Abstract
Objective
Left ventricular diastolic dysfunction (LVDD) is a common manifestation of cardiac involvement in systemic sclerosis (SSc), which is associated with increased mortality, but little is known about the risk factors. The aim is to determine the frequency and potential predictors of SSc-LVDD.
Methods
We conducted a prospective multi-center cohort study, enrolling 784 SSc patients assessed by echocardiography between April 2008 and June 2019. Diagnosis of systemic sclerosis was according to the 2013 American College of Rheumatology (ACR)/the European League Against Rheumatism (EULAR) classification criteria. Data were compared between patients with and without LVDD, while univariate and multivariate regression analysis was performed to determine the factors independently associated with LVDD.
Results
LV diastolic dysfunction was present in 246/784 (31.4%) of the subjects. There were no significant differences in gender, BMI, or disease duration between the two groups. Around 40% of the patients in the SSc-LVDD group and in the SSc-non LVDD group had diffused cutaneous involvements. Factors independently associated with LV diastolic dysfunction in multivariable analysis included age at onset (OR 1.053, 95%CI 1.021–1.086, p = 0.001), pulmonary arterial hypertension (OR 3.057, 95%CI 1.468–6.367, p = 0.003), positivity of anti-RNP antibody (OR 2.455, 95%CI 1.049–5.745, p = 0.038), increased WBC count (OR 1.156, 95%CI 1.037–1.287, p = 0.009), elevated levels of uric acid (OR 1.003, 95%CI 1.000–1.006, p = 0.036), and triglyceride (OR 1.515, 95%CI 1.106–2.077, p = 0.010).
Conclusion
LV diastolic dysfunction was prevalent in the SSc population. Advanced onset age, PAH, positive anti-RNP antibody, increased WBC count, and adverse metabolic status were independent risk factors for SSc-related LVDD.
Key Points • In this Chinese multi-center cohort of systemic sclerosis, LVDD is not a rare complication, with a prevalence of 31.4%. • The presence of advanced onset age, PAH, positive anti-RNP antibody, increased WBC count and adverse metabolic status were baseline predictors of developing LVDD in SSc. |
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis is a markedly heterogeneous systemic connective tissue disease, characterized by dysregulation of immunity, diffuse microvascular pathology, and distinctive extent of fibrosis in skin and multiple organs [1]. It is rare with a reported prevalence ranging from 3.8 per 100, 000 in Taiwan to 50 per 100, 000 in the USA [2]. Heart is one of the critical organs involved early in SSc with an incidence of 15–35% [3], but autopsy studies have inspired that cardiac involvement is far more common than suspected [4]. Despite being often asymptomatic in the preclinical phase, cardiac involvement is associated with an increased risk of death once become clinical apparent, accounting for around 30% of mortality [5, 6]. Diastolic dysfunction was thought to be the consequence of myocardial fibrosis, which is originated from coronary microcirculation anomaly and regarded as the pathological hallmark of myocardial disease in SSc. Thus, the impairment of ventricular filling, often occurring as an early finding, was deducted to be omen of the functional translation in SSc myocardial disease [7]. Considering the confounding effects of pulmonary complications, such as interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH), left ventricular diastolic function can reflect heart involvement more sensitively than right heart, making it more feasible to be concerned at an earlier stage. Besides, left ventricular diastolic dysfunction is highly prevalent in SSc patients and is associated with progression to heart failure and increased risk of mortality [8]. Therefore, precautions of LVDD may be essential for prompt detection and appropriate management of cardiac involvement in SSc patients. This study aimed to assess the prevalence of left ventricular diastolic dysfunction, describe and compare clinical features in patients with or without LVDD, as well as identify independent risk factors for the development of LVDD in a large Chinese multi-center SSc cohort.
Materials and methods
Study population
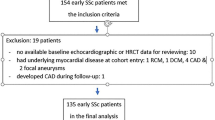
Two thousand eight hundred nine patients with systemic sclerosis who were referred to Chinese Rheumatism Data Center (CRDC) multi-center cohort were prospectively enrolled between April 2008 and June 2019. Diagnosis of systemic sclerosis was according to the 2013 American College of Rheumatology (ACR)/the European League Against Rheumatism (EULAR) classification criteria [9]. Study population was recruited from 154 centers nationwide based on the CRDC online database. Patient information were entered from paper medical record or hospital electronic medical system at enrollment or during follow-up through a specific-designed application. This study was approved by the CRDC ethics committee under number S-478, and written informed consent was obtained from each patient upon enrollment.
