Abstract
The changes of protein digestibility, the peptides in the digestive juice and angiotensin I converting enzyme (ACE) inhibitory activity after heating of oysters were investigated. The digestibility of raw oysters was 71.1%, and that of oysters heated at 100 °C was 67.9%. A total of 169 and 370 peptides were identified from the digestion of raw oysters and heated oysters, respectively. According to UPLC-Q-TOF-MS spectra, the peptides with a molecular weight below 2000 Da accounted for 87.6% of the total peptides of raw oysters and 94% of heated oysters. Testing the ACE inhibitory activity in vitro, the IC50 values of raw oyster and cooked oyster were 6.77 μg/mL and 3.34 μg/mL, respectively. Taken together, the results showed that heated oysters could produce more active peptides and provide ACE inhibitory activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As highly nourishing aquatic products, oysters are the world’s largest aquaculture shellfish. Oyster is considered a popular marine organism with high-quality nutritional and medicinal value. It is rich in proteins, active polysaccharides, and taurine, low in fats and a good source of several kinds of vitamins and minerals (Carlson-Bremer et al., 2014; Padula et al., 2015). Thus, oysters have been used widely in the general food, health food and medicine (Haberkorn et al., 2010; Martínez-Fernández et al., 2006).
Proteins of oyster not only provide a source of vital nutrition to human beings, but they also contribute with various kinds of bioactivity. By enzymes hydrolysis, oyster proteins can be decomposed into a considerable number of peptides, with remarkable bioactive activities. Oyster protein hydrolysates have been shown to exhibit strong antioxidant potential against diphenylpicrylhydrazyl and hydroxyl radicals (Asha et al., 2016; Qian et al., 2008), and they display immune activity as proved in recent years (Cai et al., 2013; Hu et al., 2014; Wang et al., 2010). The isolation of angiotensin I converting enzyme (ACE) inhibitor from oyster fermented products has been documented in recent studies (Kim et al., 2013). Wang et al. (2008) isolated and purified the ACE inhibitory peptide, Val-Tyr-Pro-Trp-THR-Gln-Arg-PHE (VVYPWTQRF) from the oyster protein hydrolysate. The peptide has good thermal stability and pH stability, and the pepsin-treated oyster protein hydrolysate can be used as an antihypertensive polypeptide (Wang et al., 2008). However, the study of ACE inhibitory activity of raw oysters and heated oysters was rarely reported.
Heat treatment is used to reduce microbes in oyster products. Apart from the great risk of pathogenic infections in raw oysters, under heating conditions, the structure of proteins could be altered by the loss of tertiary and/or secondary structures, forming new covalent or non-covalent bonds within the molecule. Nevertheless, many people choose raw food because of their unique flavor and taste (Froelich and Noble, 2016; New et al., 2014), owing to the high amino acids that attribute to the sweet and umami taste (Kitaoka et al., 2016). Moreover, the reduce of allergen potency under heat treatment is still under debate. In one study, oysters has been shown to reduce allergen reactivity after being heated (Yadzir et al., 2015), however in other studies, researchers found that the heated shellfish extracts had more allergen potency than the raw (Kamath et al., 2013; Nakamura et al., 2005). Aside, heating have showed a great impact on the nutritional and functional properties of protein hydrolysate (Menezes et al., 2017; Zielbauer et al., 2016).
Thus, the aim was to verify the effect of heat treatment on oyster protein digestibility and ACE inhibitory activity. The peptides identified in the oyster protein digest were analyzed for hydrophilicity, isoelectric point and net charge. It provides a theoretical basis for the effect of thermal processing on the digestion characteristics of oyster protein and its blood pressure lowering activity.
Materials and methods
Materials
Oyster (Crassostrea gigas) was collected from Changxing aquatic market in June 2017 (Dalian, China). Pepsin (400 U, from porcine stomach mucosa), trypsin (1000 U, from bovine pancreas) were purchased from Sigma Chemical Company (St. Louis, MO, USA).
Preparation of oyster protein with different heat treatments
The oysters was removed from the shell, and clean in deionized water. Oysters without shell were placed individually in two beakers of cold water (0 °C). Six oysters were placed into per beaker. Group A: six raw oysters; Group B: oysters was heated in boiling water for 15 min. The temperature was controlled by thermocouples connected to a data logger inserted during the experiment. Then, the sample was saved in 0 °C water after cooling to 25 °C.
