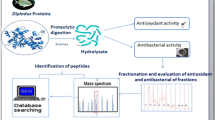
Abstract
Soft tissue from cultured farm fresh oysters (Crassostrea madrasensis) was subjected to two standard enzymatic peptide extraction procedures using pepsin and papain. The crude extracts obtained were partially purified by column chromatography and were freeze-dried. The hydrolysates obtained were compared with respect to their degree of hydrolysis (DH), antioxidant potential (AP) and total phenolic content (TPC). The hydrolysate showing better antioxidant property was further subjected to purification by high performance liquid chromatography and characterized by LC-MS/MS. Papain-digested oyster protein (OPHpap) hydrolysate showed higher DH, AP and TPC. OPHpap was further subjected to ultrafiltration and fractionated into 3 sizes namely, above 10, 3–10 and 1–3 kDa according to the molecular size. Antioxidant capacity of <3 kDa fraction OPHpap-3 evaluated by DPPH free radical scavenging assay, metal chelating activity, linoleic acid autoxidation assay showed maximum effectiveness. Of the seven fractions collected by purification of OPH-pap-3 on semi-preparative RP-HPLC, fraction 7 that showed the highest antioxidant activity was further characterized by LC-ESI-MS/MS and its sequence determined. An antioxidant peptide molecule with thirteen amino acids was identified in oyster protein hydrolysate obtained by papain digestion that may find application as a nutraceutical or may be utilized in food industry for prevention of rancidity in foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Proteins are important food group biomolecules that contribute significantly to nutrition of an individual. It is well recognized that apart from their basic nutritional role, many food proteins contain encrypted within their primary structures peptide sequences capable of modulation of specific physiological functions. Chemical or enzymatic hydrolysis in vitro and digestive proteolysis in the gastrointestinal tract are some of the processes that can release the encrypted peptides from their precursor proteins. Chemical synthesis, gene expression approaches and bacterial fermentation also can be used to generate peptides of desired sequences. Biologically active peptides, once liberated as independent entities, act as potential metabolism modulators and regulatory compounds with hormone-like activities (Shahidi and Zhong 2008). Possible bioactivities reported for peptides include antihypertensive, antioxidant, antiproliferative, anticancer, antimicrobial, and opioid activities as well as immunomodulatory and cholesterol-lowering effects (Picot et al. 2006; Cinq-Mars et al. 2008; Liu et al. 2008a, b; Anderson and Beaven 2001). Some of the areas these peptides can be put to use to improve human health and prevent disease are as functional food ingredients, or nutraceuticals and pharmaceuticals. Proteins and peptides are being considered as good candidates for use as drugs in biological systems. When used as drugs these represent a class referred to as biopharmaceuticals and are markedly different from the class of small drug molecules. The later owing to their small size react indiscriminately with molecules in the living system giving rise to side effects and making them poorly tolerated. Peptides on the other hand being analogs to, synthetic versions of, or endogenous compounds, they tend to be well-tolerated in the body. Also peptides are specialized than small molecules, typically enhancing or substituting an endogenous molecule responsible for a physiological condition or a state of disease. There are currently more than 40 peptide-based drugs on the market and more than 400 in late-stage clinical trials (Stawikowski and Fields 2002).
Marine organisms, which constitute about one half of the entire global biodiversity, are rich reservoirs of easily digestible and bioavailable proteins with high amino acid scores. Therefore it is quite reasonable to assume that they could be ideal initiating materials for the production of protein derived bioactive peptides. Fish are probably the most comprehensively explored aquatic resources as a vital source of peptides with a diverse range of bioactivities reported from them (Raghavan et al. 2008; Dong et al. 2008; Klompong et al. 2009; Bougatef et al. 2010; Hsu 2010; Mendis et al. 2004). Very few studies have been reported in shellfish especially molluscs and crustaceans (Liu et al. 2008a, b). Oysters (Crassostrea madrasensis) are a good source of high quality easily digestible protein and essential amino acids of high amino acid score and hence quite beneficial for human health (Asha et al. 2014). Though C. madrasensis is comparable to fish with respect to its nutritional attributes with its protein being of high quality and its lipids being a good source of n-3 and n-6 fatty acids, consumer preference to the fishery is scant (Asha et al. 2014). Increasing consumer knowledge of the link between diet and health has raised the awareness and demand for functional food ingredients and nutraceuticals. It was postulated that use of enzymatic methods for digesting oyster protein would result in isolating specific peptides possessing bioactive potential. Among the wide range of bioactivities reported for peptides, antioxidant potential is the most extensively researched owing to its relevance for both human health and the food industry. Several chronic diseases including diabetes, cardiovascular diseases, and neurodegenerative diseases are thought to arise due to excessive generation of free radicals in the body (Pham-Huy et al. 2008). Globally, food industries constantly strive to protect food from oxidative rancidity and off flavour development and among the strategies they employ to prevent these undesirable changes to food is the addition of synthetic antioxidants like butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), tert-butylhydroquinone (TBHQ), and propyl gallate. However increasing awareness about the safety concerns associated with these substances is limiting their use. Natural antioxidants with virtually no side-effects have gained attention in terms of their potential health benefits and also for their ability to protect food against oxidative damage. Oyster protein hydrolysate in the present study was made by enzymatic digestion and was concentrated based on molecular weight by ultrafiltration. Hydrolysates were further purified by preparative RP-HPLC and a particular RP-HPLC fraction that showed significantly higher antioxidant activity was subjected to LC-MS/MS characterization. This resulted in the deciphering the sequence of a peptide with 13 amino acids. This study contributes to the ongoing research on antioxidant peptides from marine sources and their application in food and medicine.
