Abstract
Astrocytic changes have been demonstrated in several neurodegenerative diseases, showing that these cells play an important role in functional recovery/maintenance against brain damage. Physical exercise is known to contribute to this process; however, the cellular mechanisms involved are not fully understood. This study investigated the effects of physical exercise on motor deficits and the expression of glial fibrillary acidic protein (GFAP) in a model of Parkinson’s disease (PD). Rats were divided into four groups: sham sedentary (SS) and sham trained (ST); lesioned sedentary (LS) and lesioned trained (LT). 6-OHDA was infused unilaterally into the medial forebrain bundle. Behavioral tasks were applied to evaluate motor abilities. Tyrosine hydroxylase (TH—in substantia nigra) and GFAP (in striatum) immunoreactivities (ir) were semi-quantified using optical density. The animals submitted to treadmill training completed fewer pharmacological-induced rotations when compared with sedentary animals and they also showed ameliorated motor impairments. Interestingly, although no change in TH-ir, the exercise led to restored striatal GFAP expression in the LT group while there was no effect in the ST group. This study is the first study to show data indicating the recovery of GFAP expression post-exercise in this model and further research is necessary to determine the precise action mechanisms of exercise on astrocytes in the PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Idiopathic Parkinson’s disease (PD), the second most common neurodegenerative disorder, usually manifests itself in the fifth or sixth decade of life and affects 1% of the population above the age of 65 [1]. PD is characterized by disabling motor abnormalities, such as rest tremor, muscle stiffness, paucity of voluntary movements, and postural instability [2]. Its primary neuropathological feature is the specific loss of the nigrostriatal dopaminergic neurons, whose cell bodies reside in the substantia nigra pars compacta (SNpc) and nerve terminals project to the striatum [2].
Although dopaminergic neurons are the first cells affected in PD, the involvement of astrocytes in the pathogenesis of this neurodegenerative processes has been suggested. The glial reaction, which is found in PD patients as well as in experimental models of PD, indicates its involvement in the pathophysiology of the disease [3–5]. Astrocytic activation is characterized by an increased expression of glial fibrillary acidic protein (GFAP), enlarged cell body, and projections in the injured area [6].
6-hydroxydopamine (6-OHDA) has been predominantly used to produce unilateral lesions that cause an asymmetric and quantifiable motor behavior (circling or rotational behavior) [7]. Other behavioral deficits, such as paw reach, asymmetry use of forelimbs, akinesia, bradykinesia, stepping and sensory neglect tasks, also occur in this model [8–10]. Beside the motor behavior, another common way to evaluate the degree of dopaminergic neurodegeneration is by immunohistochemical detection for tyrosine hydroxylase (TH), because TH is the limiting enzyme for dopamine synthesis and is clearly decreased after dopaminergic toxic insults [11].
Furthermore, a meta-analysis demonstrated that exercise might improve physical functions, health-related quality of life, strength, balance, and gait speed of PD patients [12]. In many animal models of PD, different kinds of exercise have been shown to induce neuroprotection and to produce beneficial effects on motor behavioral as well as on neurochemical deficits in the dopaminergic system [8, 13–16].
Due the involvement of astrocytes in neurodegenerative diseases, the main objective of this study was to investigate the effects of exercise on the GFAP expression in a model of PD and also to verify such effects on motor evaluations well established.
Methods
Animals
Male 90-day-old Wistar rats from a local breeding colony (ICBS, Universidade Federal do Rio Grande do Sul, Brazil) were housed in standard conditions. All the procedures were approved by the Ethics Committee at the Federal University of Rio Grande do Sul and all animal experiments were carried out in accordance with the Principles of Laboratory Animal Care (NIH publication no. 86-23 revised 1985).
The animals were randomly divided into four groups: (1) sham sedentary (SS, n = 7); (2) sham trained (ST, n = 8); (3) lesioned sedentary (LS, n = 8), and lesioned trained (LT, n = 8). All efforts were made to minimize the number of animals used and their suffering.