In vitro digestion of oyster protein
The in vitro digestion process was modified with reference to Njintang’s test (Njintang et al., 2001). Oysters of Groups A and B were crushed into a slurry for 1 min using beater (Baide) intermittently. Pepsin and trypsin powder (enzyme: protein = 5:1, U/mg) were sequentially added to the oyster slurry with stable temperature (37 °C) controlled by a water bath, and enzymatic hydrolysis was carried out in a solution of pH 2 and pH 8, respectively. Oyster protein was hydrolyzed by pepsin for 2 h, then by trypsin for 4 h. After the digestion was completed, the oyster digest was finally adjusted to pH 7, and boiled for 15 min to inactivate the enzyme. The pH of the mixture was adjusted with 1.0 M NaOH or 1.0 M HCl.
Protein digestibility determination
Five milliliters digest was uniformly mixed with 5 mL 20% trichloroacetic acid (TCA) solution to form a mixture containing 10% TCA to obtain the turbid liquid. Then, the mixture was centrifuged at 4000g for 10 min under 4 °C. The supernatant and precipitate were separated by being centrifuged at 4000×g for 10 min under 4 °C. The determination of protein digestibility is mainly calculated according to the related study (Carvalho et al., 2012).
High performance size exclusion chromatography
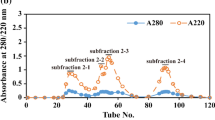
After the enzymatic hydrolysates of the two samples were centrifuged at 10,000×g for 30 min, the supernatant was taken for the determination of the molecular weight distribution by referring to the method of Yu et al. (2018). An Elite P230 HPLC system (Elite Analytical Instruments Co., Ltd., Dalian, China) equipped with a Superdex Peptide 10/300 GL column (GE Healthcare Co., Little Chalfont, Buchinghamshire, UK) was used.
UPLC-Q-TOF-MS
Peptides from the hydrolysates of oysters were identified using a Microtof-II (Bruker Daltonik, Bremen, Germany) mass spectrometer (Tu et al., 2017). The oyster protein digest was separated and identified according to a certain flow ratio and time. Signal peaks with intensity above 1,000,000 were peaked and then compared to peptides in the oyster peptide data base. After the oyster protein digest was centrifuged at 10,000×g for 30 min, the salt in the supernatant was removed by a C18 column. Then, 20 μL of a desalted sample was loaded onto the Thermo Scientific UPLC-Q-TOF-MS.
Database searching by MASCOT
The raw data of peptides identified by UPLC-Q-TOF-MS were analyzed according to Chen’s method (Chen et al., 2018). Ions score threshold for significant peptide and peptide rank cut-off was adjusted to 30 and 20, respectively.
ACE inhibitory activity assay
The ACE inhibitory activity of the oyster protein digests was measured according to the method of Tu et al. (2018).
Statistical analysis
Each experiment was conducted in triplicates, and the SPSS program was used for statistical analysis (Duncan) at a confidence interval of p < 0.05. The values were given as mean ± standard deviation (SD).
Results and discussions
Molecular weight distribution of peptides in oyster protein hydrolysates
In Fig. 1, the oyster protein hydrolysate was prepared by pepsin and trypsin. The majority of proteins were broken down into small peptides. Most of the molecular weight were between 1000 and 3000 Da. Figure 1 showed that the number of low molecule peptides (< 1000 Da) in oysters digest account for a small proportion. More than half of the peptides with molecular weights was less than 3000 Da in heated oysters (100 °C). The peptides with a molecular weight less than 3000 Da accounted for 77.0% of the raw oyster digestion, and 80.1% of oysters heated at 100 °C, explained as the hydrolysis produces more smaller molecules and the heated oyster protein were almost fully digested. It has been shown that the molecular weight of ACE inhibitory peptides is mostly below 3000 Da (Tian et al., 2017). In raw oysters, the substances with molecular weight above 10,000 Da in digestive juice accounted for 1.6%. The digest of the heated oyster contains more macromolecules whose molecular weight were above 10,000 Da where the ratio of these macromolecules in the heated digestive juice of oysters was 2.8%. That is to say, heating changes the texture of oysters, and the protein may not easily to be digested.