Materials and Methods
Sample Collection
Cultured Oysters were harvested in live condition from Moothakunnam in Ernakulam District and depurated for a few hours. They were transported to the laboratory under iced condition in insulated styrofoam boxes and were thoroughly washed to remove slime and dirt. The surface water was blotted with filter paper, edible meat (approximately 1000 g) was separated from the shells and kept on ice for immediate use.
Preparation of Oyster Protein Hydrolysate and Degree of Hydrolysis Determination
Hydrolysis was performed under the following conditions. With an enzyme-to-substrate ratio of 1/100 (w/w), substrate and glycine–HCl buffer (pH 2.0) (1:1) and either pepsin or papain were mixed in two separate reactions. The mixtures were incubated for 6 h at 37 °C and either at pH 2.2 or 5.5 with stirring and then heated in a boiling-water bath for 10 min to inactivate the enzymes. Following inactivation, the contents were centrifuged and the supernatants were spray-dried to obtain OPH-pep and OPH-pap which were saved for further studies. DH was followed for the two hydrolysis reactions and was determined by the method of Nielsen et al. (2001). which is based on the fact that the α-amino groups released by hydrolysis react with ninhydrin to form a compound that absorbs strongly at 570 nm. Samples were drawn at 30 min intervals during the reactions and inactivated by heating in a boiling water bath. Ninhydrin reaction was performed in the drawn samples as well as a standard calibration using leucine standard was carried out. All analyses were performed in triplicates.
Total Phenolic Content
The total phenolic content in OPH samples was determined according Singleton and Rossi (1965). Briefly, 1.5 mg of the spray-dried hydrolysate was dissolved in 5 mL of methanol from which a small aliquot was taken and dissolved in 1 % TFA in methanol. Folin-Ciocalteu reagent was added followed by 20 % sodium carbonate solution. The mixture was incubated at 40 °C for 30 min and absorbance was measured at 765 nm on UV/Visible spectrophotometer gallic acid standard was used to prepare the standard calibration curve. The gallic acid equivalent (GAE) was determined from standard curve and the results were expressed as µg of GAE mg−1 of OPH.
Antioxidant Activity
DPPH Free Radical Scavenging Assay
Comparison of free radical scavenging potential of oyster protein hydrolysates obtained by pepsin and papain digestions was determined by using a stable 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical (Sigma) scavenging assay following the method of von Gadow et al. (1997). The assay mixture contained 1.9 mL of 5 × 10−5 M DPPH radical dissolved in ethanol and 0.1 mL of sample solution (concentration range 0.5–10 mg mL−1). After a 30-min incubation period at room temperature, the absorbance was read against a blank at 517 nm. Inhibition of free radical by DPPH in percent (I %) was calculated according to Eq. 1
where A blank is the absorbance of the control reaction (containing all reagents except the test compound) and A sample is the absorbance of the test compound.
Hydroxyl Radical Scavenging Assay
Hydroxyl radicals were generated by a Fenton reaction (Fe3+- ascorbate-EDTA-H2O2 system), and the scavenging capacity towards the hydroxyl radicals was measured by using deoxyribose method (Halliwell et al. 1988). The reaction mixture contained 2-deoxy-2-ribose (2.8 mM), phosphate buffer (0.1 mM, pH 7.4), ferric chloride (20 μM), EDTA (100 μM), hydrogen peroxide (500 μM), ascorbic acid (100 μM) and various concentrations (10–1000 μg mL−1) of the test sample in a final volume of 1 mL. The mixture was incubated for 1 h at 37 °C. After the incubation an aliquot of the reaction mixture (0.8 mL) was added to 3 % TCA solution (1.5 mL), followed by TBA solution (1 % in 50 mM sodium hydroxide, 1 mL) and sodium dodecyl sulphate (0.2 mL). The mixture was then heated (20 min at 90 °C) to develop colour. After cooling, the absorbance was measured at 532 nm against an appropriate blank solution. All experiments were performed in triplicates. The percentage of inhibition was expressed, according to the following equation: \(\left( \% \right) = \left[ {{\text{A}}_{0} - \left( {{\text{A}}_{ 1} - {\text{A}}_{ 2} } \right)} \right]/{\text{A}}_{0} \times 100\), where: A0 is the absorbance of the control without a sample, A1 is the absorbance in the presence of the sample and deoxyribose and A2 is the absorbance of the sample without deoxyribose.
Reducing Power by FRAP Assay
The ferric reducing antioxidant power (FRAP) assay was carried out according to the protocol reported by Benzie and Strain (Iris et al. 1999). The FRAP reagent is prepared as follows: 300 mM sodium acetate buffer (pH 3.6, 25 mL), 10 mM TPTZ (2,4,6-tripyridyl-s-triazine) solution (2.5 mL) in 40 mM HCl solution (20 mL) and 20 mM FeCl3.6H2O solution (2.5 mL) were mixed thoroughly and kept at 37 °C throughout the experiment. After initial incubation for 10 min, the absorbance of the reagent was noted at 593 nm. For test, 3 mL of FRAP reagent was added to 100 µL of sample (1 mg mL−1) and absorbance was noted at 593 nm after 5 min. The blank was prepared by dissolving 100 µL of methanol in 3 mL of FRAP reagent. Ascorbic acid was used as a standard antioxidant to determine FRAP values for different concentrations and a calibration curve was plotted. The results were expressed in terms of µg of ascorbic acid equivalents (AAE) g−1 OPH.
