Abstract
The loss of nigral dopaminergic neurons typical in Parkinson’s disease (PD) is responsible for hyperexcitability of medium spiny neurons resulting in abnormal corticostriatal glutamatergic synaptic drive. Considering the neuroprotective effect of exercise, the changes promoted by exercise on AMPA-type glutamate receptors (AMPARs), and the role of activity-regulated cytoskeleton-associated protein (Arc) in the AMPARs trafficking, we studied the impact of short and long-term treadmill exercise during evolution of the unilateral 6-hydroxy-dopamine (6-OHDA) animal model of PD. Wistar rats were divided into sedentary and exercised groups, with and without lesion by 6-OHDA and followed up to 4 months. The exercised groups were subjected to a moderate treadmill exercise 3×/week. We measured the proteins tyrosine hydroxylase (TH), Arc, GluA1, and GluA2/3 in the striatum, substantia nigra, and motor cortex. Our results showed a higher reduction of TH expression in all sedentary groups when compared to all exercised groups in striatum and substantia nigra. In general, larger changes occurred in the striatum in the first and third months after training. After 1 month of exercise, there was significant increase of GluA2/3 with concomitant reduction of GluA1 and Arc. As a balanced system, these changes were reversed in the third month, showing an increase of Arc and GluA1 and decrease of GluA2/3. Similar results for GluAs and Arc were observed in the motor cortex of the exercised animals. These modifications may be relevant for corticostriatal circuits in PD, since the exercise-dependent plasticity can modulate GluAs expression and maybe neuronal excitability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, characterized by the progressive loss of the dopaminergic neurons in the substantia nigra pars compacta (SNc) and a profound loss of dopamine (DA) in the striatum (Blandini et al. 2000; Schober 2004; Logroscino et al. 2006; Blesa et al. 2012). DA acts as a neuromodulator regulating the glutamatergic inputs onto the principal neurons and controls the striatal output (Calabresi et al. 2014; Gardoni and Bellone 2015). Synaptic connections between cortical glutamatergic neurons and dopamine receptors D1- and D2-containing medium spiny neurons (MSNs) characterize the corticostriatal circuits, and interactions between these systems are important for the function of the basal ganglia (Segovia et al. 2001; Kreitzer and Malenka 2008; Calabresi et al. 2014; Toy et al. 2014; Petzinger et al. 2015). A loss of nigral dopaminergic neurons, as occurs in the pathophysiology of PD, is responsible for hyperexcitability of MSNs, reducing the ability of these neurons to modulate intracellular calcium levels and resulting in abnormal corticostriatal glutamatergic synaptic drive (Bamford et al. 2004; Day et al. 2006; Petzinger et al. 2015; Shi et al. 2016). As a result of these changes in the functional organization of the nigrostriatal pathway, the ability to control voluntary movements is also progressively lost (Blandini et al. 2000; Dirnberger and Jahanshahi 2013; Engeln 2013). Located in the postsynaptic MSN, α-amino-3 hydroxy-5-methyl-4-isooxazole-propionic acid receptors (AMPARs) convert the glutamate presynaptic chemical signal in a postsynaptic electrical signal through Na+ and Ca2+ mobilization. The increase of the GluA2 subunit expression within the AMPAR tetrameric complex creates an additional positive charge inside the pore of the channel, which prevents the flow of cations, reducing calcium conductance and decreasing the synaptic strength (Hollmann et al. 1991; Petzinger et al. 2015).
AMPARs are regulated by the activity-regulated cytoskeleton-associated protein (Arc), which is an effector immediate-early gene rapidly induced by different forms of neuronal activity (Lyford et al. 1995; Rial Verde et al. 2006; Korb and Finkbeiner 2011). Indeed, molecular studies indicate that Arc modulates the AMPAR trafficking, directly interacting with components of the endocytic pathway (endophilin 2/3 and dynamin) (Chowdhury et al. 2006; Rial Verde et al. 2006). The molecular mechanism underlying Arc selective endocytosis of AMPA receptors remains unknown, although it is clear that high levels of Arc accelerate the internalization of AMPARs, i.e., endocytosis of AMPARs is facilitated, whereas deletion of Arc slows AMPAR endocytosis (Chowdhury et al. 2006; Rial Verde et al. 2006). Arc gene expression is broadly responsive to neuronal activation by physiological stimuli (Lyford et al. 1995; Guzowski et al. 2000) and by dopamine-dependent mechanisms in the striatum (Fosnaugh et al. 1995; Berke et al. 1998), as well as it plays an active role in modifying long-term synaptic responses (Guzowski et al. 2000).