Identification of the peptides from oyster proteins hydrolysates
In Table S1, the results of amino acid sequence of the polypeptide, molecular weight, hydrophilicity, net charge at pH 7 and isoelectric point from raw oysters and oysters peptides hydrolysate treated in 100 °C for 15 min by UPLC-Q-TOF-MS/MS were illustrated. Figure 2 showed the Venn diagram of the oysters peptides identified by UPLC-Q-TOF-MS/MS in the two samples. A total of 168 and 373 unique peptides were identified in the raw and heated (100 °C) oysters digests, respectively. There were 112 peptides in raw oysters that overlapped with peptides in oysters heated at 100 °C for 15 min. They were considered to be the soluble peptides of oyster that heated at 100 °C for 15 min.
Venn diagram of the number of identified peptides in the oysters digest. The blue and yellow ellipses are peptides identified by UPLC-Q-TOF-MS/MS in digestive juices of raw oysters and 100 °C heated oysters, which was hydrolyzed by pepsin and trypsin. The middle of the intersection is the peptide that identified in both treated oyster hydrolysates
Figure 3 showed the distribution of charge at pH 7, isoelectric point, and overall hydrophilicity of peptides identified by UPLC-Q-TOF-MS/MS in oysters digest. The peptides in the digestive juices of both oysters were mostly negatively charged at pH 7. The vast majority of peptides has a value between pH 3–12, and more than half of them were charges at level of pH 3–7, further illustrating that these peptides were more easily to be precipitated under acidic conditions due to reaching the pI of them. Among the peptides identified by UPLC-Q-TOF-MS, a high proportion of peptides has average hydrophilic value more than 0 (Fig. 3C), confirming that most of the peptides in the oyster’s digestive juice are very soluble in water.
In the oysters digests identified by UPLC-Q-TOF-MS (Fig. 4), most of the peptides are below 2000 Da. The proportion of peptides with a molecular weight between 1000 and 2000 Da in the raw oyster digestive solution is higher than that of the heatd oysters. Peptides with a molecular weight of more than 3000 are still identified in raw oysters, but not in heated oysters. Heating may alter the structure of certain proteins so that the protein can be further hydrolyzed into smaller peptides. Heat treatment can expose more enzyme sites to proteins (Malgorzata et al., 2017; Singh et al., 2014).
Effect of heat treatment on digestibility of oyster protein
According to Fig. 5, the raw oysters had higher digestibility than oysters heated at 100 °C for 15 min. On the one hand, it is hard to work for enzyme when high temperature make the structure of the protein more compact. More proteins are wrapped in layers of tissue, so they are less easily hydrolyzed. On the other hand, raw oysters contain some endogenous enzymes and autolysin (Zhao et al., 2010), some of which were similar to pepsin. So that they can hydrolyze oyster protein in the solution between pH 2 and pH 4. However, in the course of consumption, many raw oysters still exist heavy metal pollution and microbial risks. Raw and untreated bivalve shellers carrying many pathogenic microorganisms and marine biotoxins. In this sense, a recent study showed the effect of heating on digestibility of protein is greater, indicating that temperature can denature proteins and facilitate digestion of the bioavailability of peptidase cleavage sites (Deb-Choudhury et al., 2014). It is a very healthy way to eat oysters after heating. This not only benefits the digestion and absorption of protein, but also avoids the risk of infection caused by raw oysters.
ACE inhibitory activity of oyster protein hydrolysates
As can be observed in Fig. 6, both the digests of raw oysters and oysters heated at 100 °C for 15 min had excellent ACE inhibitory activity. The IC50 value of ACE inhibitory activity of raw oysters was 6.77 μg/mL, which was higher than the IC50 value (3.34 μg/mL) of heated oysters at 100 °C. Oysters heated for 15 min showed higher ACE inhibitory activity than raw oysters.