Ultrafiltration
A portion of oyster protein hydrolysate obtained by papain digestion (OPHpap) that showed better antioxidant potential was fractionated by ultrafiltration (UF, Amicon® Model 8400, Millipore Corporation, Billerica, MA) with Millipore UF membranes having molecular weight cut-offs (MWCOs) of 10, 3, and 1 kDa respectively. Aliquots of 100 g L−1 OPHpap was passed through 10 kDa MWCO membrane. The flow-through-1 was less than 10 kDa fraction and the retentate OPHpap-1 was greater than 10 kDa fraction. Flow-through-1 was passed through 3 kDa MWCO membrane. Flow-through-2, was less than 3 kDa and the retentate, OPHpap-2 had MW range between 3 and 10 kDa. Flow-through-2 was passed through 1 kDa MWCO membrane. The flow-through-3 was less than 1 kDa and the retentate, OPHpap-3 had MW range between 1 and 3 kDa. Fractionates were designed as in Table 1. All UF fractions of OPH-pap recovered were lyophilized and kept at −70 °C until further use.
Amino Acid Composition and Antioxidant Potential of the UF Fractions
Amino acid composition was determined following Ishida et al. (1981). Briefly, muscle protein was hydrolyzed with 6 N hydrochloric acid at 110 °C under anaerobic condition for 24 h. The hydrolyzed samples were neutralized with 6N NaOH and injected in reverse phase high performance liquid chromatography (RP-HPLC) (Shimadzu) equipped with a C18 RP column, post column derivatization unit and a fluorescence detector. The amino acids were identified and quantified by comparing with the retention times and peak areas of standards. For the tryptophan analysis, minced meat was digested with 5 % (w/v) NaOH for 24 h and neutralized to pH 7.0 with 6 N HCl. Tryptophan content was measured spectrophotometrically at 530 nm (Sastry and Tammuru 1985). All data have been presented as mean of three determinations ± standard deviation.
Antioxidant capacity of UF fractions were compared by assaying for DPPH free radical scavenging activity, FRAP assay, and Hydroxyl scavenging assay. The fraction that showed the highest antioxidant capacity was further tested for its metal chelating affect and for its ability to inhibit linoleic acid autoxidation.
Ferrous Chelating Activity of UF Fraction
The chelating effect of UF fraction on ferrous ions was estimated by the method of Dinis with slight modifications (Dinis et al. 1994). Briefly, 100 μL of each test sample (1 mg mL−1) was taken and raised to 3 mL with methanol. 740 μL of methanol was added to 20 μL of 2 mM FeCl2. The reaction was initiated by the addition of 40 μL of 5 mM ferrozine into the mixture, which was then left at room temperature for 10 min and then the absorbance of the mixture was determined at 562 nm.
Lipid Peroxidation Inhibition by UF Fraction
Lipid peroxidation assay was carried out by method of Mitsuda et al. (1966) Linoleic acid emulsion was prepared by mixing 155 µL of the acid with 175 µg of Tween-20; the mixture was added to 50 mL of potassium phosphate buffer (0.04 M, pH 7). A small aliquot of the sample in 2.4 mL of potassium phosphate buffer was added to linoleic acid emulsion and incubated at 37 °C. An aliquot from the incubated solution was regularly taken at 24 h intervals for 4 days and allowed to react with 20 mM FeCl2 and 30 % ammonium thiocyanate. The absorbance was noted at 500 nm. A solution consisting of equal quantities of linoleic acid emulsion and potassium phosphate buffer was taken as a blank. The ability of standard butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT) to inhibit peroxidation in linoleic acid measured.
Molecular Weight Distribution of UF with Highest Antioxidant Capacity
Gel filtration column liquid chromatography was performed on a Sephadex G-25 column ideal for separation of peptides and small proteins of size range 1–5 kDa. A 200 μL aliquot of the pap-OPH (2 mg mL−1 solution) was loaded on the column that was previously equilibrated by using a 30 % acetonitrile with 0.1 % TFA (v/v) and connected to a FPLC system (Amersham Bioscience). The sample was eluted using the same buffer at a flow rate of 0.2 mL/min and elution was monitored at 214 nm characteristic absorbance maxima for peptide bonds. Phenylalanine (FW 165) and lactoferricin (FW 3196) were used as molecular weight standards.
Purification of the UF Fraction with Highest Antioxidant Capacity by RP-HPLC
The UF fraction with highest antioxidant capacity obtained previously was partially purified by column chromatography by pumping onto four Sep-Pak C-18 cartridges (Waters Associates) connected in series at a flow rate of 2 mL min−1. The retained material was eluted with 60 % methanol. The peptides were quantified by biuret method. The concentration of the extract was 16.82 mg mL−1. The eluate was concentrated; freeze dried and stored at −80 °C for further studies. Small aliquots of the freeze-dried UF fraction were re-dissolved in 60 % methanol and purified further on an analytical reverse-phase Shimadzu C-18 column equilibrated with 0.1 % (v/v) trifluoroacetic acid/water at a flow rate of 2 mL min−1. The concentration of acetonitrile in the eluting solvent was raised to 21 % (v/v) over 10 min, held at this concentration for 40 min and then raised to 49 % (v/v) over 60 min with linear gradients. Absorbance was measured at 214 and 280 nm. In order to gather enough sample for ensuing antioxidant assays and with an objective of further purification step, the process was repeated for 16 times. After removing acetonitrile and TFA under a stream of nitrogen, pooled fractions were freeze-dried and reconstituted in HPLC-grade water. For each fraction appropriate dilutions were made depending on the relative amount recovered to facilitate comparison of their relative antioxidant capacities. Antioxidative capacity was measured using the DPPH free radical scavenging assay.