Clinical manifestations
Demographic features, clinical manifestations, and organ involvements were obtained from both the initial visit and regular follow-up. Disease duration was defined as interval between the onset of the first non-Raynaud’s phenomenon symptom and first clinical visit. PAH was recorded, based on the 2015 European Society of Cardiology/European Respiratory Society guidelines[10], as mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg at rest, pulmonary artery wedge pressure (PAWP) ≤ 15 mmHg, and pulmonary vascular resistance (PVR) > 3 Wood units in right heart catheterization (RHC) or estimated pulmonary arterial systolic pressure (PASP) ≥ 45 mmHg on echocardiography when RHC was not available. Laboratory and cardiopulmonary function characteristics were also measured, including autoantibodies, complete blood count, albumin, blood urea nitrogen, creatinine, inflammatory indices, N-terminal pro B-type natriuretic peptide (NT-proBNP), as well as the NYHA heart function grades and pulmonary function parameters.
Echocardiography
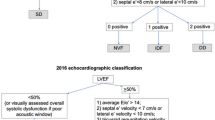
Among all the enrolled patients, 784 had underwent at least one Echo/Doppler assessments as part of their routine SSc monitoring. Comprehensive two-dimensional echocardiography with Doppler and tissue Doppler imaging were performed by sonographers blinded to all clinical and laboratory data to confirm LVDD. Assessment of diastolic function includes early and late diastolic peak velocities of mitral inflow (E and A), the E/A ratio, early diastolic deceleration time (DT) of the E wave, TDI of longitudinal velocity of the medial and lateral mitral valve annulus (Eʹ), and the ratio of mitral peak E velocity to Eʹ velocity by TDI. LV diastolic dysfunction were uniformly defined as lateral E' < 10 cm/s, E/A ratio ≤ 0.8 [11, 12]. In addition, the presence of pericardial effusion, valve dysfunction, and myocardial lesion were simultaneously obtained from the Echo/Doppler reports.
Statistical analysis
The study patients were stratified into subgroups depending on the presence or absence of LVDD. Continuous variables were expressed as mean ± SD, and Student’s t test (or Mann–Whitney U test when appropriate) was used for comparison between groups. Kolmogorov–Smirnov test and Shapiro–Wilk test were used to determine the normality of the data. Categorical variables were presented as frequencies and percentages (%), the clinically relevant differences of which were identified by chi-square test or Fischer’s exact test, as appropriate. LVDD was regarded as the dependent variable, while factors significant in the univariate analysis with p < 0.05 were included in multivariable logistic regression analyses as the independent variables. Multivariate logistic regression analyses adjusting for potential confounders were performed to identify independent risk factors for LVDD, in which odds ratios (ORs) and 95% confidence intervals (CIs) were also estimated to determine their contributory effects of LVDD. A p value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS statistics version 24.0 (IBM, Armonk, NY, USA).
Results
Demographic characteristics
Among the 2809 SSc patients enrolled in the CRDC cohort, 784 patients had undergone echocardiography assessments at baseline and during follow-ups. Two hundred forty-six patients (31.4%) had left ventricular diastolic dysfunction, among which 67 (27.2%) cases had no LVDD but were found during follow-up with a mean interval of 31.4 months, and their distribution by subsets is shown in Table 1. Patients with LVDD were significantly older at SSc onset (45.9 ± 11.6 years vs. 37.3 ± 12.9 years, p < 0.001) and at enrollment (53.5 ± 10.5 years vs. 43.8 ± 12.7 years, p < 0.001) than patients without LVDD. Hypertension was more frequently observed in SSc patients with LVDD (17.0 vs. 8.0%, p < 0.001). Detailed description of the SSc medication is outlined in Fig. 1.