Stereotaxic surgery
Rats weighing 300–350 g at the time of surgery (Day 0) were anesthetized with equithesin i.p. (pentobarbital, 25 mg/kg; chloral hydrate, 150 mg/kg) followed by atropine sulfate (0.1 mg/kg i.p.) prior to being placed in the stereotaxic apparatus. 6-OHDA hydrobromide (10 μg) was dissolved in 3 μL of vehicle solution (0.9% sterile saline containing 0.02% ascorbic acid), and infused unilaterally (0.5 μL/min) into the medial forebrain bundle (3.3 mm posterior and 1.8 mm to bregma, and 8.1 mm ventral to dura-mater) [17] using a syringe micro-pump connected to a 10-μL Hamilton syringe. Control-operated rats (sham) received the vehicle solution alone (3 μL).
Maximal exercise test
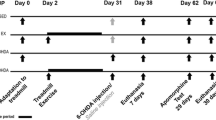
The maximal exercise test (MET) was used to determine the maximal exercise capacity (MEC). Three days prior to surgery (pre-lesion), all animals were adapted on the treadmill during 10 min at 5 m/min. Two days after the surgery (Day 2) they were submitted to the MET. The test consisted of a graded exercise on the treadmill, with speed increments of 5 m/min every 3 min, starting at 5 m/min and continuing up to the MEC of each rat [18]. The values attained in the MET were used to plan the treadmill training program. A new MET was repeated at the end of treadmill training (Day 31).
Treadmill training
This training program began 3 days (Day 3) after the surgery and consisted of running on the treadmill for 20 min on the first day; this period was progressively increased every day up to 50 min on the fifth day and 60 min in the next 3 weeks. This was repeated in five sessions per week, once a day during 4 weeks [19].
Drug-induced rotation
Animals were injected with methylphenidate (40 mg/kg, i.p.) 22 days after the surgery (Day 22). The number of ipsilateral rotations to the lesioned side was determined in 30 min [20].
Narrow beam test
The beam was suspended 80 cm from the ground by wooden supports at either end. The wooden supports at the “starting” end of the beam formed a sheer drop while a platform was located at the other end. A line was drawn 20 cm from the start end of the beam. During a test the rat was entirely placed within this 20-cm starting zone and a stopwatch immediately started upon release of the animal. The time was recorded when the animal placed a weight-bearing step entirely over the start line and represented the latency to begin the task. The total time taken to cross the beam was also recorded. A testing session consisted of three trials on the beam [10]. This evaluation was conducted 2 days before the lesion (pre-lesion), and on the 8th and 29th days after the lesion.
Immunohistochemistry
After the end of the experimental period (Day 32), the rats were anesthetized with thiopental (200 mg/kg, i.p.), injected with 1,000 IU heparin, and transcardially perfused with 150 mL of saline solution, followed by 150 mL of 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) at room temperature. After perfusion the brains were removed, post-fixed in the same fixative solution for 4 h, and cryoprotected in 30% sucrose solution in PB at 4°C. The brains were then frozen by immersion in isopentane followed by immersion in nitrogen and stored in a freezer (−70°C) for later analyses.
Serial coronal sections (40 μm) were obtained using a cryostat. For immunohistochemistry, the free-floating sections were pretreated in 3% hydrogen peroxidase (H2O2) for 30 min, washed and blocked with 2% bovine serum albumin in PBS containing 0.3% Triton X-100 (PBS-Tx) for 30 min, and incubated with the chosen antibodies (TH or GFAP).
For TH immunohistochemistry, tissue sections containing the SNpc were incubated in a monoclonal TH antibody conjugated with Peroxidase Anti-Peroxidase (PAP) Complex produced in mouse (1:750; Sigma, USA), diluted in PBS-Tx for 48 h at 4°C. After washing several times, sections were incubated in an Anti-Mouse IgG antibody produced in rabbit (1:200; Sigma, USA), diluted in PBS-Tx at room temperature for 2 h.