The molecular weight distributions identified by UPLC-Q-TOF in the two treated digests was shown in Table S2. According to the analysis of the existing ACE inhibitory peptides database, most of the molecular weights were below 2000 Da (Park and Song, 1997; Ren et al., 1996). Table S3 showed the isoelectric point distribution of oyster digests. Peptides with an isoelectric point below pH 7 accounted for 74.6% of the total peptides in the raw oysters digest and 70.2% in the digestive juices of the oysters at 100 °C. Raw and heated oysters have peptides occupying 14.8% and 15.8% of the peptides between pH 5–7, and 1.8% and 3.5% between pH 8–10, respectively. Since most of the ACE-inhibitory peptides have an isoelectric point between pH 5–7 and pH 8–10 (Roskar et al., 2009), 100 °C heated oyster digests had higher ratios of peptides in this interval than raw oysters. Therefore, from the isoelectric point of the peptide, it was explained that the ACE inhibitory activity of heated oysters at 100 °C was higher than raw oysters. Table S4 showed the overall hydrophilicity data of raw oyster derived peptides. The percentage of the peptides in the raw oyster hydrolysate with the hydrophilicity of greater than or equal to zero occupied 72.8%, which was higher than that of heated oyster hydrolysate. Therefore, the hydrolysate for heating oyster has more hydrophobic peptides, that is, the heated oyster has a greater possibility of having ACE inhibitory activity. Table S5 showed the percentage of peptides with a hydrophilic amino acid content of less than 50% and more than 40%. In the digestive juice heated at 100 °C, both the proportions of hydrophobic peptides and the peptides with higher hydrophobic amino acids were higher than that of raw oysters. Table S6 showed the analysis of peptides identified from oyster hydrolysate according to different N-terminal amino acids. Data results analysis showed that peptides with N-terminal amino acids L, V, and Y are more likely to have ACE inhibitory activity. Raw oysters have 23.6% of total peptides of these amino acids at the N-terminus and 25.9% of those at 100 °C. Thus, Table S6 provided evidence that extracts of oysters heated at 100 °C for 15 min have possibility with higher ACE inhibitory activity.
Heating the food leads to protein unfolding and exposure to linear epitopes. Environmental changes in lysine, which affect the products of the Maillard reaction, and subsequently alter the action of proteases, resulting in different peptide components after enzymatic hydrolysis. It was similar to previous study, a significant difference in ACE inhibitory activity before and after cooking obtained in the previous study (Escudero et al., 2014; Mora et al., 2017). However, there are some differences between aquatic proteins and other meats proteins, for example, they are more susceptible to temperature. At the same time, the presence of large amounts of internal enzymes in oysters is also one of the main causes of this difference (Fu et al., 2012).
In summary, the oysters heated at 100 °C for 15 min showed better ACE inhibitory activity, IC50 of the digest of heated and raw oyster were 6.77 μg/mL and 3.34 μg/mL, respectively. Moreover, the oyster heated showed relatively lower digestibility compared with raw oyster (67.9% vs 71.1%), under the conditions of simulated gastrointestinal digestion in vitro. Raw oysters have a slightly higher digestibility, but heat-treated oysters are more likely to be hydrolyzed into smaller peptides. The changes of protein digestibility and ACE inhibitory activity of digestive juice before and after heat treatment were verified. This study provided a theoretical basis for utilizing the oyster proteins as a potential source of ACE-inhibitory peptides, which is beneficial for the development of functional foods especially aiming for the hypotensive humans.
