Abstract
To assess the long-term use of l-dopa alone vs l-dopa-sparing therapy, as initial treatment, provides the most efficient long-term control of symptoms and best quality of life for people with early Parkinson’s disease (PD). PubMed; Google scholar; Cochrane Central Register of Controlled Trials and the Web of Science were searched for randomised, placebo-controlled trials (RCTs) on l-dopa alone and l-dopa sparing as initial treatment in early PD patients. We used a random effects model rather than a fixed effects model because of this takes into account heterogeneity between multi-studies. Eleven RCTs were included. The results showed that l-dopa alone could evidently improve the UPDRS part I (p = 0.005), part II (p < 0.0001), part III (p < 0.0001) and UPDRS total score (p = 0.004) compared with l-dopa-sparing therapy in PD patients. Meanwhile, a reduced risk of dyskinesia (p < 0.0001, RR = 1.88, 95 % CI 1. 37–2.59) and wearing-off phenomenon (p < 0.00001, RR = 1.36, 95 % CI 1. 20–1.55) in patients treated initially with l-dopa-sparing therapy compared to l-dopa has been consistently reported. What is more, we found more patients on al-dopa-sparing therapy were more than triple as likely to discontinue treatment prematurely due to adverse events than l-dopa treatment patients (43.7 vs 15.8 %). l-Dopa alone is the most effective medication available for treating the motor symptoms of PD patients, despite the greater incidence of involuntary movements. Meanwhile, more patients on dopamine agonists or MAOBI were more likely to discontinue treatment prematurely than l-dopa alone treatment patients within the long follow-up period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is a progressive disorder affecting over six million people worldwide, making it the most common neurodegenerative disease after Alzheimer’s disease [1]. Clinical symptoms of PD generally occur when at least 50 % of the dopamine neurons within the substantia nigra have died, thus implying the existence of a relatively long preclinical period during which several disease-induced compensatory changes are active [2]. Of the classical classes of drug widely used as initial therapy, levodopa (l-dopa) achieves somewhat better control of motor symptoms of PD than do dopamine agonists or monoamine oxidase type B inhibitors (MAOBI) [3], but long-term l-dopa use is associated with the development of motor complications such as dyskinesia, ‘wearing-off’ phenomenon and unpredictable ‘on–off’ fluctuations [4]. Motor complications are seen less frequently with dopamine agonists or MAOBI than with l-dopa, suggesting that longer term symptomatic control could be better with l-dopa-sparing therapy than with l-dopa [5]. However, non-motor side effects such as nausea, hallucinations, edema, and sleep disturbance are more common with dopamine agonists than with l-dopa, and could be more important for patients and caregivers than are motor complications [6].
Following this, attention focused on the possibility of using l-dopa-sparing therapy (dopamine agonists or MAOBI) as initial treatment for PD. These treatment strategies would allow either a delayed start or lower dose of l-dopa, thus potentially preventing or delaying the onset of the late complications of l-dopa therapy [7]. In recent years, a major shift in this treatment approach is occurring, on the basis of results of longer follow-up of earlier trials and recognition of important differences in side effects condition [8]. Rascol et al. showed early PD patients can be managed successfully for up to 5 years with a reduced risk of dyskinesia by initiating treatment with dopamine agonists alone and supplementing it with l-dopa if necessary [9]. However, PD MED trial reported very small but persistent benefits are shown for patient-rated mobility scores when treatment was initiated with l-dopa compared with dopamine agonists or MAOBI [10]. The overall balance of benefits and risks favours l-dopa over l-dopa-sparing therapy with better patient-rated quality of life both in the short and long time [10]. On the contrary, PELMOPET study demonstrated both l-dopa and dopamine agonists seem to be suitable options as initial PD therapy. The choice remains with the treating physician based on the different efficacy and adverse event profiles [11].
Despite of numerous studies have been conducted to determine the initial treatment for early PD patients. Uncertainty remains regarding the comparative balance of risks and benefits of initiation of treatment with these different classes of drugs. In this scenario, this review aims to pool all the relevant trials and assess whether l-dopa alone or l-dopa-sparing therapy should be used as first-line treatment in patients with early PD. Therefore, we undertook a meta-analysis of data from all published randomised controlled trials (RCTs) comparing l-dopa alone with l-dopa-sparing therapy to quantify more reliably the benefits and risks of these two kinds of drug in early PD patients.