In addition, for GFAP immunohistochemistry, sections of dorsal striatum were incubated in polyclonal anti-GFAP from rabbit (1:150; Sigma, USA) and diluted in PBS–Tx for 48 h at 4°C. After washing, tissue sections were incubated in a rabbit PAP-conjugated anti-rabbit IgG (1:150; Sigma, USA), diluted in PBS at room temperature for 2 h. Immunohistochemical reactions were revealed by incubating the sections in a histochemical medium that contained 0.06% 3,3-diaminobenzidine (DAB) dissolved in PBS for 10 min and then, in the same solution containing 1 μM of 3% H2O2 per mm of DAB medium for approximately 10 min. Afterwards, the sections were rinsed in PBS, dehydrated in ethanol, cleared with xylene and covered with synthetic balsam and coverslips.
Image analysis
From each rat, ten images of the lesioned side (left hemisphere, 200×) of each SNpc and dorsal striatum were obtained with Nikon E600 light microscope coupled to a USB 2.0 Digital Camera Eyepiece. Images were processed and analyzed with Image J 1.40 software (Wayne Rasband, National Institute of Health, USA). Briefly, RGB (24-bits) color images (640 × 480 pixels) were converted to 8-bit grayscale images (0–255 gray levels). All lighting conditions and magnifications were held constant and the investigator was unaware of the experimental groups. A reference image of an empty field was recorded and Image J’s Calculator Plus Plugin ‘divide’ operation was used for correction of unequal illumination (shading correction).
Rectangular areas of interest (AOI) were set for the images of the SNpc (480 × 220 pixels) and dorsal striatum (450 × 20 pixels). The optical density (OD) of each AOI was measured in the form of uncalibrated OD [10/log(255/255-pixel value)] [21].
Statistical analysis
Data (MEC and evaluated parameters in the narrow beam test) were analyzed using two-way repeated measures analysis of variance (ANOVA). The other data (OD of TH and GFAP and methylphenidate-induced rotation) were analyzed using two-way ANOVA. All analyses were followed by Fisher’s Least Significant Difference (LSD) post hoc test. Data were expressed as mean ± S.E.M. Probability values less than 5% were considered significant. The statistical analyses were made using the Statistica 6.0 software.
Results
The MEC and efficacy of the treadmill training
No difference was found between the groups in the MEC before the treadmill training (Fig. 1). The treadmill training significantly increased the MEC in both trained groups after the exercise period (ST: 28 ± 2.0 m/min; LT: 23 ± 1.3 m/min) when compared with values before the training (ST: 19 ± 2.0 m/min, P = 0.001; LT: 16 ± 1.8 m/min, P = 0.001). Nevertheless, the effects of treadmill training were more robust in the ST (28 ± 2.0 m/min) group when compared with LT group (23 ± 1.3 m/min, P = 0.02). In the LS group, the 6-OHDA lesion had no effect on MEC when the values before (16 ± 1.27 m/min) and after the training (18 ± 1.7 m/min, P = 0.5) were compared.
The MEC before and after the 6-OHDA lesion and treadmill training. Two-way repeated ANOVA revealed significant effects of the factors 6-OHDA lesion [F(1,27) = 9.126; P < 0.005] and exercise [F(1,27) = 7.911; P < 0.009]. All values are expressed as mean ± S.E.M. a (P = 0.001) when comparing the ST and LT groups pre and post-training; b (P = 0.02) when comparing the ST group to the LT group post-training; c (P = 0.02) when comparing the LS group to the LT group post-training
Methylphenidate-induced rotation
The number of ipsilateral rotations in animals submitted to 6-OHDA lesion (LS: 221 ± 25; LT: 145 ± 12) was significantly higher than the respective sham groups (SS: 41 ± 15; ST: 46 ± 10, P = 0.001) (Fig. 2). After 4 weeks of treadmill training the number of rotations in the LT group (145 ± 12) was significantly smaller than the values from the LS group (221 ± 25, P = 0.008).