References
Asha KK, Kumari KRR, Kumar KA, Chatterjee NS, Anandan R, Mathew S. Sequence determination of an antioxidant peptide obtained by enzymatic hydrolysis of oyster Crassostrea madrasensis (Preston). Int. J. Pept. Res. Ther. 22: 421-433 (2016)
Cai B, Pan J, Wu Y, Peng W, Sun H. Immune functional impacts of oyster peptide-based enteral nutrition formula (OPENF) on mice: a pilot study. Chin. J. Oceanol. Limn. 31: 813-820 (2013)
Carlson-Bremer D, Norton TM, Sanders FJ, Winn B, Spinks M, Glatt BA, Mazzaro L, Jodice P, Chen TC, Dierenfeld ES. Circulating fat-soluble vitamin concentrations and nutrient composition of aquatic prey eaten by American oystercatchers (Haematopus palliatus palliatus) in the Southeastern United States. J. Avian Med. Surg. 28: 216-224 (2014)
Carvalho AFU, de Sousa NM, Farias DF, da Rocha-Bezerra LCB, da Silva RMP, Viana MP, Gouveia ST, Sampaio SS, de Sousa MBD, de Lima GPG. Nutritional ranking of 30 Brazilian genotypes of cowpeas including determination of antioxidant capacity and vitamins. J. Food Compos. Anal. 26: 81-88 (2012)
Chen H, Shi P, Fan F, Tu M, Xu Z, Xu X, Du M. Complementation of UPLC-Q-TOF-MS and CESI-Q-TOF-MS on identification and determination of peptides from bovine lactoferrin. J. Chromatogr. B. 1084: 150-157 (2018)
Deb-Choudhury S, Haines S, Harland D, Clerens S, Van KC, Dyer J. Effect of cooking on meat proteins: mapping hydrothermal protein modification as a potential indicator of bioavailability. J. Agr. Food Chem. 62: 8187-8196 (2014)
Escudero E, Mora L, Toldrá F. Stability of ACE inhibitory ham peptides against heat treatment and in vitro digestion. Food Chem. 161: 305-311 (2014)
Froelich B A, Noble R T. Vibrio bacteria in raw oysters: managing risks to human health. Philos. Trans. R. Soc. Lond. B Biol. Sci. 371: 20150209 (2016)
Fu J, Lin J, Zheng H, Chen Z, Tan A, Xie Y. Optimization of conditions for autolysis hydrolysis of Jinjiang oyster protein. Mod. Food Sci. Technol. 28:195-199 (2012)
Haberkorn H, Lambert C, Goïc NL, Moal J, Suquet M, Guéguen M, Sunila I, Soudant P. Effects of Alexandrium minutum exposure on nutrition-related processes and reproductive output in oysters Crassostrea gigas. Harmful Algae 9: 427-439 (2010)
Hu X, Wu H, Fan X, Liu Q. Immunomodulatory activity of glycosaminoglycans from Jinjiang oyster Crassostrea rivularis. Mod. Food Sci. Technol. 12: 16-24 (2014)
Kamath SD, Abdel Rahman AM, Komoda T, Lopata AL. Impact of heat processing on the detection of the major shellfish allergen tropomyosin in crustaceans and molluscs using specific monoclonal antibodies. Food Chem. 141: 4031-4039 (2013)
Kim S, Bang C, Kim A, Ha J, Choi Y, Choung S. Inhibitory effect of active peptide from oyster hydrolysate on Angiotensin-I Converting Enzyme (ACE). Planta Med. 79: 1665-1682 (2013)
Kitaoka C, Hosoe J, Hakamatsuka T, Araya K, Habara M, Ikezaki H, Hamada-Sato N, Shinagawa A, Yamamoto J, Kato-Yoshinaga Y. Taste component analysis of Pacific oysters cultured in Konagai, Nagasaki and taste evaluation using a taste-sensing system. Jpn. J. Food Chem. Saf. 23: 63-71 (2016)
Malgorzata T, Joost VN, Huub S. Food processing: the influence of the Maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients 9: 835-852 (2017)
Martínez-Fernández E, Acosta-Salmón H, Southgate PC. The nutritional value of seven species of tropical microalgae for black-lip pearl oyster (Pinctada margaritifera L.) larvae. Aquaculture 257: 491-503 (2006)
Menezes EA, Oliveira AF, França CJ, Souza GB, Nogueira A. Bioaccessibility of Ca, Cu, Fe, Mg, Zn, and crude protein in beef, pork and chicken after thermal processing. Food Chem. 240: 75-83 (2017)
Mora L, Bolumar T, Heres A, Toldrá F. Effect of cooking and simulated gastrointestinal digestion on the activity of generated bioactive peptides in aged beef meat. Food Funct. 8: 4347-4355 (2017)