Comparison of clinical features in SSc-LVDD
As shown in Table 2, patients with LVDD tended to present more digital pits (30.9 vs. 23.6%, p = 0.035), while other microangiopathies demonstrated no remarkable differences between groups. LVDD was also associated with a higher rate of pulmonary arterial hypertension (40.3 vs. 26.4%, p = 0.002). Laboratory features of the 784 SSc patients with or without LVDD are described in Table 3. LVDD was connected with a significant increase of uric acid (304.1 ± 117.6 vs. 280.2 ± 94.0, p = 0.009) and triglyceride (2.0 ± 1.2 vs. 1.6 ± 0.9, p = 0.007), as well as a lower proportion of elevated IgG (32.2 vs. 43.6%, p = 0.005) and hypocomplementemia (10.9 vs. 20.9%, p = 0.005).
Pulmonary and cardiac complications in SSc patients
No significant differences were found between the groups regarding both the ventilation and diffusion function. In all study populations, the mean 6MWD was 467.8 m. The 6MWD was shorter in SSc patients with LVDD as compared with those without LVDD (442.8 ± 97.2 m vs. 482.1 ± 87.7 m, p ≤ 0.01). There were no correlations between LVDD and other cardiac complications, including myocardial involvement, pericardial effusion, valve lesions, and arrhythmia.
Independent risk factors for LVDD
As is shown in Table 4, multivariate logistic regression analysis was performed. Results indicated an increased risk of LVDD in patients with late onset disease (OR 1.053, 95% CI 1.021–1.086, p = 0.001). Likewise, the presence of anti-RNP antibody and pulmonary arterial hypertension were associated with an increased risk of LVDD (OR 2.445, 95%CI 1.049–5.745, p = 0.038 and OR 3.057, 95%CI 1.468–6.367, p = 0.003, respectively). Furthermore, rise of WBC count would indicate a trend to develop LVDD (OR 1.156, 95%CI 1.037–1.287, p = 0.009). Moreover, metabolism-related factors including uric acid (OR 1.003, 95%CI 1.000–1.006, p = 0.036) and triglyceride (OR 1.515, 95%CI 1.106–2.077, p = 0.010) demonstrated a high-risk effect on development of LVDD.
Discussion
Heart involvements were first identified by Heine et al. [13] in SSc patients in 1926, when an autopsy demonstrated pathological changes in coronary arteries, pericardium, and myocardium. Left ventricular diastolic dysfunction (LVDD) were found in 31.4% of our SSc population, a lower prevalence compared with 40% reported by Andreu et al. [14] and 44% by Vemulapalli et al. [15] but higher than 27% by Akdogan [16] and 23% by Hinchcliff [17], which may represent actual divergence from other cohorts. Data from general populations concluded an overall prevalence of LVDD ranging from 11.1 to 27.3% [18,19,20], which is lower than that in our cohort, disclosing a latent inclination in SSc patients to develop LVDD. There is strong evidence that LVDD is related to irreversible patchy myocardial fibrosis possibly secondary to repeated focal ischemic injuries and immune-inflammatory damage [21]. As the diastolic compliance of the left ventricle decreases, manifest diastolic heart failure may evolve, and multiple characteristic symptoms of SSc patients, such as decreased exercise capacity, dyspnea, and decompensation will consequently emerge [16, 22]. In many other studies, LVDD has been considered as a harbinger of increased mortality [8, 23].
In our study, SSc patients with LVDD were much older both at onset and enrollment than those without LVDD, and the significance remains after adjusting for potential confounders in multivariable regression analysis, which was in accordance with existing studies [15, 17]. Interestingly, one of the mentioned studies also discovered that the prevalence of LVDD remarkably varied according to age, from 2.8% in individuals aged 25–35 years to 15.8% among those older than 65 years [18]. Similar tendencies with an increase of LVDD in elders were also pronounced by some other investigators [19, 24]. Although the mechanism implicated are not fully understood, it is well known that aging itself leads to normal physiological changes in the heart. As the ages grow, vascular stiffness attributing to myocardial collagen accumulation, calcification, and fragmentation of elastin gradually impairs vascular compliance[25], which is a similar process as happens in scleroderma myocardial disease [26, 27]. Besides, myocardial hypertrophy due to afterload increases modulates ventricular wall tension, contributing to structural or functional adaptations of heart and subsequently ventricular stiffness and impairment of ventricular filling, which may act as another potential explanation.