Characterization of a Preparative RP-HPLC Fraction by LC-MS/MS
The preparative RP-HPLC fraction showing the highest antioxidant capacity was further sequenced by liquid chromatography- positive electron spray ionization tandem mass spectrometry (LC-ESI-MS/MS). Total ion chromatogram was acquired in information dependent acquisition mode with a linear ion trap mass spectrometer (AB Sciex 4000 QTrap®) hyphenated to a UPLC (Waters Acquity®), equipped with a C18 column (BEH 130 peptide, 1.7 µ, 2.1 × 100 mm). A gradient elution programme of 99 % eluent A (water containing 0.1 % TFA) and 1 % eluent B (acetonitrile containing 0.1 % TFA) at 0 min to 1 % eluent A and 99 % eluent B at 33 min was used. Enhanced mass spectra (EMS) and enhanced resolution spectra of the prominent peak eluting at 23.8 min were acquired. Further enhanced product ion (EPI) spectra of the tentative molecular ion (m/z 646) was acquired and exported to Protein pilot® software (ABSciex) to determine the amino acid sequence.
Statistical Analysis
Statistical analysis was performed using SPSS software, version 10.0. The comparison of different biochemical parameters were tested using Duncan’s test (95 % confidence interval) with one-way analysis of variance (ANOVA).
Results and Discussion
In our study, soft tissues from oyster were directly treated with commercial enzymes pepsin and papain and no action was taken to inactivate the endogenous enzymes present in oyster soft tissues. Thus the peptides generated and the bioactivities demonstrated here are combined functions of the peptide populations produced by digestion by added commercial as well as endogenous enzymes. But the ratio of the endogenous enzymes to the soft tissue muscle must be quite low here unlike for substrates made entirely of fish processing discards which predominantly contain gut entrails rich in proteolytic enzymes. In addition, the effect of these endogenous enzymes on protein digestion would be negligible if not entirely absent owing to the large amount of the substrate available for enzyme action compared to the very low amounts of the endogenous enzymes. We also think that, peptides generated by endogenous enzyme action would only add to the richness of the peptide population and is not a deterrent in any way.
Yield and Degree of Hydrolysis
The activity of peptides in hydrolysates is a direct consequence of their amino acid composition and sequence. In vitro, the nature of peptides formed following digestion depends on the amino acid sequence of the protein, the type of enzyme used and the degree of hydrolysis that takes place. The latter is in turn influenced by the enzyme substrate ratio, pH, temperature and time of digestion. Yield, percentage recovery and degree of hydrolysis obtained for papain and pepsin digested oyster are given in Table 2. Lesser yields than what were obtained in this study were reported for sardine hydrolysates by Souissi et al. (2007).
Papain-digested oyster protein hydrolysate (OPHpap) showed small but significant increase in all the three measured aspects namely yield, percentage recovery and degree of hydrolysis than pepsin- digested oyster protein hydrolysate (OPHpep). Degree of hydrolysis (Table 2) obtained for OPHpap and OPHpep was 27.3 and 22.4 respectively and agrees with DH reported for fish protein hydrolysates by Sathivel et al. ((2005). in red salmon and by Souissi et al. (2007). in sardines. Hydrolysis for both the enzymes proceeded at a faster rate during the initial stages of the reaction, generating rapidly increasing concentration of soluble peptides with time for up to 3 h when the maximum recovery of peptides was observed. Following this period, a more static response was seen where hydrolysis time did not significantly affect the reaction rate and a steady-state phase was reached.
Many of the physiological and functional properties of proteins are believed to be attributed to biologically active peptides encrypted in the protein molecules. Pepsin has a very broad specificity and is believed to often cleave after bulky hydrophobic residues (Fruton et al. 1961). The multiple cleavage sites means that the peptides produced are usually small, around 3–30 residues in length. Despite its broad specificity pepsin is a very reproducible enzyme, indicating it will yield the same peptides when digestion of a protein is performed at identical conditions (Zhang and Smith 1993). Several studies show that pepsin cleaves after most bulky hydrophobic residues such as leucine and phenylalanine and has a low probability of cleaving after the residues arginine, cysteine, proline and histidine (Palashoff 2008). Papain cleaves next to either Arg or Lys residue that is subsequent to any of the hydrophobic amino acids. Proteins in oyster used in this study occur in their native state and are folded in three-dimensional space in a manner that keep the bioactive amino acids particularly the aromatic and other hydrophobic residues buried in pockets away from exposure to aqueous environment. Enzymatic digestion that specifically cleaves after basic amino acids in the case of papain and bulky hydrophobic residues in case of pepsin, exposes these sites making them amenable to react with various biomolecules giving rise to bioactivities. Hence degree of hydrolysis is an important influence that determines the type and extent of bioactivities observed in protein hydrolysates.
Antioxidant Activity
There is an increased incidence of oxidative stress-related ailments and so also a rising awareness about the benefits of naturally derived nutraceuticals. This has intensified research efforts for developing antioxidant peptides from natural sources in recent times. Also due to strict regulations against use of synthetic chemicals in the food industry to prevent oxidative damage to food, search for natural alternatives is ongoing. Many fish protein hydrolysates such as those from Allaska Pollack (Je et al. 2005), mackerel (Wu et al. 2003), tilapia (Kristinsson and Leeuwenburgh 2008) etc. have been demonstrated to show antioxidant property. In the current study, the hydrolysates, when tested for their antioxidant potential, showed that OPHpap had significantly better DPPH free radical scavenging ability, hydroxyl free radical scavenging activity, FRAP than OPHpep (Table 3). Among the several type of amino acids that occur in proteins, aromatic amino acids containing the phenolic groups are responsible for the bulk of their antioxidant potential. In the present study, the hydrolysates were estimated for their total phenolic content to correlate their antioxidant capacity with their total phenolic content (TPC). OPHpap also showed significantly (P < 0.05) high TPC (Table 3) when compared to OPHpep.