Nakamura A, Watanabe K, Ojima T, Ahn DH, Saeki H. Effect of Maillard reaction on allergenicity of scallop tropomyosin. J. Agric. Food Chem. 53: 7559-7564 (2005)
New CY, Kantilal HK, Tan MTH, Nakaguchi Y, Nishibuchi M, Son R. Consumption of raw oysters: a risk factor for Vibrio parahaemolyticus infection. Int. Food Res. J. 21: 2459-2472 (2014)
Njintang NY, Mbofung CMF, Waldron KW. In vitro protein digestibility and physicochemical properties of dry red bean (Phaseolus vulgaris) flour: effect of processing and incorporation of soybean and cowpea flour. J. Agric. Food Chem. 49: 2465-2471 (2001)
Padula D, Greenfield H, Cunningham J, Kiermeier A, Mcleod C. Australian seafood compositional profiles: a pilot study. Vitamin D and mercury content. Food Chem. 193: 106-111 (2015)
Park E, Song KB. Isolation of angiotensin converting enzyme inhibitors from pig blood. Agric. Chem. Biotechnol. 40: 39-42 (1997)
Qian Z, Jung W, Byun H, Kim S. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 99: 3365-3371 (2008)
Ren W, Li Y, Geng X, Wang S, Guo J. Isolation of angiotensin-converting enzyme inhibitor from enzymatic hydrolysate of hog bone collagen. Chin. Biochem. J. 12: 693-697 (1996)
Roskar R, Simoncic Z, Gartner A, Kmetec V. Stability of new potential ACE inhibitor in the aqueous solutions of different pH. J. Pharm. Biomed. Anal. 49: 295-303 (2009)
Singh TK, Øiseth SK, Lundin L, Day L. Influence of heat and shear induced protein aggregation on the in vitro digestion rate of whey proteins. Food Funct. 5: 2686-2698 (2014)
Tian L, Liu J, Ma L, Zhang L, Wang S,Yan E, Zhu H. Isolation and purification of antioxidant and ACE-inhibitory peptides from Yak (Bos grunniens) skin. J. Food Process. Preserv. 41(5): 1-6 (2017)
Tu M, Feng L, Wang Z, Qiao M, Shahidi F, Lu W, Du M. Sequence analysis and molecular docking of antithrombotic peptides from casein hydrolysate by trypsin digestion. J. Funct. Foods 32: 313-323 (2017)
Tu M, Wang C, Chen C, Zhang R, Liu H, Lu W, Jiang L, Du M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 256: 98-104 (2018)
Wang J, Hu J, Cui J, Bai X, Du Y, Miyaguchi Y, Lin B. Purification and identification of a ACE inhibitory peptide from oyster proteins hydrolysate and the antihypertensive effect of hydrolysate in spontaneously hypertensive rats. Food Chem. 111: 302-308 (2008)
Wang YK, He HL, Wang GF, Wu H, Zhou BC, Chen XL, Zhang YZ. Oyster (Crassostrea gigas) hydrolysates produced on a plant scale have antitumor activity and immunostimulating effects in BALB/c mice. Mar. Drugs 8: 255-268 (2010)
Yadzir ZHM, Misnan R, Bakhtiar F, Abdullah N, Murad S. Tropomyosin, the major tropical oyster Crassostrea belcheri allergen and effect of cooking on its allergenicity. Allergy Asthma Clin. Immunol. 11: 30 (2015)
Yu Y, Fan F, Wu D, Yu C, Wang Z, Du M. Antioxidant and ACE inhibitory activity of enzymatic hydrolysates from Ruditapes philippinarum. Molecules 23: 1189 (2018)
Zhao H, Zhu B, Zhou D, Liu Z, Qin L, Jiang X, Response surface methodology optimization of enzymatic hydrolysis of oyster. J. Dalian Univ. Technol. 29: 421-425 (2010)
Zielbauer BI, Franz J, Viezens B, Vilgis TA. Physical aspects of meat cooking: time dependent thermal protein denaturation and water loss. Food Biophys. 11: 34-42 (2016)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771926), the State Key Research and Development Plan “Modern Food Processing and Food Storage and Transportation Technology and Equipment” (2018YFD0400105).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, Z., Zhao, F., Chen, H. et al. Heat treatments of peptides from oyster (Crassostrea gigas) and the impact on their digestibility and angiotensin I converting enzyme inhibitory activity. Food Sci Biotechnol 29, 961–967 (2020). https://doi.org/10.1007/s10068-020-00736-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-020-00736-4