While digital pits were markedly more common in SSc patients with LVDD, it cannot be ignored that PAH also show a significant higher coexistence with LVDD. Accordingly, no less than one prior studies have elaborated that the presence of PAH was independently associated with onset of LVDD in SSc and often accompanied by a worse severity [15, 17, 28]. A study concerning idiopathic PAH also indicates a high prevalence of LVDD, which leads to worse hemodynamics and outcomes [29]. Although no significant difference of nailfold-capillaroscopy abnormality was uncovered in our study, it has been repeatedly reported as a predictor of major organ damage in SSc because of common pathogenesis [30, 31]. All these findings concerning microangiopathies may imply a potential pathophysiological process of LVDD development in SSc, which has rarely been concluded in existing literatures. In this study, six-minute walking distance (6MWD) was found to be obviously decreased in SSc patients with LVDD compared with those without LVDD. Since 6MWD is likely to be impaired by a variety of disorders concerning the heart and pulmonary other than LVDD, such as PAH and ILD, it was explainable to be excluded from the independent correlations in multivariable analysis. Nevertheless, 6MWD could still serve as a clinical implication and prompt the investigation of LVDD in SSc patients when reduced.
As a marker of immunological dysregulation, the presence of circulating autoantibodies, is one of the hallmarks and prominent features of systemic sclerosis. In our study cohort, despite the fact that anti-centromere, anti-Sci-70, and anti-RNA-topoisomerase III antibodies were evenly distributed among patients with and without LVDD, which was the same as previous reports [15, 17], an insignificant higher positivity of anti-RNP antibody was observed between the subgroups, and independent correlation with LVDD was obtained. But till now, no evidences could be taken out to prove the effects of anti-RNP antibody on SSc-LVDD directly. Remarkable elevation of uric acid and triglyceride, which act as hallmark of metabolic syndrome, was described in the study population with LVDD and then be defined as independent indicators in the multivariable regression analysis. A research undergone in mice reminded that increased production of uric acid promotes cardiomyocyte hypertrophy, inflammation, and oxidative stress that lead to myocardial fibrosis and associated impaired diastolic relaxation [32]. Georgios et al. [33] made an analysis in hypertensive subjects without heart failure, proving that UA is independently associated with the presence of diastolic dysfunction. Several studies [34, 35] made in obesity demonstrated that metabolic syndrome was associated with subclinical decrement in LV diastolic function, in which myocardial energetics and steatosis play an equally important role with myocardial remodeling. An observational study suggests that metabolic syndrome (MS) can lead to the development of diastolic dysfunction through mechanisms of ventricular hypertrophy [36], highlighting the potential importance of early risk factor modification and preventive strategies in MS, but leaving the gap of diverse hypothesized pathways and undefined components of MS. In a nutshell, the current conclusions on metabolic factors are drawn from general population or patients of other diseases [37], calling for further explorations in SSc cohort.
Several limitations should be interpreted for this study. First of all, due to the fact that echocardiography is an operator-dependent procedure, the assessment of LVDD may be biased by the center specific differences, which could hardly be avoided in multi-center study. Moreover, since echocardiography was not evaluated for every single patient enrolled in the database, at least some of them may get the investigation due to the presence of manifestations suggestive of cardiac involvement, which may introduce selection bias into this study. In addition, since the data were prospectively collected from real-world practice, there were several variables with a data missing rate over 50%. They were remained in statistical analysis in order to investigate their correlation with LVDD, which unavoidably introduced biases. Finally, although we have data on clinical presentations and vital status for all patients in our study, the survival analyses, concerning the overall survival rate and major causes of deaths, were absent. Thus, prognostic study should be taken very cautiously in the future study.
In conclusion, we deeply investigate the clinical characteristics of systemic sclerosis patients with LVDD in this study. Relying on the largest SSc cohort in China, our study probes the prevalence of LVDD, and identified advanced onset age, PAH, positive anti-RNP antibody, increased WBC count and adverse metabolic status as independent risk factors. Further studies are needed for subgroup analysis in severe LVDD population and to develop risk stratification model for patient management.
Availability of supporting data and materials
All data generated or analyzed during this study are included in the article.