Exercise has been the subject of many studies because it promotes important changes generating neuroplasticity and potential lasting effects on both molecular and behavioral levels (Dietrich et al. 2005; Arida et al. 2011). These exercise-induced changes in brain connectivity may occur at molecular and circuit levels and include essential components that modulate neuroplasticity: neurotransmission, synaptogenesis, and neurogenesis (Klein et al. 1996; Fahimi et al. 2016; Klein et al. 2016; Zhao et al. 2016). In studies with animal models of PD, neuroprotection caused by exercise involves angiogenesis (Al-Jarrah et al. 2010), changes in neurotransmitters and receptors (Henderson et al. 2003; Petzinger et al. 2015), increased neurotrophins such as brain-derived neurotrophic factor (BDNF) and glial-derived neurotrophic factor (GDNF) (Yoon et al. 2007; Zigmond et al. 2009; Real et al. 2013; Tuon et al. 2012), and enhanced functional recovery after striatal lesions (Lau et al. 2011). In clinical studies with PD patients, physical exercise improves motor performance and daily activity with consequent improvement of quality of life and reduces both the risk and mortality of PD (Logroscino et al. 2006; Rafferty et al. 2016). Through activation of molecular and cellular cascades that support brain plasticity, exercise has been shown to be inversely related with neurodegenerative diseases (Alonso-Frech et al. 2011). Considering the neuroprotective effect of treadmill exercise, the changes promoted by exercise on AMPARs in animal models of PD, and the role of Arc in the AMPARs trafficking described above, we analyze here the impact of short and long-term treadmill exercise in the expression of Arc and AMPARs during evolution of the unilateral 6-hydroxy-dopamine (6-OHDA) animal model of PD.
Materials and Methods
Animals
Adult male Wistar rats (280–300 g, 3 months of age, from the Institute of Biomedical Sciences of the University of São Paulo) were housed in groups of three to four animals per cage and were maintained on an inverted 12 h light/dark cycle (Salgado-Delgado et al. 2008), with free access to food and water. Rats were divided into experimental groups: (1) sedentary animals group without injury by 6-OHDA (control or SED), (2) exercised animals group without injury by 6-OHDA (Ex), (3) sedentary animals group with injury by 6-OHDA (6-OHDA), and (4) exercised animals group with injury by 6-OHDA (6-OHDA + Ex). All the groups were followed up to 1–4 months. The experiments were carried out in accordance with the guidelines of the National Council for the control of Animal Experimentation (CONCEA, Brazil), a constituent body of the Ministry of Science, Technology, and Innovation (MCTI, Brazil). All protocols were approved by the Ethics Committee for Animal Research of the Institute of Biomedical Sciences of the University of São Paulo (CEUA-ICB/USP, Brazil) (Protocol number 113/2012).
Surgical Procedure
Rats were anesthetized with 2.2.2-tribromoethanol at 2.5% (1 ml/100 g–250 mg/kg ip—Sigma®) and received unilateral stereotaxic injections of 6-OHDA hydrochloride (Sigma®) in saline and 0.3% ascorbic acid or vehicle in two different places in the right striatum, using a micropipette connected to a microinfusion pump (Real et al. 2013). Two points were injected with 0.5 μl with 6 μg of 6-OHDA each, which results in a total dose of 12 μg of 6-OHDA. Injections were performed in the striatum at the following coordinates according to a stereotaxic atlas (Paxinos and Watson 2005) with references to bregma and dura mater: (1) anteroposterior (AP) 0.5 mm, lateral (LAT) 3 mm, and vertical (VERT) −4.5 mm and (2) AP 1.2 mm, LAT 2.5 mm, and VERT −5 mm. The micropipette was left in place for additional 5 min before slowly retracting it. After finishing the infusion, the incision was sutured and the animals were kept isolated in cages until complete recovery.
Treadmill Exercise
The animals that were selected to the exercised groups were adapted for 2 days on a treadmill (KT 3000—IMBRAMED) before surgery. Rats that refused running were excluded and transferred to other studies. After adaptation, the animals were subjected to a moderate treadmill exercise protocol (Garcia et al. 2012; Real et al. 2013; Real et al. 2015) at a 10-m/min speed for 40 min, three times a week, for 1, 2, 3, or 4 months. The time of the training was the same for all training groups, during the active period of the animal (Salgado-Delgado et al. 2008).
Immunoblotting
The animals were decapitated, and the midbrain, motor cortex, and striatum (n = 5–8 per group) were quickly collected in tubes, frozen in liquid nitrogen, and stored at −80 °C until use. The procedure was performed according to the protocol described previously in studies from our group (Garcia et al. 2012; Real et al. 2013; Real et al. 2015). Protein amount equivalent to 50 μg was subjected to separation by electrophoresis with constant current of 25 mA on acrylamide gels of 8 and 12% sodium dodecyl sulfate SDS (Bio-Rad, USA). After electrophoretic separation, the proteins were transferred to a nitrocellulose membrane. The membranes were incubated with antibodies against TH 56–60 kDa (MAB 5280) (1:1000), Arc 55 kDa (SC-15325) (1: 250), GluA1 99 kDa (AB 1504) (1:1000), and GluA2/3 98 kDa (AB1506) (1:1000). An antibody against β-actin 42 kDa (1:10,000) was used as an internal control. The bound antibodies were developed using the ECL kit (Bio-Rad, USA) for chemiluminescence. Bands were captured with the scanner SCAN-C (Li-Cor, USA) and analyzed for optical density of the immunoreactivity using its own software (C-Digit, USA).