Methylphenidate-induced rotation 22 days after the 6-OHDA lesion and 20 days after the beginning of exercise. Two-way ANOVA revealed significant effects of the factor 6-OHDA lesion [F(1,27) = 51.898; P < 0.001], with a significant 6-OHDA lesion × exercise interaction [F(1,27) = 4.443; P < 0.04]. All values are expressed as mean ± S.E.M of ipsilateral rotations in 30 min after an injection of methylphenidate (40 mg/kg, i.p.). a (P = 0.001) when comparing the LS and LT groups with the SS and ST groups, respectively; b (P = 0.008) when comparing the LT with the LS group
Narrow beam test
No difference was seen between the groups in the latency before the lesion (pre-lesion), and there was no significant difference between the two sham groups on any evaluation day (Fig. 3). Whereas, on the 8th post-lesion day, the latencies in the LS (4.73 ± 0.7 s) and LT (4.63 ± 0.64 s) groups were significantly higher than the respective pre-lesion values (1.97 ± 0.2; 2.17 ± 0.34 s, P = 0.001) and there was no difference between them (P = 0.9). In addition, on the 29th post-lesion day, the values from both lesioned groups (LS: 6.44 ± 0.5; LT: 3.94 ± 0.4 s) were significantly higher than the pre-lesion values (P = 0.001; P = 0.003, respectively). The value from the LS group on the 29th day was significantly greater than the scores from all other groups. In addition, the LT (3.94 ± 0.4 s) group was not different from the ST (3.19 ± 0.5 s, P = 0.3) group on the 29th post-lesion day.
The latency to begin crossing the beam on the 8th and 29th days after the surgery. Two-way repeated ANOVA revealed significant effects of the factor 6-OHDA lesion [F(1,27) = 22.217; P < 0.001], with a significant 6-OHDA lesion × exercise interaction [F(1,27) = 4.245; P < 0.04]. The data represent the mean ± S.E.M. a (P = 0.001) when comparing the latencies of the same group to pre-lesion; b (P = 0.001) when comparing the LS group on 29th day post-lesion to all the other values
As with the latency values, there was no difference between the groups in the total time to cross the beam before the lesion (pre-lesion) and there was also no significant difference between the two sham groups on any day (Fig. 4). Nevertheless, on the 8th post-lesion day, a significant difference was found between the LS (11.79 ± 0.93 s) and LT (7.42 ± 0.87 s) groups (P = 0.001), as well as when compared with pre-lesion values (4.77 ± 0.61 s, P = 0.001; 4.88 ± 0.61 s P = 0.008, respectively). In addition, there was no difference either in the LS group between the 8th (11.79 ± 0.93 s) and 29th post-lesion days (10.52 ± 0.54 s, P = 0.2) or in the LT group on the 8th (7.42 ± 0.87 s) and 29th post-lesion days (7.88 ± 0.75 s, P = 0.6). On the 29th day, the total time to cross the beam from the LT group (7.88 ± 0.75 s) was significantly lower than the values from the LS group (10.52 ± 0.54 s, P = 0.02).
Total time to cross the beam on 8th and 29th days after the surgery. Two-way repeated ANOVA revealed significant effects of the factors 6-OHDA lesion [F(1,27) = 49.634; P < 0.001] and exercise [F(1,27) = 5.983; P < 0.02], with a significant 6-OHDA lesion × exercise interaction [F(1,27) = 7.904; P < 0.009]. The data represent the mean ± S.E.M. a (P = 0.001) when comparing the total time of the same group with pre-lesion; b (P = 0.001) when comparing the LS and LT groups on 8th day post-lesion; c (P = 0.001) when comparing the LS and LT groups on 29th day post-lesion
Optical densitometry of TH and GFAP
TH-immunoreactive (TH-ir) neurons were found in all the groups; however, the OD scores from the 6-OHDA lesioned animals were considerably lower than the scores from the sham animals. TH-ir was observed in neuronal cell bodies and their processes. Under light microscopy, the TH-ir showed good resolution, allowing precise delineation of the anatomical boundaries of the SNpc (Fig. 5a).