References
Hughes M, Herrick AL (2019) Systemic sclerosis. Br J Hosp Med (Lond) 80:530–536. https://doi.org/10.12968/hmed.2019.80.9.530
Zhong L, Pope M, Shen Y, Hernandez JJ, Wu L (2019) Prevalence and incidence of systemic sclerosis: A systematic review and meta-analysis. Int J Rheum Dis 22:2096–2107. https://doi.org/10.1111/1756-185X.13716
Allanore Y, Meune C (2010) Primary myocardial involvement in systemic sclerosis: evidence for a microvascular origin. Clin Exp Rheumatol 28:S48–53
Bulkley BH, Ridolfi RL, Salyer WR, Hutchins GM (1976) Myocardial lesions of progressive systemic sclerosis. A cause of cardiac dysfunction. Circulation 53:483–490. https://doi.org/10.1161/01.cir.53.3.483
Wangkaew S, Prasertwitayakij N, Phrommintikul A, Puntana S, Euathrongchit J (2017) Causes of death, survival and risk factors of mortality in Thai patients with early systemic sclerosis: inception cohort study. Rheumatol Int 37:2087–2094. https://doi.org/10.1007/s00296-017-3846-7
Ferri C, Valentini G, Cozzi F, Sebastiani M, Michelassi C, La Montagna G, Bullo A, Cazzato M, Tirri E, Storino F, Giuggioli D, Cuomo G, Rosada M, Bombardieri S, Todesco S, Tirri G; Systemic Sclerosis Study Group of the Italian Society of Rheumatology (SIR-GSSSc) (2002) Systemic sclerosis: demographic, clinical, and serologic features and survival in 1,012 Italian patients. Medicine (Baltimore) 81:139–153. https://doi.org/10.1097/00005792-200203000-00004
Aguglia G, Sgreccia A, Bernardo ML, Carmenini E, Giusti De Marle M, Reali A, Morelli S (2001) Left ventricular diastolic function in systemic sclerosis. J Rheumatol 28:1563–1567
Faludi R, Költő G, Bartos B, Csima G, Czirják L, Komócsi A (2014) Five-year follow-up of left ventricular diastolic function in systemic sclerosis patients: determinants of mortality and disease progression. Semin Arthritis Rheum 44:220–227. https://doi.org/10.1016/j.semarthrit.2014.04.001
Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, Varga J (2015) Systemic sclerosis. Nat Rev Dis Primers 23:15002. https://doi.org/10.1038/nrdp.2015.2
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group (2015) 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 37:67–119. https://doi.org/10.1093/eurheartj/ehv317
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelisa A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 10:165–193. https://doi.org/10.1093/ejechocard/jep007
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD; Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17:1321–1360. https://doi.org/10.1093/ehjci/jew082
Heine JJVAFPAUPUFKM (1926) Über ein eigenartiges Krankheitsbild von diffuser Skelerosis der Haut und innerer Organe. Virchows Arch Pathol Anat Physiol Klin Med 262:351–382
Fernández-Codina A, Simeón-Aznar CP, Pinal-Fernandez I, Rodríguez-Palomares J, Pizzi MN, Hidalgo CE, Guillén-Del Castillo A, Prado-Galbarro FJ, Sarria-Santamera A, Fonollosa-Plà V, Vilardell-Tarrés M (2017) Cardiac involvement in systemic sclerosis: differences between clinical subsets and influence on survival. Rheumatol Int 37:75–84. https://doi.org/10.1007/s00296-015-3382-2
Vemulapalli S, Cohen L, Hsu V (2016) Prevalence and risk factors for left ventricular diastolic dysfunction in a scleroderma cohort. Scand J Rheumatol 46:281–287. https://doi.org/10.1080/03009742.2016.1206963
Akdogan A, Kaya EB, Sahin A, Okutucu S, Yakut E, Kalyoncu U, Aksoy H, Karadag O, Calguneri M, Tokgozoglu L, Kiraz S, Ertenli I (2011) Relationship between left ventricular diastolic dysfunction and six minute walk test in patients with systemic sclerosis. Int J Rheum Dis 14:379–383. https://doi.org/10.1111/j.1756-185X.2011.01672.x