In the DPPH and hydroxyl free radical scavenging assays, OPHpap at a concentration of 1 mg mL−1 showed IC50 values of 69 and 82 μg when compared to 143 and 109 μg for OPHpep. In ferric reducing antioxidant power (FRAP) assay, ferric ions are reduced to ferrous ions in the presence of an antioxidant and a coloured ferrous tripyridyltriazine complex is formed whose intensity is measured at 593 nm. OPH-pap showed significantly high AAE g−1 than OPHpep. Considering the higher yield, degree of hydrolysis, antioxidant potential and higher phenolic content of the hydrolysate obtained by using papain, pap-OPH was subjected to ultrafiltration in order to fractionate the peptides according to their molecular weight.
Ultrafiltration (UF) and Antioxidant Activity of the UF Fractions
Ultrafiltration (UF) is usually used to refine hydrolysates and also to fractionate them in order to obtain a peptide population enriched in a selection of sizes (Kim et al. 2007). In this study, successive fractionation on UF membranes allowed concentration of peptides of designated sizes which were labelled as OPHpap-1, 2 and 3 (Table 1).
The basic intent of the study was to successively concentrate and purify peptides in the OPH on the basis of the antioxidant capacity of fractions of the hydrolysate. Ultrafiltration fractions, were compared with respect to their antioxidant potential. Hydroxyl free radical scavenging activity of the UF fractions is shown in Fig. 1.
OPHpap-3 required just 128.1 μg mL−1 to achieve 100 % inhibition of hydroxyl free radical and its IC50 concentration was 65.2 μg mL−1 while nearly 200 μg mL−1 of OPHpap-1 (IC50-102 μg mL−1) was required to give 100 % inhibition. In this assay, the hydroxyl free radicals oxidize the substrate 2 deoxy 2 ribose producing oxidation products that are then made to react with TBA to give rise to pink chromogens that absorb at 532 nm. In the presence of OPHpap-3, the intensity of the chromogen forming oxidation products decreased. Antioxidant capacity was further validated by DPPH free radical scavenging and FRAP assays and was correlated with TPC. IC50 concentrations of UF fractions required to inhibit DPPH free radical, FRAP assay in terms of mg AAE g−1 and TPC of the UF fractions are shown in Table 4.
41 μg of UF fraction OPHpap-3 was required to give 50 % inhibition of DPPH radical and the fraction showed 0.4 mg AAE g−1 indicating that OPHpap-3 was the most potent of the three fractions in terms of its antioxidant capability. OPHpap showed a certain degree of hydroxyl free radical scavenging activity, DPPH free radical scavenging activity, and ferric reducing antioxidant potential (FRAP) that were increased in fractions obtained following ultrafiltration, the maximum activity being detected in OPHpap-3 (Table 4). Results expressed as mg of antioxidant power g−1 of OPH showed that the fractions had widely different in vitro antioxidant power and that the antioxidant capacity was strongly correlated (r = 0. 998) with the TPC of the OPH. Ting et al. (2001) showed that ORAC values were increased by more than threefold in UF-fractions of egg yolk protein enzymatic hydrolysates.
For further confirmation of the antioxidative capacity of OPHpap-3, its metal chelating activity, and its ability to inhibit linoleic auto-oxidation were evaluated. Significant metal chelating effect was observed in OPHpap-3 and the percentage metal chelating effect increased with increasing sample concentration (Fig. 2). Ferrous ion (Fe2+) is the most powerful pro-oxidant among metal ions. It can interact with hydrogen peroxide in a Fenton reaction to produce the reactive oxygen species and hydroxyl free radical (OH), leading to the initiation and/or acceleration of lipid oxidation. As a consequence of chelation of metal ions by peptides in hydrolysates the oxidative reaction would be impeded. Results indicated that OPH-3 was able to interact with pro-oxidative iron (II) ions and metals, resulting in lowered oxidation. Ferrous ion (Fe2+) is the most powerful pro-oxidant among metal ions and significant ferrous chelating activity has been reported for hydrolysates of silver carp(Klompong et al. 2009), threadfin bream (Nalinanon et al. 2001) and yellow stripe trevally (Klompong et al. 2008).
Protecting food from oxidation is very important in terms of preserving its quality and nutritional attributes. Protein hydrolysates derived from various sources like fish, shellfish, milk, meat etc. are tested for antioxidant potential by using linoleic acid (Zhu et al. 2006), an unsaturated fatty acid which serves as an ideal compound for lipid oxidation and antioxidant studies in an ethanol/water emulsion system. Lipid peroxidation of linoleic acid is thought to progress via radical-mediated withdrawal of hydrogen from methylene carbons(Ramarathnam et al. 1988) which was effectively prevented by peptides molecules in OPHpap-3. Cheng et al. (2003) reported that in a similar model system, phenolic compounds could perform antioxidative activity by scavenging lipid-derived radicals and thereby breaking free radical chain reaction of peroxidation. Rajapakse et al. (2005) showed that giant squid muscle protein hydrolysates inhibit lipid peroxidation of linoleic acid.
Percentage inhibition of linoleic acid auto-oxidation by control, OPHpap-3, BHT and BHA is shown in Fig. 3. There is no significant difference (P > 0.05) in the percentage inhibition of linoleic auto-oxidation among the samples. In fact OPHpap-3 has shown inhibition similar to that of known synthetic antioxidants, BHT and BHA. The antioxidant ability of OPHpap-3 may be attributed to the presence of amino acid residues capable of extracting free radicals from the hydroperoxides formed during the process of linoleic acid auto-oxidation. Amino acids with such a property are most likely to be of hydrophobic (Rajapakse et al. 2005) type, which have enhanced affinity to the hydrophobic fatty acid. The probability of interaction of the amino acid residues in the hydrolysate with free radicals and their subsequent chain-breaking action on the free radical chain mechanism could have contributed to the anti-oxidative property. Off flavour and unpleasant odour development termed as rancidity, in foods especially rich in fats and oils can be prevented by proper application of antioxidant formulations.