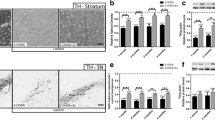
a Digitalized images of coronal sections of substantia nigra showing TH immunoreaction. Sham sedentary (SS); sham trained (ST); lesioned sedentary (LS); lesioned trained (LT); substantia nigra pars compacta (SNpc); substantia nigra pars reticulate (SNpr); ventral tegmental area (VTA). Bar = 300 μm. b Optical density of TH immunoreactivity in SNpc after 6-OHDA lesion and treadmill training. Two-way ANOVA revealed significant effects of the factor 6-OHDA lesion [F(1,27) = 57.259; P < 0.002]. Mean ± S.E.M., a (P = 0.001) when comparing the LS and LT groups with the SS and ST groups, respectively
The OD of the TH-ir neurons from the lesioned side of the SNpc in both lesioned groups (LS: 0.136 ± 0.017; LT: 0.171 ± 0.019) was significantly lower than the OD from the respective sham groups (SS: 0.338 ± 0.02; ST: 0.321 ± 0.02, P = 0.001) (Fig. 5b). The treadmill training did not have any effect on OD of TH-ir in the ST group (0.321 ± 0.02) when compared with SS group (0.338 ± 0.02, P = 0.6). Also, there was no difference in the LT group (0.171 ± 0.019) when compared with the LS group (0.136 ± 0.017, P = 0.3).
GFAP-immunoreactive (GFAP-ir) astrocytes were found in the dorsal striatum in all the experimental groups (Fig. 6a). GFAP-ir was seen in astrocytic cell bodies and their processes. In both lesioned groups the GFAP-ir in the cell body and/or processes were significantly higher than the GFAP-ir from the sham groups and this may be indicative of astroglial reaction. This same GFAP-ir was also found in the sham groups, though only bordering the needle track.
a Digitalized images from the dorsal striatum showing the GFAP-ir in astrocytic cell bodies (arrows) and their processes (thin arrows). Sham sedentary (SS); sham trained (ST); lesioned sedentary (LS); lesioned trained (LT). Bar = 40 μm. b Optical density of GFAP immunoreactivity in dorsal striatum after 6-OHDA lesion and treadmill training. Two-way ANOVA revealed significant effects of the factor 6-OHDA lesion [F(1,27) = 10.826; P < 0.002] with a significant 6-OHDA lesion × exercise interaction [F(1,27) = 4.095; P < 0.04]. Mean ± S.E.M.; a (P = 0.001) when comparing the LS with the SS group; b (P = 0.03) when comparing the LT with the LS group
In the LS group the score (0.083 ± 0.005) obtained for the GFAP-ir OD was significantly higher than the score from the SS group [0.058 ± 0.005; P = 0.001] (Fig. 6b). The OD from the LT (0.068 ± 0.005) group presented no difference when compared with the ST (0.063 ± 0.005) and SS groups (0.058 ± 0.005). However, the value from the LT group (0.068 ± 0.005) was significantly lower than the OD values from the LS group (0.083 ± 0.005, P = 0.03).
Discussion
Effects of 6-OHDA lesion
Experimental models of PD must mimic in animals both the dopaminergic cell loss and the behavioral deficits associated with idiopathic PD [22]. Our results indicate that 6-OHDA infusion was able to lesion the nigrostriatal system as measured by OD of TH, methylphenidate-induced rotation, and also by motor deficit (narrow beam test).
In both lesioned groups the number of ipsilateral rotations was significantly higher than the number of rotations from the sham groups. When animals are unilaterally lesioned by 6-OHDA, they present rotational activity after administration of a dopamine-acting drug, and this rotational behavior is usually considered an index of dopamine depletion [23, 24]. Our results suggest that the lesioned animals lost at least half their TH-ir neurons, because methylphenidate challenging at 40 mg/kg promotes rotational activity in animals with at least a 50% loss of TH-ir neurons [25]. The OD of the TH-ir from both 6-OHDA lesioned groups was significantly smaller than the OD from the sham groups. These data confirm the neurotoxicity of 6-OHDA in dopaminergic neurons. Furthermore, concomitantly to the loss of neurons as indicated by TH-ir data from the SNpc, in the LS group the value from the striatal OD of GFAP-ir was significantly higher than the value from the SS group. These findings are consistent with previous studies that describe astroglial activation in the nigrostriatal system after infusion of 6-OHDA [26, 27].
The lesioned animals appeared more rigid and more cautious when moving across the beam. Many rats would begin to cross but stop and then reinitiate some movement, or remain stationary, analogous to the freezing behavior seen in Parkinson’s patients. However, the 6-OHDA lesion does not appear to affect the balance of the animals. These results are in agreement with a previous study where 6-OHDA lesioned animals demonstrated a fourfold increase in both the latency to initiate the task and the total time to cross the beam when compared with the sham group [10].
