zambito Marsala S, Vitaliani R, Volpe D, Capozzoli F, Baroni L, Belgrado E, Borsato C, Gioulis M, Marchini C, Antonini A (2013) Rapid onset of efficacy of rasagiline in early Parkinson’s disease. Neurol Sci 34:2007–2013. https://doi.org/10.1007/s10072-013-1437-2
Getz SJ, Levin B (2017) Cognitive and neuropsychiatric features of early Parkinson’s disease. Arch Clin Neuropsychol 32(7):769–785. https://doi.org/10.1093/arclin/acx091
Lew MF, Somogyi M, McCague K, Welsh M (2011) Immediate versus delayed switch from levodopa/carbidopa to levodopa/carbidopa/entacapone: effects on motor function and quality of life in patients with Parkinson’s disease with end-of-dose wearing off. Int J Neurosci 121(11):605–613. https://doi.org/10.3109/00207454.2011.598982
Hauser RA, Panisset M, Abbruzzese G, Mancione L, Dronamraju N, Kakarieka A, Grp F-SS (2009) Double-blind trial of levodopa/carbidopa/entacapone versus levodopa/carbidopa in early Parkinson’s disease. Mov Disord 24(4):541–550. https://doi.org/10.1002/mds.22343
Stocchi F, Rascol O, Kieburtz K, Poewe W, Jankovic J, Tolosa E, Barone P, Lang AE, Olanow CW (2010) Initiating levodopa/carbidopa therapy with and without entacapone in early Parkinson disease: the STRIDE-PD study. Ann Neurol 68(1):18–27. https://doi.org/10.1002/ana.22060
Tolosa E, Hernández B, Linazasoro G, López-Lozano JJ, Mir P, Marey J, Kulisevsky J (2014) Efficacy of levodopa/carbidopa/entacapone versus levodopa/carbidopa in patients with early Parkinson’s disease experiencing early wearing-off: a randomised, double-blind trial. Journal of neural transmission (vienna, austria : 1996) 121(4):357–366. https://doi.org/10.1007/s00702-013-1114-x
Brooks DJ, Agid Y, Eggert K, Widner H, Ostergaard K, Holopainen A (2005) Treatment of end-of-dose wearing-off in parkinson’s disease: stalevo (levodopa/carbidopa/entacapone) and levodopa/DDCI given in combination with Comtess/Comtan (entacapone) provide equivalent improvements in symptom control superior to that of traditional levodopa/DDCI treatment. Eur Neurol 53(4):197–202. https://doi.org/10.1159/000086479
Larsen JP, Worm-Petersen J, Siden A, Gordin A, Reinikainen K, Leinonen M (2003) The tolerability and efficacy of entacapone over 3 years in patients with Parkinson’s disease. Eur J Neurol 10(2):137–146
Myllyla VV, Kultalahti ER, Haapaniemi H, Leinonen M (2001) Twelve-month safety of entacapone in patients with Parkinson’s disease. Eur J Neurol 8(1):53–60
Poewe WH, Deuschl G, Gordin A, Kultalahti ER, Leinonen M (2002) Efficacy and safety of entacapone in Parkinson’s disease patients with suboptimal levodopa response: a 6-month randomized placebo-controlled double-blind study in Germany and Austria (Celomen study). Acta Neurol Scand 105(4):245–255
Kuoppamaki M, Vahteristo M, Ellmen J, Kieburtz K (2014) Pooled analysis of phase III with entacapone in Parkinson’s disease. Acta Neurol Scand 130(4):239–247. https://doi.org/10.1111/ane.12278
Yi ZM, Qiu TT, Zhang Y, Liu N, Zhai SD (2018) Levodopa/carbidopa/entacapone versus levodopa/dopa-decarboxyiase inhibitor for the treatment of Parkinson’s disease: systematic review, meta-analysis, and economic evaluation. Ther Clin Risk Manag 14:709–719. https://doi.org/10.2147/tcrm.S163190
Rosendaal FR, Reitsma PH (2014) Meta-analysis. J Thromb Haemost 12(7):1009. https://doi.org/10.1111/jth.12616
Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J et al (2011) The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis—a simulation study. PLoS One 6(10):e25491. https://doi.org/10.1371/journal.pone.0025491
Funding
This study is supported by the Sichuan Science and Technology Program (No. 18PTDJ0117 and 2018ZDYF0569).
Author information
Authors and Affiliations
Contributions
Liao Xiaoli, Wu Nianyue, and Liu Dongfeng contributed equally. Data for each study were extracted independently by two researchers (Wu and Liu). If there is any ambiguity, consult a third investigator (Liao). Similarly, three researchers made similar contributions to the writing of the manuscript. Wu and Liu completed the preliminary manuscript. Liao is responsible for the final revision.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, X., Wu, N., Liu, D. et al. Levodopa/carbidopa/entacapone for the treatment of early Parkinson’s disease: a meta-analysis. Neurol Sci 41, 2045–2054 (2020). https://doi.org/10.1007/s10072-020-04303-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04303-x