Methods
Literature search
We electronically searched databases of PubMed, Google scholar, Cochrane Central Register of Controlled Trials (CENTRAL) and the Web of Science. We identified completed trials by searching the Meta register of Controlled Clinical Trials (mRCT). The publication time is from January 1990 of each database up to August 2014 for all English language publications. The search terms were ‘‘levodopa”, “l-dopa”, “Parkinson”, or “Parkinson’s disease”. Reference lists from the resulting publications and reviews were used to identify further relevant publications.
Inclusion and exclusion criteria
Types of studies
We included all truly randomised, properly concealed, controlled trials (RCTs) comparing l-dopa alone with l-dopa-sparing therapy (mainly dopamine agonists or MAOBI) in early PD patients. Studies in which the method of randomisation or concealment was unknown were included. Cross-over studies were excluded.
Types of participants
Patients with early PD entered into the relevant RCTs. Early disease was defined as idiopathic PD (Hoehn–Yahr stage 3 or less), with no history of motor complications, either untreated or with limited (generally less than 6 months) exposure to anti-parkinsonian medication. There were no age and gender restrictions.
Types of interventions
We included long-term (treatment and follow-up at least 1 year) trials comparing any dose of l-dopa alone with l-dopa-sparing therapy (currently dopamine agonists or MAOBI). Other treatments had to be the same in both arms. We included trials in which additional l-dopa or dopamine agonists were introduced to either arm according to clinical need as the disease progressed. Trials of less than 1 year duration were excluded because we were interested in the long-term effects of treatment rather than short-term symptomatic effects.
Types of outcome measures
Data extracted included clinician-rated disability scales, for example. unified Parkinson’s disease rating scale (UPDRS), Parkinson’s disease questionnaire (PDQ-39), EuroQol (EQ-5D), motor complications (dyskinesia, wearing-off phenomenon and on–off fluctuation) and withdrawals rate between two groups.
Data extraction
Data from each study was extracted independently by two authors. For each study, information was carefully extracted from all eligible studies, including first author, year of publication, number of subjects, sex ratio, mean age of subjects, diagnostic criteria of PD; treatment schedule (including dose, duration), outcomes reported. If more than one paper with the same study was found, the one that contained the more detailed was reviewed. If outcomes were presented from the studies at different time points, we extracted data from the last time point. Disagreements between authors on the eligibility of studies were discussed with another specialized investigator to resolve the dispute.
Quality assessment of RCTs
The methodological quality of RCTs was assessed independently using the Cochrane Handbook for Systematic Reviews of Interventions. Two investigators independently evaluated the methodological quality of the included studies. Disagreements were resolved through consensus or discussed with a third author.
Statistical analysis
We combined results of each trial using standard meta-analytic methods to estimate an overall treatment effect for l-dopa alone vs l-dopa-sparing treated patients. For event data (such as UPDRS) was considered as continuous data, and then an estimate of the combined effect sizes utilizing weighted mean difference (WMD) was given and its standard error, with 95 % confidence interval. WMD is a standard statistic that measures the absolute difference between the mean values in two groups. It estimates the amount by which the experimental intervention changes the outcome on average compared with the control. It can be used as a summary statistic in meta-analysis when outcome measurements in all studies are made on the same scale. On the contrary, standardised mean difference (SMD) is used as a summary statistic in meta-analysis when the studies all assess the same outcome but measure it in a variety of ways [12]. Meanwhile, for event data (such as motor complications) was considered as dichotomous data, and then were given an estimate of the combined effect sizes utilizing relative risk (RR) with a random effects model. We used a random effects model rather than a fixed effects model because of this takes into account heterogeneity between multi-studies. Difference between n groups was assessed by partitioning heterogeneity and using the χ 2 distribution with n-1 degrees of freedom (df), where n equals the number of groups. Publication bias was assessed by visual inspection of a funnel plot. A very common and simple version of the meta-analysis procedure is commonly referred to as the inverse variance method. The inverse variance method is so named because the weight given to each study is chosen to be the inverse of the variance of the effect estimate. All analyses were performed with Revman version 5.1. Probability value p < 0.05 was considered significant.
Result
Study inclusion
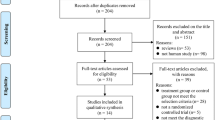
We identified 481 references, from which we excluded 273 due to the duplicates. After screening the titles and abstracts, 141 were excluded because they failed to meet the inclusion criteria. By reading the full text of the remaining 67 articles, 29 studies were excluded because a result of without control groups, 24 were excluded because of not testing the effect of comparing l-dopa alone with l-dopa-sparing therapy in early PD patients, three studies were eliminated due to short time follow-up. Ultimately, just leaving 11 qualified studies satisfied the pre-established inclusion criteria [10, 13–22] (Fig. 1).