Taken together, these results suggest that dopamine depletion in the dorsal striatum, as consequence of lesion in the nigrostriatal system, resulted in both an increased delay in the latency and an increase in total time to crossing the beam, which would be consistent with akinesia and bradykinesia previously observed in animal models of PD [10]. Furthermore, our data are the second results using this kind of evaluation to show motor deficits in this model.
Effects of treadmill training
The beneficial effects of forced exercise on general brain disorders are quite well documented, including neurodegenerative diseases, as PD [8, 13, 15, 16, 28]. Treadmill training is a kind of forced exercise commonly used in animal experiments and was applied to this study to investigate the effects of exercise on motor deficits and on astrocytes in this PD model.
Our results showed that the treadmill training was able to improve the MEC in the ST and LT groups, indicating that training can effectively increase this performance in rats. In the ST group the MEC value was higher than that from the SS group and that from the LT group; it was also higher than the value from the LS group. We assume that the effects of exercise in our study may have been mediated, at least in part, by improving the exercise performance on treadmill, as indicated by the increase in the MEC. These data from the MEC together with the results from the narrow beam test, the methylphenidate-induced rotation and the GFAP-ir indicate that exercise produces beneficial effects in animals subjected to this model of PD. Specifically, our results from striatal GFAP expression can contribute to a better understanding of the neurobiological mechanisms by which exercise has been shown to be beneficial for patients with PD.
Moreover, it seems that the 6-OHDA lesion impeded a better performance in the MEC, since the ST group showed a higher value in the MEC when compared with the value from the LT group and our results also showed no difference between the ST and SS groups after the exercise, suggesting that the physical exercise had no per se effect.
Whereas treadmill training was able to ameliorate motor deficits in the animals lesioned by 6-OHDA, it had no such effect on the sham animals. These results are in agreement with previous reports [8, 13, 14, 28], indicating that exercise is able to improve motor deficits in a 6-OHDA model of PD. Indeed, exercise can induce effects through compensatory mechanisms in the nigrostriatal system of lesioned runners, by attenuating the DA depletion and its metabolites (3,4-dihydroxiphenylacetic acid and homovanillic acid) as well as several proteins characteristic of DA terminals (dopamine transporter, vesicular monoamine transporter-2 and tyrosine hydroxylase) [13]. In addition, other mechanisms such as improved mitochondrial function and an increase in the brain region-specific levels of brain-derived and glial cell line-derived neurotrophic factors have been shown the beneficial effects of exercise [16].
Our data from GFAP-ir showed no difference between the LT group and the ST group and this fact indicates that physical exercise can restore changes in GFAP expression associated with neurodegenerative disorders as PD. Thus, it is possible that reduced astrocytic responses to lesion are a neurobiological mechanism that mediates the exercise-induced functional recovery in PD.
Furthermore, there is a growing body of evidence to suggest the importance of neuron-glial interactions at synapses in the CNS. Astrocytes form “tripartite” complexes with pre and postsynaptic structures and regulate synaptic transmission and plasticity as well as actively participating in synaptic function [29]. Several external stimuli, such as exercise, exert important influence on rat brain plasticity that affects neuron-glial interactions which can modulate behavioral responses. We hypothesized that the reduction in GFAP expression, observed in rats lesioned by 6-OHDA and submitted to exercise, is due the beneficial effects of exercise on brain plasticity. This reduction in GFAP expression may be related to the reduced expansion of astrocytes, probably due to an increase in synaptic function in the dorsal striatum induced by exercise.
Conclusion
Our data suggest that treadmill training improves motor deficits in a 6-OHDA model of PD. In addition, our results showed that exercise can restore the expression of GFAP in the dorsal striatum, indicating that astrocytes may play a role in producing the beneficial effects of exercise in PD. Nevertheless, these are the first data that show a reduction in the post-exercise expression of striatal GFAP in this model and further investigation is needed to determine the precise action of exercise on astrocytes in this model of Parkinson’s disease.