Amino Acid Composition of the Oyster Protein Hydrolysates
In our study, OPHpap-3 that was demonstrated to have highest antioxidant potential showed higher levels of amino acid residues like Try, Tyr, Met, Glu, Cys, Arg (Fig. 4) all of which are known to possess antioxidant potential by virtue of their chemical structure. An amino acid acts as an antioxidant provided it has the critical ability to itself get oxidized prior to the oxidation of the macromolecules like lipid or protein thereby sparing them from degradation.
It has been reported (Li and Li 2013) that amino acid susceptibility to hydroxyl-mediated oxidation followed the order Cys > Trp, Tyr > Met > Phe > His > Ile > Leu > Pro (Sharp et al. 2003). Also charged amino acids like Glu and Arg, aromatic amino acids Try and Tyr also have antioxidant potential as reported in earlier studies. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates by Udenigwe and Aluko (2011) showed that sulfur-containing (SCAA), acidic and hydrophobic amino acids had strong positive effects on scavenging of 2,2-diphenyl-1-picrylhydrazyl (DPPH) and H2O2 radicals in addition to ferric reducing antioxidant power. The indole group of Try and phenol group of Tyr are responsible for the oxidizability of these amino acid residues making them capable of limiting oxidation processes. Previous studies indicate a relationship between the physicochemical properties of the C-terminal and N-terminal regions of the peptides and antioxidant potency in addition to the electronic property of the peptides (Li and Li 2013).
Molecular Weight Distribution Profile of Ultrafiltration Fraction
Results from GF-column chromatography OPHpap-3 are shown in Fig. 5. Most of the peptides present were eluted between the 3200 and 165 Da molecular weight standards. The relationship between molecular size of peptides in protein hydrolysates and the extent of their bioactivities has been reported by several authors. It has been shown that generally strong antioxidant properties are exhibited by peptides with low molecular weight (Kumar et al. 2011).
Purification of UF Fraction Using Semi-Preparative RP-HPLC
The isolation and purification of bioactive peptides are very important for exploration of their physicochemical properties and evaluation of their in vitro and in vivo bioactivities. Bioactive peptides can be separated from a protein hydrolysate mixture by a number of approaches, mainly, different kinds of chromatography and membrane-based separation techniques. Prior to the separation process in our study, the UF fraction OPHpap-3 was subjected to solid phase extraction to remove co-extractives. Seven peaks were seen with absorption maxima at 214 characteristic for peptide bond (Fig. 6). Antioxidant potential of the seven fractions pooled from 16 RP-HPLC runs were tested by DPPH free radical assay and the results are shown in Fig. 7. The area under each RP-HPLC peak was used to estimate the relative amounts of peptides present in each fraction since it was very difficult to collect enough peptides to get actual sample weights.
Since peak seven showed the maximum DPPH free radical inhibition, the fraction was subjected to further characterization. Since the unfractionated OPHpap-3 is a crude mixture of many peptides and free amino acids, the MS spectrum was complex (not shown). However, this spectrum also confirmed the fact that the majority of the peptides present in FPH are less than about 3000 Da in size. Total ionic chromatogram (TIC), enhanced mass spectrum (EMS), enhanced resolution (ER) and enhance product ion (EPI) of RP-HPLC fraction F-7 of OPHpap-3 is shown in Fig. 8.
The precursor MS region, production spectra and the Y type and B type fragments for the elucidation of the amino acid sequence are shown in Figs. 9, 10 and Table 5 respectively for ease of comprehension. The amino acid sequence (Table 6) as determined in the study is Ile-Ser-Ile-Gly-Gly-Gln-Pro-Ala-Gly-Arg-Ile-Val-Met. Using online peptide analysis tools,Footnote 1 the hydrophobicity was determined to be about 53.85 %. Dong et al. (2008) reported that higher antioxidant activity of silver carp protein hydrolysate was due to its higher content of hydrophobic amino acids. Ile and Val the hydrophobic and branched chain amino acids (BCAA) (Tanaka et al. 2014) in the peptide identified are widely recognized as having a significant antioxidant potential. Ichikawa et al. (2012) showed that BCAA upregulated DNA repair enzyme in carbon tetrachloride-induced cirrhosis in rats where the DNA damage was due to oxidative stress. Met is an efficient scavenger of almost all oxidizing molecules like H2O2, hydroxyl radicals, chloramines, hypochlorous acid, peroxynitrite etc. under physiological conditions. In the event of oxidative stress, Met residues within a protein exposed to surface get oxidized without causing loss of protein function and is the only amino acid apart from Cys that is restored to its reduced state (Levine et al. 1999). Gly present in the purified peptide is known to be a direct proton donor which is critical to the free radical scavenging property of antioxidant peptides due to their capacity to reduce unpaired electrons or free radicals by donating protons (Qian et al. 2008). Glutamine was shown to act as a potent antioxidant that protected the gastric mucosa and decreased oxidative damage to the gastric mucosa in a rat model of portal hypertension (Marques et al. 2013). Arginine supplementation enhanced antioxidant defence in experimental rats (Shan et al. 2013). The anti-atherogenic effects of the amino acid l-arginine are thought to be due to its antioxidant effects mediated by its alpha amino group (Wallner et al. 2001). Several studies have shown that fish protein hydrolysates were purified to obtain proline rich peptides (Bougatef et al. 2009). Salmon protein hydrolysate was shown to contain a dipeptide Pro-Arg that had maximum scavenging property (Wang et al. 2008). Studies have shown that l-serine (Maralani et al. 2012) has antioxidant and cytoprotective effects through the elevation of some crucial antioxidant factors such as Nrf2, HO-1 and NO.