Description of studies
Eleven RCTs, with a total of 3584 patients, met the inclusion criteria and were included in this review. Of whom, 1594 were randomised to l-dopa alone groups (62.4 % males), and 1990 were randomised to l-dopa-sparing groups (64.4 % males). Among them, seven studies adopted the UK Brain Bank Diagnostic Criteria of idiopathic PD disease; the remaining five studies were restricted to those with a clinical diagnosis of PD. The mean age of those included studies was relatively young at about 60 years, most were in Hoehn and Yahr stage I or II and there were slightly more males than females. The number of participants included in this meta-analysis ranged from 35 [22] to 1406 [10] subjects. Meanwhile, the time of follow-up was ranged from 1 year [22] to 10 years [19]. l-Dopa-sparing in this study including dopamine agonists or MAOBI, but only one trial used MAOBI [10], the remaining trials adopted dopamine agonists. In which, pramipexole was administrated in five trials (5/11, 45.4 %), cabergoline was used in three trials (3/11, 27.2 %), ropinirole and bromocriptine as the intervention therapy in two and one trial, respectively. In terms of outcome measure, UPDRS as the outcome measure was observed in nine studies, the motor complications (at least one type of dyskinesia, wearing-off phenomenon and on–off fluctuation) were also observed in nine studies, withdrawal rate was reported in all studies. PDQ-39 and EQ-5D were reported as outcome measure in five and four studies, respectively. The basic characteristics of the 11 selected studies are summarized in Table 1.
Study quality
Table 2 showed the methodological quality of the included trials. All trials described the method of randomisation used (e.g. random number table, computer generated). Nine trials gave information that allowed the assessment of whether an adequate concealment of allocation procedure was used as well as reported the blinding of participants. All trials described intention-to-treat analyses (ITT) and reported follow-up data. Therefore, all of the included trials were deemed to have a low risk of bias.
Updrs
UPDRS part I data were available from three trials of l-dopa alone compared with l-dopa-sparing therapy. We pooled the whole data to process and found significant difference when l-dopa alone compared to l-dopa-sparing (p = 0.005, WMD = −0.30, 95 % CI −0.51 to −0.09; heterogeneity test: τ 2 = 0.00, χ 2 = 1.74, p = 0.42, I 2 = 0 %, Fig. 2a). Six trials reported significant effect of l-dopa alone for reducing UPDRS part II compared with l-dopa-sparing (p < 0.0001, WMD = 0.95, 95 % CI 0.51–1.39; heterogeneity test: τ 2 = 0.00, χ 2 = 3.41, p = 0.64, I 2 = 0 %, Fig. 2b). Two studies were excluded for pool analysis due to the data represented in the form of graphically [13, 22]. We did not have access to obtain the changes in UPDRS scores between baseline and at the end of follow-up. Data on the clinical associated UPDRS part III score were available from seven trials with 1320 subjects included. The result showed the overall effects of l-dopa alone were more effective than l-dopa-sparing treatment (p < 0.0001, WMD = 2.89, 95 % CI 1.56–4.21; heterogeneity test: τ 2 = 1.41, χ 2 = 11.43, p = 0.08, I 2 = 47 %, Fig. 2c). In terms of UPDRS total score, four trials reported significant effects of l-dopa alone for reducing the score compared with l-dopa-sparing (p = 0.004, WMD = 3.33, 95 % CI 1. 04–5.61; heterogeneity test: τ 2 = 2.11, χ 2 = 4.91, p = 0.18, I 2 = 39 %, Fig. 2d). Meanwhile, all the results showed homogeneity by heterogeneity test. The funnel plot was roughly symmetric for the effects of l-dopa on UPDRS score, which did not suggest an obvious publication bias (Fig. 3).