Immunohistochemistry
The animals (n = 3–5 per group) were anesthetized with ketamine (100 mg/kg) and xylazine (25 mg/kg) intraperitoneally and subjected to transcardiac perfusion with 0.9% saline followed by fixative solution of 4% paraformaldehyde dissolved in phosphate buffer (0.1 M PB, pH 7.4). After perfusion, the brains were collected and postfixed for 4–6 h. After this period, the material was transferred to a cryoprotectant solution of 30% sucrose in 0.1 M PB. Tissues were cut to a thickness of 30 μm on a sliding freezing microtome. For immunostaining of SNc, motor cortex, and striatum, we used a protocol previously described in other studies of our group (Garcia et al. 2012; Real et al. 2013; Real et al. 2015). We used the same antibodies used in the immunoblotting technique. The brain sections were incubated overnight at 22 °C, with a primary antibody solution (5% normal goat serum in 0.3% Triton X-100 in PB). The secondary antibody solution was diluted 1:200 and incubated with the sections for 2 h. The sections were processed with ABC Elite Kit (Vector Labs, CA, USA) for 2 h, and the labeling was developed with 0.05% diaminobenzidine tetrahydrochloride and 0.03% (final concentration) hydrogen peroxide in PB. The qualitative analysis of the material and quantification by densitometry was performed using an optical microscope (E1000, Nikon) coupled to a digital camera and the ImageJ software (NIH, USA). The final data in the graphs and tables represent the ratio between cell number and optical density mean for experimental and for control hemispheres. For each hemisphere, five histological sections for each region per animal were analyzed. For the striatum, the analysis was performed in the dorsolateral area between bregma 1.52 and −0.48 mm. For the motor cortex, data were collected from both the primary and secondary motor cortex (between bregma 2.52 and 1.44 mm), and for the substantia nigra, from the area between bregma −4.68 and −5.28 mm. Analysis was performed using the ImageJ Analyze Particle plugin, cells between 50 and 300 pixels, in two areas of ca. 0.40 mm2 in motor cortex for Arc, GluA1, and GluA2/3, as well as two areas of ca. 0.10 mm2 in CPu for GluA1 and GluA2/3 and of ca. 0.55 mm2 in the CPu for Arc. TH staining in the CPu was evaluated by relative optical density in five areas of ca. 0.10 mm2 each. In the SNc, analysis of Arc and TH was performed by counting manually the number of labeled cells per square millimter using the cell counter plugin.
Statistical Analysis
Data were normalized to sedentary animals group without injury by 6-OHDA 1 m (control group). To compare all groups (6-OHDA and 6-OHDA + Ex) with control group, we used one-way ANOVA and Dunnett’s post hoc test. To compare 6-OHDA groups with 6-OHDA + Ex groups, we used two-way ANOVA and Tukey’s post hoc test; p < 0.05 was adopted as the significance level. Data were expressed in the table as mean ± SEM (Statistica 13). F value described in the figure legends shows the significance of two-way ANOVA between the categorical factors: exercise and time after injury by 6-OHDA (1, 2, 3, and 4 months). In text, the results were expressed in percentage of control, between groups (6-OHDA and 6-OHDA + Ex), between different times in the same group (6-OHDA 1, 2, 3, and 4 m or 6-OHDA + Ex 1, 2, 3, and 4 m), and between Ex and 6-OHDA + Ex groups.
Results
Tables 1and 2, in supplementary data, summarize the immunohistochemistry and immunoblotting data (mean ± SEM), respectively. Below, the data will be divided by proteins analyzed, by structures, and by groups of interest.
Tyrosine Hydroxylase
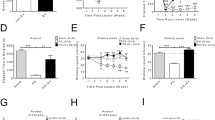
After the 6-OHDA injection, there were changes in TH in the striatum and substantia nigra, as shown by immunohistochemistry and immunoblotting techniques (Fig. 1). There was a variation between the data for immunohistochemistry and immunoblotting, possibly because larger areas were analyzed in immunoblotting (all the striatum and midbrain). TH decreased in the following groups: 6-OHDA 1, 2, 3, and 4 m groups compared to the control group (ca. 40% in all groups). In the 6-OHDA + Ex 1 and 2 m groups, TH staining was also reduced compared to the control group (ca. 20% in both groups). After 3 and 4 months of exercise, there was no change in TH staining in relation to control. Comparing the 6-OHDA with 6-OHDA + Ex groups, a larger reduction (between 40 and 50%) was observed in the TH levels in 6-OHDA groups. Comparing the exercise groups in different times, there was less neuronal death in 6-OHDA + Ex 3 and 4 m groups compared to 6-OHDA + Ex 1 m group (19%) (Fig. 1b). In the immunoblotting data, all sedentary groups revealed a high reduction in the TH levels compared to the control group (ca. 65%). The 6-OHDA + Ex 1, 2, and 3 m groups showed a decrease of expression of ca. 40% when compared to the control group, and 6-OHDA + Ex 4 m has a lower expression of ca. 30% compared to control group. The 6-OHDA + Ex 1, 2, 3, and 4 m groups showed an increased expression compared to the 6-OHDA 1, 2, 3, and 4 m groups (111, 88, 83, and 100%, respectively) (Fig. 1c).