Conclusions
Oyster protein hydrolysates (OPHs) were prepared by using two proteases, papain and pepsin. Papain-digested OPH showed better yield, degree of hydrolysis, total phenolic content and antioxidant activity. The same was subjected to ultrafiltration and the fraction below 3 kDa that had significantly higher antioxidant activity was further purified by preparative RP-HPLC. The UF fraction analysed for its molecular weight distribution by get filtration chromatography showed that the peptides ranged from about 200–3000 Da. One of the seven fractions (fraction 7) obtained by RP-HPLC that showed good antioxidant property was subjected to LC-MS/MS and sequence was determined as Ile-Ser-Ile-Gly-Gly-Gln-Pro-Ala-Gly-Arg-Ile-Val-Met (1298 Da). Research in the field of antioxidants has garnered much interest due to the ever-increasing substantiation of the importance of antioxidants in human health. Food industries confront the issue of rancidity or oxidation induced spoilage of foods by relying on synthetic antioxidants, whose use is being discouraged due to their toxicity. In the light of these observations, it is appropriate to state that peptide identified in our study and also oyster protein hydrolysates in general may have the potential to be developed into health foods and also to be used as antioxidant formulations in the food industry.
References
Anderson RS, Beaven AE (2001) Antibacterial activities of oyster (Crassostrea virginica) and mussel (Mytilus edulis and Geukensia demissa) plasma. Aquat Living Resour 14:343–349
Asha KK, Anandan R, Mathew S, Lakshmanan PT (2014) Biochemical profile of oyster Crassostrea madrasensis and its nutritional attributes. Egypt J Aquat Res 40:35–41
Bougatef A, Hajji M, Balti R, Lassoued I, Triki-Ellouz Y, Nasri M (2009) Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem 114:1198–1205
Bougatef A, Nedjar-Arroume N, Manni L, Ravallec R, Barkia A, Guillochon D, Nasri M (2010) Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem 118:559–565
Chay Pak Ting BP, Mine Y, Juneja LR, Okubo T, Gauthier SF, Pouliot Y (2001) Comparative composition and antioxidant activity of peptide fractions obtained by ultrafiltration of egg yolk protein enzymatic hydrolysates. Membranes 1:149–161
Cheng Z, Ren J, Li Y, Chang W, Chen Z (2003) Establishment of a quantitative structure-activity relationship model for evaluating and predicting the protective potentials of phenolic antioxidants on lipid peroxidation. J Pharm Sci 92:475–484
Cinq-Mars CD, Hu C, Kitts DD, Li-Chan EC (2008) Investigations into inhibitor type and mode, simulated gastrointestinal digestion, and cell transport of the angiotensin I-converting enzyme-inhibitory peptides in Pacific hake (Merluccius productus) fillet hydrolysate. J Agric Food Chem 56:410–419
Dinis TCP, Madeira VMC, Almeida LM (1994) Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch of Biochem Biophys 315:161–169
Dong SY, Zeng MY, Wang DF, Liu ZY, Zhao YH, Yang HC (2008) Antioxidant and biochemical properties of protein hydrolysates prepared from Silver carp (Hypophthalmichthys molitrix). Food Chem 107:1485–1493
Fruton JS, Fujii S, Knappenberger MH (1961) The mechanism of pepsin action. Proc Natl Acad Sci USA 15(47):759–761
Halliwell B, Grootveld M, Gutteridge JM (1988) Methods for the measurement of hydroxyl radicals in biomedical systems: deoxyribose degradation and aromatic hydroxylation. Methods Biochem Anal 33:59–90
Hsu KC (2010) Purification of antioxidative peptides prepared from enzymatic hydrolysates of tuna dark muscle by-product. Food Chem 122:42–48
Ichikawa K, Okabayashi T, Shima Y, Iiyama T, Takezaki Y, Munekage M, Namikawa T, Sugimoto T, Kobayashi M, Mimura T, Hanazaki K (2012) Branched-chain amino acid-enriched nutrients stimulate antioxidant DNA repair in a rat model of liver injury induced by carbon tetrachloride. Mol Biol Rep 39:10803–10810
Iris F, Benzie F, Strain JJ (1999) Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. In: Packer L (ed) Methods in enzymology. Academic Press, New York, pp 15–27
Ishida Y, Fujita T, Asai K (1981) New detection and separation method for amino acids by high-performance liquid chromatography. J Chromatogr 204:143–148
Je JY, Park PJ, Kim SK (2005) Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res Int 38:45–50
Kim SY, Je JY, Kim SK (2007) Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J Nutri Biochem 18:31–38
Klompong V, Benjakul S, Kantachote D, Hayes KD, Shahidi F (2008) Comparative study on antioxidative activity of yellow stripe trevally protein hydrolysate produced from Alcalase and Flavourzyme. Int J Food Sci Technol 43:1019–1026
Klompong V, Benjakul S, Yachai M, Visessanguan W, Shahidi F, Hayes K (2009) Amino acid composition and antioxidative peptides from protein hydrolysates of yellow stripe trevally (Selaroides leptolepis). J Food Sci 74:C126–C133
Kumar NSS, Nazeer RA, Jaiganesh R (2011) Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 32:1496–1501
Levine RL, Bertlet BS, Moskovitz J, Mosoni L (1999) Methionine residues may protect proteins from critical oxidative damage. Mech Ageing Dev 107:323–332
Li Y-W, Li B (2013) Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: key sequence positions and their amino acid properties. J Theor Biol 318:29–43