References
Lang AE, Lozano AM (1998) Parkinson’s disease. First of two parts. N Engl J Med 339:1044–1053
Fahn S, Przedborski S (2000) Parkinsonism. In: Rowland LP (ed) Merritt’s Neurology. Lippincott Williams & Wilkins, New York, pp 679–693
Forno LS, DeLanney LE, Irwin I et al (1992) Astrocytes and Parkinson’s disease. Prog Brain Res 94:429–436
Sheng JG, Shirabe S, Nishiyama N et al (1993) Alterations in striatal glial fibrillary acidic protein expression in response to 6-hydroxydopamine-induced denervation. Exp Brain Res 95:450–456
Yokoyama H, Uchida H, Kuroiwa H et al (2011) Role of glial cells in neurotoxin-induced animal models of Parkinson’s disease. Neurol Sci 32:1–7
Eddleston M, Mucke L (1993) Molecular profile of reactive astrocytes: implications for their role in neurologic disease. Neuroscience 54:15–36
Beal MF (2001) Experimental models of Parkinson’s disease. Nat Rev Neurosci 2:325–334
Tillerson JL, Cohen AD, Philhower J et al (2001) Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci 21:4427–4435
Cohen AD, Tillerson JL, Smith AD et al (2003) Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem 85:299–305
Allbutt HN, Henderson JM (2007) Use of the narrow beam test in the rat 6-hydroxydopamine model of Parkinson’s disease. J Neurosci Meth 159:195–202
Emborg ME (2004) Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. J Neurosci Meth 139:121–143
Goodwin VA, Richards SH, Taylor RS et al (2008) The effectiveness of exercise interventions for people with Parkinson’s disease: a systematic review and meta-analysis. Mov Disord 23:631–640
Tillerson JL, Caudle WM, Reverón ME et al (2003) Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience 119:899–911
O’Dell SJ, Gross NB, Fricks AN (2007) Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience 144:1141–1151
Yoon MC, Shin MS, Kim TS et al (2007) Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett 423:12–17
Lau YS, Patki G, Das-Panja K et al (2011) Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson’s disease with moderate neurodegeration. Eur J Neurosci 33:1264–1274
Paxinos G, Watson C (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Melo RM, Martinho E, Michelini LC (2003) Training-induced, pressurelowering effect in SHR: Wide effects on circulatory profile of exercised and nonexercised muscles. Hypertension 42:851–857
Ilha J, Araujo RT, Malysz T et al (2008) Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair 22:355–366
Silvestrin RB, Oliveira LF, Batassini C et al (2008) The footfault test as a screening tool in the 6-hydroxydopamine rat model of Parkinson’s disease. J Neurosci Meth 177:317–321
Xavier LL, Viola GG, Ferraz AC et al (2005) A simple and fast densitometric method for the analysis of tyrosine hydroxylase immunoreactivity in the substantia nigra pars compacta and in the ventral tegmental area. Brain Res Protoc 16:58–64
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Ungerstedt U, Arbuthnott GW (1970) Quantitative recording of rotational behavior in rats after 6-hydroxydopamine lesions of the nigrostriatal dopamine system. Brain Res 24:485–493
Chopin P, Colpaert FC, Marien M (1999) Effects of alpha-2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway. J Pharmacol Exp Ther 288:798–804
Kuczenski R, Segal DS (1997) Effects of methylphenidate on extracellular dopamine, serotonin, and norepinephrine: comparison with amphetamine. J Neurochem 68:2032–2037
Gomide VC, Bibancos T, Chadi G (2005) Dopamine cell morphology and glial cell hypertrophy and process branching in the nigrostriatal system after striatal 6-OHDA analyzed by specific sterological. Int J Neurosci 115:557–582
Gomide VC, Silveira GA, Chadi G (2005) Transient and widespread astroglial activation in the brain after a striatal 6-OHDA-induced partial lesion of the nigrostriatal system. Int J Neurosci 115:99–117
Tajiri N, Yasuhara T, Shingo T et al (2009) Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310:200–207
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dutra, M.F., Jaeger, M., Ilha, J. et al. Exercise improves motor deficits and alters striatal GFAP expression in a 6-OHDA-induced rat model of Parkinson’s disease. Neurol Sci 33, 1137–1144 (2012). https://doi.org/10.1007/s10072-011-0925-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0925-5