a, d Digital images of coronal sections stained for TH in the striatum and SNc, respectively. b Relative optical density for TH in the striatum (F(3,24) = 6.771, p = 0.002 for time after injury and F(1,24) = 183.6, p < 0.0001 for exercise). c Mean ratio of TH densitometry density data in relation to β-actin in the striatum and typical immunoblots in each condition (F(3,37) = 4.986, p = 0.005 for time after injury and F(1,37) = 90.17, p < 0.0001 for exercise). e Ratio between experimental and control hemispheres for TH in the SNc ( F(1,24) = 146,8, p < 0.0001 for exercise). f Mean ratio of TH densitometry density data in relation to β-actin in the midbrain and typical immunoblots in each condition. TH—56 kDa. **p < 0.01; ***p < 0.001; ****p < 0.0001 vs control group. ●● p < 0.001; ●●● p < 0.001; ●●●● p < 0.0001 vs corresponding 6-OHDA group. ■ p < 0.05 vs 6-OHDA + Ex 1 m. Scale: 100 μm
In the SNc, there was a decrease of TH staining of approximately 60% in the 6-OHDA + Ex groups compared to the control group and approximately 75% in 6-OHDA groups compared to control groups. Comparing the 6-OHDA with 6-OHDA + Ex groups, a larger reduction was observed for TH levels in 6-OHDA groups (1 m 79%, 2 m 104%, 3 m 48%, and 4 m 59%) (Fig. 1e). Immunoblotting data showed a decrease of TH staining of approximately 50% in the 6-OHDA + Ex groups compared to control group and approximately 60% in 6-OHDA groups compared to control groups. There was no difference between groups (Fig. 1f).
Arc
In striatum, data showed that the 6-OHDA + Ex 3 m group has a higher expression of Arc compared to control group (54%) and 6-OHDA 3 m group (44%). In addition, the 6-OHDA + Ex 1 m group showed a lower Arc expression compared to 6-OHDA + Ex 2, 3, and 4 m groups (39, 53, and 45%, respectively). 6-OHDA + Ex 4 m has a higher expression of Arc compared with control group (33%) (Fig. 2b). The immunoblotting results showed an increased expression of Arc in the 6-OHDA + Ex 3 m group compared to control group (89%) and 6-OHDA 3 m group (200%). The 6-OHDA + Ex 3 m group showed a higher expression of Arc compared to 6-OHDA + Ex 1, 2, and 4 m groups (262, 192, and 169%, respectively). The 6-OHDA 1, 2, 3, and 4 m groups and 6-OHDA + Ex 1, 2, and 4 m groups showed a lower expression of Arc compared with the control group (ca. 40%) (Fig. 2c).
a Ratio between experimental and control hemispheres for Arc in the striatum (interaction between factors: F(3,24) = 8.614, p = 0.0005). b Mean ratio of Arc densitometry density data in relation to β-actin in the striatum and typical immunoblots in each condition (interaction between factors: F(3,34) = 44.57, p < 0.0001). c, d Digital images of coronal sections of the striatum and motor cortex, respectively, stained for Arc. e Ratio between experimental and control hemispheres for Arc in the motor cortex (interaction between factors: F(3,40) = 71.81, p < 0.0001). f Mean ratio of Arc densitometry density data in relation to β-actin in the motor cortex and typical immunoblots in each condition (interaction between factors: F(3,24) = 7.089, p = 0.0008). Arc—55 kDa. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs control group. ●● p < 0.01; ●●● p < 0.001; ●●●● p < 0.0001 vs corresponding 6-OHDA group. $$ p < 0.0001 vs all exercise groups. ■■ p < 0.01; ■■■ p < 0.001; ■■■■ p < 0.0001 vs 6-OHDA + Ex 1 m. Scale: 100 μm
In addition, we noted changes in Arc expression in the motor cortex of the animals. The 6-OHDA + Ex 1 m group showed a decreased expression of Arc compared to 6-OHDA 1 m group (25%), and the 6-OHDA + Ex 2, 3, and 4 m groups (24, 29, and 17%, respectively). The 6-OHDA + Ex 2 m group showed an increased expression compared to control group (23%). The 6-OHDA + Ex 3 m group showed increased expression of 32% when compared to control group and 43% when compared to the 6-OHDA 3 m group. The 6-OHDA + Ex 4 m group showed a reduced expression of Arc compared to 6-OHDA 4 m group (29%) and higher expression when compared to control group (13%). The 6-OHDA 1, 2, and 4 m groups showed an increased expression of Arc when compared to the control group (24, 30, and 60%, respectively). The 6-OHDA 3 m group showed a lower expression of Arc compared to 6-OHDA 1, 2, and 4 m groups (26, 29, and 42%, respectively) (Fig. 2e). The immunoblotting results showed an increased expression of Arc in the 6-OHDA + Ex 3 m group compared to the control group (64%) and 6-OHDA 3 m group (34%). The 6-OHDA 1, 2, and 3 m groups and 6-OHDA + Ex 2 and 4 m groups showed higher expression compared to control group (ca. 20%). The 6-OHDA 3 m group exhibited a lower expression compared to control group (ca. 10%) (Fig. 2f).
AMPA Glutamate Receptors
We observed in the motor cortex and striatum the two types of AMPA receptors evaluated, namely those containing GluA1 and GluA2/3.