Motor complications
There were nine trials reported the motor complications as the outcome measure with 3269 subjects included in the analysis, with fewer motor complications in the l-dopa-sparing arm than the l-dopa alone arm (24.4 vs 33.7 %, p < 0.0001, RR = 1.53, 95 % CI 1. 25–1.87; heterogeneity test τ 2 = 0.05, χ 2 = 28.29, p = 0.0002, I 2 = 75 %, Fig. 4a). Meanwhile, dyskinesia was reported by eight trials, the result showed significant difference in the incidence of dyskinesia between the l-dopa alone and l-dopa-sparing (p < 0.0001, RR = 1.88, 95 % CI 1. 37–2.59; heterogeneity test τ 2 = 0.17, χ 2 = 46.20, p < 0.00001, I 2 = 83 %, Fig. 4b). A 11.6 % reduction in wearing-off phenomenon occurred in patients randomised to l-dopa-sparing arm (41.2 vs 29.6 %, p < 0.00001, RR = 1.36, 95 % CI 1. 20–1.55, Fig. 4b). However, we found no difference in the incidence of on–off fluctuation between the l-dopa alone and l-dopa-sparing (6.5 % vs 3.1 %, p = 0.19, RR = 2.07, 95 % CI 0.70–6.16, Fig. 4b).
Withdrawal rates
In this study, there were significantly more withdrawals amongst those in the l-dopa-sparing arm than in those in the l-dopa alone arm (43.7 vs 15.8 %, p < 0.0001, RR = 0.51, 95 % CI 0.28–0.95, Fig. 5), largely because of lack of efficacy or intolerable side effects with dopamine agonists or MAOBI. Nevertheless, there was severe heterogeneity for the analysis of withdrawals between studies (τ 2 = 0.94, χ 2 = 199.69, p < 0.00001, I 2 = 95 %, Fig. 5). Consequently, we should interpret the pool result prudently. What is more, non-motor side effects were more frequent among participants randomised to l-dopa-sparing arm compared to l-dopa alone, with clinically significant increases in the risk of edema, somnolence, constipation et al. (data were not showed).
PDQ-39 and EQ-5D
There were five trials reported PDQ-39 as outcome measure, among them, three trials showed no significant difference between trials (p > 0.05, Fig. 6a) except two trials. The PD MED trial [10] showed PDQ-39 scores averaged 1.8 points better in patients randomly assigned to l-dopa than those assigned to l-dopa-sparing therapy (p < 0.05). In addition, Hollowy et al. [2] reported PDQ-39 improved in both groups initially and then declined over time. At end point, the mean change score were significantly different between l-dopa alone and l-dopa sparing (p = 0.006). The remaining three trials showed no significance difference between the l-dopa alone and l-dopa-sparing at any follow-up assessment (p > 0.05). In terms of EQ-5D, data were available from only four trials. However, we found no difference between l-dopa alone arm and l-dopa-sparing arm (p > 0.05, Fig. 6b) except one trial. Meanwhile, the time of follow-up was ranged from 1 year [22] to 10 years [19] with a median follow-up of 4.3 years (Fig. 6c).
Discussion
Summary of evidence
It is a systematic review and meta-analysis to assess l-dopa alone compared with l-dopa-sparing therapy as initial treatment for PD and the most comprehensive in the range of subjects included. It provides the most reliable available summary of the current evidence from clinical trials of l-dopa alone and l-dopa-sparing in the treatment of early PD and clarifies previous uncertainties about the role of these drugs which is better to be chosen as initial drugs. Our meta-analysis found that l-dopa alone could evidently improve the UPDRS part I, part II, part III and UPDRS total score compared with l-dopa-sparing therapy in PD patients. There was also a significant difference in withdrawal rates reduction by the l-dopa therapy. Therefore, it was no question that l-dopa therapy was beneficial in the treatment of PD patients. However, this meta-analysis once again confirmed reports from individual trials that patients with early PD treated with a policy of initial dopamine agonist therapy, are less likely to develop motor complications than l-dopa-treated patients. An obvious reduced risk of dyskinesia and wearing-off phenomenon in patients treated initially with a dopamine agonist compared to l-dopa has been consistently reported. In addition, the crucial question we should highlight is that sometimes non-motor side effects could be more important for patients and caregivers than are motor complications. This was the main reason more patients on l-dopa-sparing therapy were more than triple as likely to discontinue treatment prematurely due to adverse events than l-dopa treatment patients, suggesting that the side effects were of sufficient severity to have a profound impact on patients’ quality of life and were perhaps at least as clinically important as the motor complications [23]. In terms of PDQ-39 and EQ-5D, we found similar effects between l-dopa alone arm and l-dopa-sparing arm.