Liu Z, Dong S, Xu J, Zeng M, Song H, Zhao Yuanhui (2008) Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 19:231–235
Maralani MN, Movahedian A, Javanmard SH (2012) Antioxidant and cytoprotective effects of l-serine on human endothelial cells. Res Pharm Sci 7:209–215
Marques C, Licks F, Zattoni I, Borges B, de Souza LER, Marroni CA, Marroni NP (2013) Antioxidant properties of glutamine and its role in VEGF-Akt pathways in portal hypertension gastropathy. World J Gastroenterol 19:4464–4474
Mendis E, Rajapakse N, Kim SK (2004) Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J Agric Food Chem 53:581–587
Mitsuda H, Yasumoto K, Iwami I (1966) Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyoto Shokuryo 19:210–214
Nalinanon S, Benjakul S, Kishimura H, Shahidi F (2001) Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem 124:1354–1362
Nielsen PM, Petersen D, Dambmann C (2001) Improved method for determining food protein degree of hydrolysis. J Food Sci 66:642–646
Palashoff MH (2008) Determining the specificity of pepsin for proteolytic digestion. Department of Chemistry and Chemical Biology, Northeastern University, Boston, MS thesis, p. 78
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Inter J Biomed Sci 4:89–96
Picot L, Bordenave S, Didelot S, Fruitier-Arnaudin I, Sannier F, Thorkelsson G et al (2006) Antiproliferative activity of fish protein hydrolysates on human breast cancer cell lines. Process Biochem 41:1217–1222
Qian ZJ, Jung WK, Byun HG, Kim SK (2008) Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster. Crassostrea gigas against free radical induced DNA damage. Bioresour Technol 99:3365–3371
Raghavan S, Kristinsson HG, Leeuwenburgh C (2008) Radical scavenging and reducing ability of tilapia (Oreochromis niloticus) protein hydrolysates. J Agric Food Chem 56:10359–10367
Rajapakse N, Mendis E, Byun HG, Kim SK (2005) Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J Nutr Biochem 16:562–569
Ramarathnam N, Osawa T, Namiki M, Kawakishi S (1988) Chemical studies on novel rice hull antioxidants. I. Isolation, fractionation partial characterization. J Agric Food Chem 36:732–737
Sastry CSP, Tammuru MK (1985) Spectrophotometric determination of tryptophan in proteins. J Food Sci Tech 22:146–147
Sathivel S, Smiley S, Prinyawiwatkul W, Bechtel PJ (2005) Functional and nutritional properties of red salmon (Oncorhynchus nerka) enzymatic hydrolysates. J Food Sci 70:401–406
Shahidi F, Zhong Y (2008) Bioactive peptides. J AOAC Int 91:914–931
Shan L, Wang B, Gao G, Cao W, Zhang Y (2013) l-Arginine supplementation improves antioxidant defenses through l-arginine/nitric oxide pathways in exercised rats. J Appl Physiol 115:1146–1155
Sharp JS, Becker JM, Hettich RL (2003) Protein surface mapping by chemical oxidation: structural analysis by mass spectrometry. Anal Biochem 313:216–225
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16:144–158
Souissi N, Bougatef A, Triki-Ellouz Y, Nasri M (2007) Biochemical and functional properties of Sardinella (Sardinella aurita) by-product hydrolysates. Food Technol Biotechnol 45:187–194
Stawikowski M, Fields GB (2002) Introduction to peptide synthesis. In: Coligan JE et al (eds) Current protocols in protein science. Wiley, New York
Tanaka H, Fukahori S, Baba S, Ueno T, Sivakumar R, Yagi M, Asagiri K, Ishii S, Tanaka Y (2014) Branched-chain amino acid-rich supplements containing microelements have antioxidant effects on nonalcoholic steatohepatitis in mice. J Parenter Enteral Nutr
Udenigwe CC, Aluko RE (2011) Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Molecular Sci 12:3148–3161
von Gadow A, Joubert E, Hansmann CF (1997) Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), alpha-tocopherol, BHT, and BHA. J Agric Food Chem 45:632–638
Wallner S, Hermetter A, Mayer B, Wascher TC (2001) The alpha-amino group of l-arginine mediates its antioxidant effect. Eur J Clin Invest 31:98–102
Wang Y, Zhu F, Han F, Wang H (2008) Purification and characterization of antioxidative peptides from salmon protamine hydrolysate. J Food Biochem 32:654–671
Wu HC, Chen HM, Shiau CY (2003) Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res Int 36:949–957
Zhang Z, Smith DL (1993) Determination of amide hydrogen exchange by mass spectrometry: a new tool for protein structure elucidation. Protein Sci 2:522–553
Zhu K, Zhou H, Qian H (2006) Antioxidant and free radical scavenging activities of wheat germ protein hydrolysates WGPH prepared with Alcalase. Process Biochem 41:1296–1302
Acknowledgment
Funding
This study was funded by Indian Council of Agricultural research (ICAR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each of the authors, namely, Asha K. K., Remyakumari K. Raghavan, Ashok Kumar K., Niladri S. Chatterjee, R. Anandan and Seseela Mathew declare that he/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. This article also does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Asha, K.K., Remya Kumari, K.R., Ashok Kumar, K. et al. Sequence Determination of an Antioxidant Peptide Obtained by Enzymatic Hydrolysis of Oyster Crassostrea madrasensis (Preston). Int J Pept Res Ther 22, 421–433 (2016). https://doi.org/10.1007/s10989-016-9521-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10989-016-9521-0