In the striatal immunoblotting, the 6-OHDA + Ex 4 m group showed higher expression of GluA1 compared to 6-OHDA 4 m group (30%); 6-OHDA + Ex 1, 2, and 3 m groups (40, 30, and 29%, respectively); and control group (80%). The 6-OHDA 4 m group and 6-OHDA + Ex 2 and 3 m groups showed a higher expression compared to control group (ca. 30%) (Fig. 3a, b).
a Ratio between experimental and control hemispheres for GluA1 in the striatum. b Mean ratio of GluA1 densitometry density data in relation to β-actin in the striatum and typical immunoblots in each condition (interaction between factors: F(3,20) = 7.005, p = 0.002). c, d Digital images of coronal sections of the striatum and motor cortex, respectively, stained for GluA1. e Ratio between experimental and control hemispheres for GluA1 in the motor cortex (interaction between factors: F(3,16) = 6.337, p = 0.005). f Mean ratio of GluA1 densitometry density data in relation to β-actin in the motor cortex and typical immunoblots in each condition (interaction between factors: F(3,26) = 4.824, p = 0.008). GluA1–99 kDa. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 vs control group. ●●● p < 0.001 vs corresponding 6-OHDA. ■■■ p < 0.001; ■■■■ p < 0.0001 vs 6-OHDA + Ex 4 m group. $ p < 0.05; $$ p < 0.01 vs 6-OHDA + Ex 3 m. # p < 0.05; ## p < 0.01 vs 6-OHDA + Ex 1 m. Scale: 100 μm for mCx and 50 μm for striatum
In the motor cortex, there was an increase of GluA1 expression in the 6-OHDA + Ex 3 m group compared to the control group (69%) and 6-OHDA + Ex 1, 2, and 4 m groups (69, 56, and 50%, respectively) (Fig. 3e). The immunoblotting technique showed a lower expression of GluA1 in the 6-OHDA + Ex 1 m group compared to 6-OHDA + Ex 2, 3, and 4 m groups (ca. 65%) and control group (ca.70%) (Fig. 3f).
The striatum data showed an increased expression of GluA2/3 in the 6-OHDA + Ex 1 m group compared to the 6-OHDA 1 m group (68%) and the 6-OHDA + Ex 3 m group (86%). The 6-OHDA 4 m group and 6-OHDA + Ex 3 m group showed reduced expression compared to control group (ca. 35%) (Fig. 4a). In the immunoblotting, the 6-OHDA + Ex 1 m group showed a higher expression of GluA2/3 compared to 6-OHDA 1 m group (41%), 6-OHDA + Ex 3 m group (33%), and control group (48%) (Fig. 4b).
a Ratio between experimental and control hemispheres for GluA2/3 in the striatum (interaction between factors: F(3,16) = 5.715, p = 0.007). b Mean ratio of GluA2/3 densitometry density data in relation to β-actin in the striatum and typical immunoblots in each condition (interaction between factors: F(3,22) = 3.135, p = 0.05). c, d Digital images of coronal sections of the striatum and motor cortex, respectively, stained for GluA2/3. e Ratio between experimental and control hemispheres for GluA2/3 in the motor cortex (F(3,16) = 7.789, p = 0.002 for time after injury and F(1,16) = 22.64, p = 0.0002 for exercise). f Mean ratio of GluA2/3 densitometry density data in relation to β-actin in the motor cortex and typical immunoblots in each condition (F(3,27) = 6.155, p = 0.002 for time after injury and F(1,27) = 6.666, p = 0.01 for exercise). GluA2/3—98 kDa. *p < 0.05; **p < 0.01 vs control group. ●● p < 0.01 vs corresponding 6-OHDA group. ■ p < 0.05; ■■ p < 0.01 vs 6-OHDA + Ex 3 m. Scale: 100 μm for mCx and 50 μm for striatum
The motor cortex data showed an increased expression of GluA2/3 in the 6-OHDA + Ex 2 m group compared to the control group (40%). The 6-OHDA + Ex 3 m group showed an increased expression compared to the control group (52%) and 6-OHDA 3 m group (51%). In addition, the 6-OHDA + Ex 3 m group showed a higher expression compared to 6-OHDA + Ex 1 and 4 m groups (50 and 48%, respectively) (Fig. 4e). In the immunoblotting, the 6OHDA 2 m group and 6-OHDA + Ex 2 and 3 m groups showed higher expression compared to the control group (66, 106, and 100%, respectively) (Fig. 4f).
Discussion
The present study was designed to compare the effects of physical exercise performed for short and long periods in the brain of rats subjected to unilateral 6-OHDA injections. We decided to use the present exercise protocol (intermittent exercise) because it is closer to the reality and more feasible to PD patients. We could then correlate and/or observe the synchrony of the changes that occur during physical training over long periods after dopaminergic depletion.