Interpretation of the results
l-Dopa provides the greatest symptomatic benefit for PD and is associated with less somnolence, edema, freezing, hallucinations, and risk of impulse control disorders than dopamine agonists [24]. However, dopamine agonists are also efficacious in early PD and are less likely than l-dopa to cause dopaminergic motor complications, particularly dyskinesia [24]. Therefore, consistent with previous results, in this paper we found l-dopa could significantly improve the UPDRS score compared with dopamine agonists in PD patients. Meanwhile, a reduced risk of motor complications in patients treated initially with a l-dopa-sparing therapy (mainly dopamine agonist) compared to l-dopa alone has been consistently reported. Nevertheless, to date, dopamine agonists are usually introduced as initial treatment for patients younger than 60 years [25]. However, there is increasing evidence in open-label, observational, naturalistic follow-up studies that the early advantage of dopamine agonists over l-dopa diminishes over time [26]. This was the main reason in these long follow-up (median 4.3 years) RCTs more patients on dopamine agonists or MAOBI were more likely to discontinue treatment prematurely than l-dopa treatment patients. Consequently, it is important that cost effectiveness, as well as clinical effectiveness and safety are assessed in the future studies.
Limitations
Several limitations of this study should be considered. First, a large proportion of the studies included in this review are of l-dopa vs dopamine agonists, only one trial aim to investigate the difference effect between l-dopa compared with MAOBI. Consequently, further large, well-designed RCTs that evaluate the long-term balance of benefit and harm, comparing l-dopa with MAOBI is urgently needed. Second, all the trials were carried out in Europe and US, suggesting that there are insufficient data on the Asian and Africa. In such a worldwide disease as PD, it would inevitably be valuable to know whether the initial treatment is discrepancy in different regions. Third, this meta-analysis also highlighted the lack of data on the long-term balance of benefits and risks of l-dopa vs l-dopa-sparing therapy, as well as inadequate patient-rated quality of life data, limited the interpretation and applicability of these results. Finally, few participants were younger than 60 years at this meta-analysis, particularly in the l-dopa vs l-dopa-sparing comparison. Therefore, the study provided little direct evidence for how such patients should be treated result in unable to offer definite age-specific treatment recommendations. In spite of these limitations, our meta-analysis also had possessed some advantages. First, all of the included trials were well designed and deemed to have a low risk of bias and provided hopefully evidences. Second, the long follow-up period between initiation and availability of the results provided the comprehensive information between l-dopa and l-dopa-sparing therapy.
Implications for future research
The treatment of PD aims to limit the gradually increasing amount of disability. In this regard, the most effective strategy is the treatment with l-dopa, which improves some of the PD symptoms. However, for several years of l-dopa treatment, the number of patients that will develop motor complications increases. These complications contribute to an additional disease burden and are source of increased medical care. Therefore, the efficacy of l-dopa-sparing (presently dopamine agonists or MAOBI) treatment for delaying the onset of motor complications is being concerned. According to our meta-analysis, a number of implications for research arise from this paper. First, the balance of risks and beets between two groups remain unclear, as few trials the overall quality of life and cost effectiveness as outcome measures. Patient-rated quality of life outcomes measures, which assess all aspects of the patient’s life is an urgent need in the future trials [27]. What is more, there is also a need for better evidence on the cost effectiveness of dopamine agonists and l-dopa, which have previously become widely used as initial treatment for PD. Consequently, future RCTs should highlight this issue as the main investigate target. Second, in this meta-analysis, research has concentrated on comparing dopamine agonists with l-dopa, with few trials having directly compared the l-dopa with MAOBI, therefore, it is essential that this contrast should evaluate in the future. Finally, we included long-term trials comparing l-dopa alone with dopamine agonists or MAOBI, so the difference in outcomes between those receiving initial combination l-dopa and dopamine agonists or MAOBI vs initial therapy with l-dopa or dopamine agonists or MAOBI is unclear. All these issues should be confirmed by the future of RCTs.
Conclusion
The current meta-analysis provides evidence that l-dopa alone is the most effective medication available for treating the motor symptoms of PD patients, despite the greater incidence of involuntary movements. Meanwhile, more patients on dopamine agonists or MAOBI were more likely to discontinue treatment prematurely than l-dopa treatment patients within the long follow-up period, suggesting that lack of efficacy or non-motor side effects appear at least as clinically important as the motor complications. Whatever, further large sample size and rigorously designed RCTs are still necessary and are indeed underway to confirm these trends.