We found that when compared to sedentary groups, the exercised animals showed a dopaminergic neuroprotection observed by less cell death in the SNc and larger TH expression in the striatum that occurred during the first month, when the 6-OHDA is still promoting cell death. In the following months, this neuroprotection remains, since the same percentage of dopaminergic cells is apparent within the SNc and may be an improvement in dopamine release produced by the larger TH expression in the striatum. Studies revealed no changes in TH expression levels compared to the control group in the striatum of an exercised animal model of PD (Tajiri et al. 2010; Real et al. 2013). On the other hand, in our study, only in the third month, the changes in TH levels were not significant when compared to control animals. Maybe this is due to the dose of 6-OHDA injected in animals. The models used in other studies were mild lesions (5–6 μg/μl of 6-OHDA), whereas in this study, we used a moderate dose (12 μg/μl) to induce a PD-like state, taking a longer time for exercised animals to recover the dopaminergic system to baseline levels. The beneficial effects of exercise evidenced in the cellular techniques accompany the functional benefit evidenced in behavioral tests. Several studies have shown that regardless of the type and the time of exercise performed, it is able to decrease the rotational asymmetry (rotational tests) caused by dopamine depletion (Yoon et al. 2007; Tajiri et al. 2010; Real et al. 2013). This improvement in TH expression in the striatum after long-term exercise may be due to compensatory mechanisms of remaining dopaminergic cells (Zigmond et al. 1990; McCallum et al. 2006), considering that the number of cells in the substantia nigra did not alter in exercised animal model of PD during the 4 months of analysis. These mechanisms try to maintain a sufficient concentration of dopamine in the striatum, which include responses such as increased synthesis, metabolism, and release of dopamine (Zigmond et al. 1990; Deumens et al. 2002).
The depletion of dopamine in the basal ganglia leads to glutamatergic corticostriatal hyperexcitability at the level of striatal MSN, which contributes to the PD motor deficits (VanLeeuwen et al. 2010; Calabresi et al. 2014). Exercise may reduce corticostriatal hyperactivity by diminishing AMPAR-mediated conductance, since when the AMPARs include the GluA2 subunit in its constitution, there is a reduction in permeability to calcium and consequently low conductance (Isaac et al. 2007; VanLeeuwen et al. 2010; Petzinger et al. 2013). Our data showed that physical exercise on a treadmill, even at short-term, already promotes an increase of GluA2/3 receptors in the striatum. This increased expression in the first month occurs only in the PD-like animals, suggesting that this change in GluA2/3 can occur in response to dopamine depletion. In the motor cortex, this change begins to be observed from the second month of exercise. Concomitant with these results, we noted that especially after 1 month of exercise, the GluA1 expression in the striatum of PD-like animals had an inverse relationship with GluA2/3. This reduction of GluA1 suggests a reduction of hyperexcitability, since the presence of homotetramer GluA1 channels produces higher conductance due to their permeability to calcium (Plant et al. 2006). Our GluA2/3 data corroborate another study in which MPTP mouse models of PD submitted to short-term exercise (28 days) exhibited increased GluA2 expression, as well as there was an increase in the phosphorylated state of GluA2 at serine 880, which has been associated with increased trafficking of AMPARs, no increase in the amplitude and frequency of excitatory postsynaptic current (EPSC), and thus a decrease of glutamatergic excitability (VanLeeuwen et al. 2010). With the continuity of the exercise for a few months, it was noted that expression of AMPARs still had oscillations. Three months of exercise decrease GluA2/3 and increase GluA1 positive cells, and after 4 months of exercise, the AMPAR levels were similar to those at 1 month. These GluA2/3 receptor oscillations appear to be synchronized with the Arc protein oscillations. For instance, the group of PD-like animals trained for 1 month exhibited an intense reduction of Arc expression. After 3 months of training, a clear reversal of the levels occurred, suggesting a possible homeostatic function. In both striatum and motor cortex, the Arc expression occurs increasingly in control animals that performed exercise (data not shown), showing no oscillations as those observed in animals with dopaminergic depletion. This may suggest that these variations in Arc levels seem to occur to compensate the dopaminergic dysfunction. Studies showed that changes in Arc expression are capable of modifying the trafficking of AMPA type receptors (Chowdhury et al. 2006; Rial Verde et al. 2006). This occurs due to the binding of the Arc protein to two major proteins responsible for regulating the kinetics, spatial distribution, and induction of endocytosis. Thus, high levels of Arc can accelerate the internalization of AMPARs (mainly GluA1 and GluA2) whereas deleted or decreased Arc expression slows endocytosis (Chowdhury et al. 2006; Rial Verde et al. 2006). As an immediate-early gene, Arc is dynamically regulated by neuronal activity (Steward et al. 1998; Rao et al. 2006); however, we observed that when there was a dopaminergic striatal depletion, Arc tends to remain in a reduced amount due to the possible interactions with glutamatergic receptors. This relationship between Arc and AMPARs also occurs in the motor cortex. Interestingly, in general, in the time points in which there was an increase of GluA2/3 with reduction of Arc in the striatum, there was a reduction of GluA2/3 with an increase of Arc in the motor cortex. This homeostatic balance between Arc and AMPARs seems to be involved with exercise-dependent plasticity, through the optimal control of the levels of those receptors in the membrane, which could modulate the neuronal excitability. Thus, our AMPAR and Arc data suggest that other mechanisms may participate in the beneficial effects of exercise in PD, complementing previous studies that describe the role of neurotrophic factors, such as BDNF, as demonstrated by other authors (Lau et al. 2011; Wu et al. 2011; Tuon et al. 2012; Zoladz et al. 2014) and by our group (Real et al. 2013), angiogenesis (Al-Jarrah et al. 2010), and axonal and dopaminergic sprouting (Finkelstein et al. 2000; Song and Haber 2000), among other mechanisms, such as changes in nitric oxide levels (Tuon et al. 2015).