References
Marsh L, Dawson TM (2000) Treatment of early Parkinson’s disease. BMJ 321(7252):1–2
Brotchie J, Fitzer-Attas C (2009) Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology 72(7 Suppl):S32–S38
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311(16):1670–1683
Fahn S, Oakes D, Shoulson I et al (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351(24):2498–2508
Stowe RL, Ives NJ, Clarke C, et al. (2008) Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev (2):CD006564. doi:10.1002/14651858.CD006564
Stern MB (2004) Dopamine agonists modify the course of Parkinson disease. Arch Neurol 61(12):1969–1971
Utsumi H, Okuma Y, Kano O et al (2013) Evaluation of the efficacy of pramipexole for treating levodopa-induced dyskinesia in patients with Parkinson’s disease. Intern Med 52(3):325–332
Lang AE, Marras C (2014) Initiating dopaminergic treatment in Parkinson’s disease. Lancet 384(9949):1164–1166
Rascol O, Brooks DJ, Korczyn AD et al (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342(20):1484–1491
Pd Med Collaborative Group (2014) Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet 384(9949):1196–1205
Oertel WH, Wolters E, Sampaio C et al (2006) Pergolide vs levodopa monotherapy in early Parkinson’s disease patients: the PELMOPET study. Mov Disord 21(3):343–353
Vesterinen HM, Sena ES, Egan KJ et al (2014) Meta-analysis of data from animal studies: a practical guide. J Neurosci Methods 221:92–102
Rinne UK, Bracco F, Chouza C et al (1998) Early treatment of Parkinson’s disease with cabergoline delays the onset of motor complications. Results of a double-blind levodopa controlled trial. The PKDS009 Study Group. Drugs 55(Suppl 1):23–30
Parkinson Study Group (2000) Pramipexole vs levodopa as initial treatment for Parkinson disease: a randomized controlled trial. JAMA 284(15):1931–1938
Rascol O, Brooks DJ, Korczyn AD et al (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study Group. N Engl J Med 342(20):1484–1491
Caraceni T, Musicco M (2001) Levodopa or dopamine agonists, or deprenyl as initial treatment for Parkinson’s disease. A randomized multicenter study. Parkinsonism Relat Disord 7(2):107–114
Parkinson Study Group (2002) Dopamine transporter brain imaging to assess the effects of pramipexole vs levodopa on Parkinson disease progression. JAMA 287(13):1653–1661
Holloway RG, Shoulson I, Fahn S et al (2004) Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61(7):1044–1053
Hauser RA, Rascol O, Korczyn AD et al (2007) Ten-year follow-up of Parkinson’s disease patients randomized to initial therapy with ropinirole or levodopa. Mov Disord 22(16):2409–2417
Parkinson Study Group CALM Cohort Investigators (2009) Long-term effect of initiating pramipexole vs levodopa in early Parkinson disease. Arch Neurol 66(5):563–570
Utsumi H, Cabergoline as the Starting Treatment and its Long-term Effects (CASTLE) Study Group (2012) Long-term effects of cabergoline and levodopa in Japanese patients with early Parkinson’s disease: a 5-year prospective study. Acta Med Okayama 66(2):163–170
Storch A, Wolz M, Beuthien-Baumann B et al (2013) Effects of dopaminergic treatment on striatal dopamine turnover in de novo Parkinson disease. Neurology 80(19):1754–1761
Oguh O, Kwasny M, Carter J et al (2013) Caregiver strain in Parkinson’s disease: national Parkinson Foundation Quality Initiative study. Parkinsonism Relat Disord 19(11):975–979
Ferreira JJ, Katzenschlager R, Bloem BR et al (2013) Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur J Neurol 20(1):5–15
Olanow CW, Schapira AH (2013) Therapeutic prospects for Parkinson disease. Ann Neurol 74(3):337–347
Katzenschlager R, Head J, Schrag A et al (2008) Fourteen-year final report of the randomized PDRG-UK trial comparing three initial treatments in PD. Neurology 71(7):474–480
Parashos SA, Luo S, Biglan KM et al (2014) Measuring disease progression in early Parkinson disease: the National Institutes of Health Exploratory Trials in Parkinson Disease (NET-PD) experience. JAMA Neurol 71(6):710–716
Acknowledgments
We gratefully acknowledge Dr. Jie Chen for his help in guiding and revising the manuscript. We also thank all the study participants. The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declared that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
C. Xie and Y.-Y. Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xie, Cl., Zhang, YY., Wang, XD. et al. Levodopa alone compared with levodopa-sparing therapy as initial treatment for Parkinson’s disease: a meta-analysis. Neurol Sci 36, 1319–1329 (2015). https://doi.org/10.1007/s10072-015-2253-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2253-7