Conclusions
This study suggests that the exercise-dependent neuroprotection in the 6-OHDA animal model of PD involves the balance of expression of AMPARs possibly regulated by Arc, which are both directly involved with neuronal plasticity. During 4 months of this protocol, different mechanisms can be involved in the neuroprotection considering that the changes in the receptors were different for each time point. In addition, possible compensatory mechanisms appear to be additive, since improvement in TH levels in the striatum was observed after long-term exercise which suggests progressive beneficial effects of exercise in the model used here.
References
Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO (2010) Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson’s disease. NeuroRehabilitation 26:369–373
Alonso-Frech F, Sanahuja JJ, Rodriguez AM (2011) Exercise and physical therapy in early management of Parkinson disease. Neurologist 17:S47–S53
Arida RM, Scorza FA, Gomes da Silva S, Cysneiros RM, Cavalheiro EA (2011) Exercise paradigms to study brain injury recovery in rodents. American Journal of Physical Medicine & Rehabilitation/Association of Academic Physiatrists 90:452–465
Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK (2004) Dopamine modulates release from corticostriatal terminals. J Neurosci 24:9541–9552
Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR (1998) A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci 18:5301–5310
Blandini F, Nappi G, Tassorelli C, Martignoni E (2000) Functional changes of the basal ganglia circuitry in Parkinson's disease. Prog Neurobiol 62:63–88
Blesa J, Phani S, Jackson-Lewis V, Przedborski S (2012) Classic and new animal models of Parkinson's disease. J Biomed Biotechnol 2012:845618
Calabresi P, Picconi B, Tozzi A, Ghiglieri V, Di Filippo M (2014) Direct and indirect pathways of basal ganglia: a critical reappraisal. Nat Neurosci 17:1022–1030
Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF (2006) Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron 52:445–459
Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ (2006) Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci 9:251–259
Deumens R, Blokland A, Prickaerts J (2002) Modeling Parkinson’s disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol 175:303–317
Dietrich MO, Mantese CE, Porciuncula LO, Ghisleni G, Vinade L, Souza DO, Portela LV (2005) Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Res 1065:20–25
Dirnberger G, Jahanshahi M (2013) Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol 7:193–224
Engeln M (2013) Throwing some light on executive function in Parkinson’s disease. Mov Disord 28:1052
Fahimi A, Baktir MA, Moghadam S, Mojabi FS, Sumanth K, McNerney MW, Ponnusamy R, Salehi A (2016) Physical exercise induces structural alterations in the hippocampal astrocytes: exploring the role of BDNF-TrkB signaling. Brain Struct Funct
Finkelstein DI, Stanic D, Parish CL, Tomas D, Dickson K, Horne MK (2000) Axonal sprouting following lesions of the rat substantia nigra. Neuroscience 97:99–112
Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM (1995) Activation of arc, a putative “effector” immediate early gene, by cocaine in rat brain. J Neurochem 64:2377–2380
Garcia PC, Real CC, Ferreira AF, Alouche SR, Britto LR, Pires RS (2012) Different protocols of physical exercise produce different effects on synaptic and structural proteins in motor areas of the rat brain. Brain Res 1456:36–48
Gardoni F, Bellone C (2015) Modulation of the glutamatergic transmission by dopamine: a focus on Parkinson, Huntington and addiction diseases. Front Cell Neurosci 9:25
Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA (2000) Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci 20:3993–4001
Henderson JM, Watson S, Halliday GM, Heinemann T, Gerlach M (2003) Relationships between various behavioural abnormalities and nigrostriatal dopamine depletion in the unilateral 6-OHDA-lesioned rat. Behav Brain Res 139:105–113
Hollmann M, Hartley M, Heinemann S (1991) Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science 252:851–853
Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54:859–871
Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT (1996) Synaptogenesis and FOS expression in the motor cortex of the adult rat after motor skill learning. J Neurosci 16(14):4529–4535
Klein C, Rasinska J, Empl L, Sparenberg M, Poshtiban A, Hain EG, Iggena D, Rivalan M, Winter Y, Steiner B (2016) Physical exercise counteracts MPTP-induced changes in neural precursor cell proliferation in the hippocampus and restores spatial learning but not memory performance in the water maze. Behav Brain Res 307:227–238
Korb E, Finkbeiner S (2011) Arc in synaptic plasticity: from gene to behavior. Trends Neurosci 34:591–598
Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60:543–554
Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO (2011) Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci 33:1264–1274
Logroscino G, Sesso HD, Paffenbarger RS Jr, Lee IM (2006) Physical activity and risk of Parkinson's disease: a prospective cohort study. J Neurol Neurosurg Psychiatry 77:1318–1322
Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF (1995) Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14:433–445
McCallum SE, Parameswaran N, Perez XA, Bao S, McIntosh JM, Grady SR, Quik M (2006) Compensation in pre-synaptic dopaminergic function following nigrostriatal damage in primates. J Neurochem 96:960–972
Paxinos G, Watson C (2005) The rat in stereotaxic coordinates. Academic, Ed San Diego, 456p
Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW (2013) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol 12:716–726
Petzinger GM, Holschneider DP, Fisher BE, McEwen S, Kintz N, Halliday M, Toy W, Walsh JW, Beeler J, Jakowec MW (2015) The effects of exercise on dopamine neurotransmission in Parkinson’s disease: targeting neuroplasticity to modulate basal ganglia circuitry. Brain Plast 1:29–39
Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT (2006) Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9:602–604
Rafferty MR, Schmidt PN, Luo ST, Li K, Marras C, Davis TL, Guttman M, Cubillos F, Simuni T (2016) Regular exercise, quality of life, and mobility in Parkinson’s disease: a longitudinal analysis of national parkinson foundation quality improvement initiative data. J Parkinson’s Dis. 1–10
Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S (2006) AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci 9:887–895
Real CC, Ferreira AF, Chaves-Kirsten GP, Torrao AS, Pires RS, Britto LR (2013) BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson’s disease. Neuroscience 237:118–129
Real CC, Garcia PC, Britto LR, Pires RS (2015) Different protocols of treadmill exercise induce distinct neuroplastic effects in rat brain motor areas. Brain Res 1624:188–198
Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT (2006) Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron 52:461–474
Salgado-Delgado R, Angeles-Castellanos M, Buijs MR, Escobar C (2008) Internal desynchronization in a model of night-work by forced activity in rats. Neuroscience 154:922–931
Schober A (2004) Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res 318:215–224
Segovia G, Porras A, Del Arco A, Mora F (2001) Glutamatergic neurotransmission in aging: a critical perspective. Mech Ageing Dev 122:1–29
Shi W, Liu X, Qiao D, Hou L (2016) Effects of treadmill exercise on spontaneous firing activities of striatal neurons in a rat model of Parkinson’s disease. Motor Control 1–23
Song DD, Habber SN (2000) Striatal responses to partial dopaminergic lesion: evidence for compensatory sprouting. J Neurosci 20(13):5102–5114
Steward O, Wallace CS, Lyford GL, Worley PF (1998) Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron 21:741–751
Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I (2010) Exercise exerts neuroprotective effects on Parkinson’s disease model of rats. Brain Res 1310:200–207
Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vučković MG, Jakowec MW (2014) Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mousemodel of Parkinson's disease. Neurobiol Dis 63:201–209
Tuon T, Valvassori SS, Lopes--Borges J, Luciano T, Trom CB, Silva LA, Queveda J, Souza CT, Lira FS, Pinho RA (2012) Physical training exerts neuroprotective effects in the regulation of neurochemical factors in an animal model of Parkinson’s disease. Neuroscience 227:305–312
Tuon T, Souza PS, Santos MF, Pereira FT, Pedroso GS, Pereira TF, De Souza CT, Dutra RC, Silveira PCL, Pinho RA (2015) Physical training regulates mitochondrial parameters and neuroinflammatory mechanisms in an experimental model of Parkinson’s disease. Oxidative Med Cell Longev 2015:261809
VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW (2010) Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res 88:650–668
Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, Wu CW, Kuo YM (2011) Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun 25:135–146
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, Kim SE, Lee HH, Kim YP, Kim CJ (2007) Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett 423:12–17
Zhao Y, Pang Q, Liu M, Pan J, Xiang B, Huang T, Tu F, Liu C, Chen X (2016) Treadmill exercise promotes neurogenesis in Ischemic rat brains via caveolin-1/VEGF signaling pathways. Neurochem Res
Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM (1990) Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci 13:290–296
Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD (2009) Triggering endogenous neuroprotective processes through exercise in models of dopamine deficienc. Parkinsonism and Related Disorders 15S3:S42–S45
Zoladz JA, Majerczak J, Zeligowska E, Mencel J, Jaskolski A, Jaskolska A, Marusiak J (2014) Moderate-intensity interval training increases serum brain-derived neurotrophic factor level and decreases inflammation in Parkinson’s disease patients. J Physiol Pharmacol 65:441–448
Acknowledgements
This study was supported by FAPESP, CAPES, University of São Paulo—NAPNA and CNPq (Brazil). Thanks are also due to Adilson S. Alves for technical assistance and Fernanda Crunfli for helpful comments in relation to data analysis. PCG was the recipient of a fellowship from CAPES, and CCR was the recipient of a fellowship from FAPESP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiments were carried out in accordance with the guidelines of the National Council for the control of Animal Experimentation (CONCEA, Brazil), a constituent body of the Ministry of Science, Technology, and Innovation (MCTI, Brazil). All protocols were approved by the Ethics Committee for Animal Research of the Institute of Biomedical Sciences of the University of São Paulo (CEUA-ICB/USP, Brazil) (Protocol number 113/2012).
Rights and permissions
About this article
Cite this article
Garcia, P.C., Real, C. & Britto, L. The Impact of Short and Long-Term Exercise on the Expression of Arc and AMPARs During Evolution of the 6-Hydroxy-Dopamine Animal Model of Parkinson’s Disease. J Mol Neurosci 61, 542–552 (2017). https://doi.org/10.1007/s12031-017-0896-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-017-0896-y