Hinchcliff M, Desai CS, Varga J, Shah SJ (2012) Prevalence, prognosis, and factors associated with left ventricular diastolic dysfunction in systemic sclerosis. Clin Exp Rheumatol 30:S30–37
Fischer M, Baessler A, Hense HW, Hengstenberg C, Muscholl M, Holmer S, Döring A, Broeckel U, Riegger G, Schunkert H (2003) Prevalence of left ventricular diastolic dysfunction in the community. Results from a Doppler echocardiographic-based survey of a population sample. Eur Heart J 24:320–328. https://doi.org/10.1016/s0195-668x(02)00428-1
Kloch-Badelek M, Kuznetsova T, Sakiewicz W, Tikhonoff V, Ryabikov A, González A, López B, Thijs L, Jin Y, Malyutina S, Stolarz-Skrzypek K, Casiglia E, Díez J, Narkiewicz K, Kawecka-Jaszcz K, Staessen JA; European Project On Genes in Hypertension (EPOGH) Investigators (2012) Prevalence of left ventricular diastolic dysfunction in European populations based on cross-validated diagnostic thresholds. Cardiovasc Ultrasound 19:10. https://doi.org/10.1186/1476-7120-10-10
Kuznetsova T, Herbots L, López B, Jin Y, Richart T, Thijs L, González A, Herregods MC, Fagard RH, Díez J, Staessen JA (2009) Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail 2:105–112. https://doi.org/10.1161/CIRCHEARTFAILURE.108.822627
Meune C, Avouac J, Wahbi K, Cabanes L, Wipff J, Mouthon L, Guillevin L, Kahan A, Allanore Y (2008) Cardiac involvement in systemic sclerosis assessed by tissue-doppler echocardiography during routine care: A controlled study of 100 consecutive patients. Arthritis Rheum 58:1803–1809. https://doi.org/10.1002/art.23463
Ciurzyński M, Bienias P, Irzyk K, Kostrubiec M, Bartoszewicz Z, Siwicka M, Kurzyna M, Demkow U, Pruszczyk P (2013) Exaggerated increase of exercise-induced pulmonary artery pressure in systemic sclerosis patients predominantly results from left ventricular diastolic dysfunction. Clin Res Cardiol 102:813–820. https://doi.org/10.1007/s00392-013-0594-x
Tennøe AH, Murbræch K, Andreassen JC, Fretheim H, Garen T, Gude E, Andreassen A, Aakhus S, Molberg Ø, Hoffmann-Vold AM (2018) Left Ventricular Diastolic Dysfunction Predicts Mortality in Patients With Systemic Sclerosis. J Am Coll Cardiol 72:1804–1813. https://doi.org/10.1016/j.jacc.2018.07.068
Desai CS, Colangelo LA, Liu K, Jacobs DR Jr, Cook NL, Lloyd-Jones DM, Ogunyankin KO (2013) Prevalence, prospective risk markers, and prognosis associated with the presence of left ventricular diastolic dysfunction in young adults: the coronary artery risk development in young adults study. Am J Epidemiol 177:20–32. https://doi.org/10.1093/aje/kws224
Hajdu MA, Heistad DD, Siems JE, Baumbach GL (1990) Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res 66:1747–1754. https://doi.org/10.1161/01.res.66.6.1747
Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS (2011) Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal 16:1492–1526. https://doi.org/10.1089/ars.2011.4179
Carreira PE, Carmona L, Joven BE, Loza E, Andréu JL, Riemekasten G, Vettori S, Balbir-Gurman A, Airò P, Walker U, Damjanov N, Matucci-Cerinic M, Ananieva LP, Rednic S, Czirják L, Distler O, Farge D, Hesselstrand R, Corrado A, Caramaschi P, Tikly M, Allanore Y (2019) Differences associated with age at onset in early systemic sclerosis patients: a report from the EULAR Scleroderma Trials and Research Group (EUSTAR) database. Scand J Rheumatol 48:42–51. https://doi.org/10.1080/03009742.2018.1459830
Jaeger VK, Wirz EG, Allanore Y, Rossbach P, Riemekasten G, Hachulla E, Distler O, Airò P, Carreira PE, Balbir Gurman A, Tikly M, Vettori S, Damjanov N, Müller-Ladner U, Distler JH, Li M, Walker UA; EUSTAR co-authors (2016) Incidences and Risk Factors of Organ Manifestations in the Early Course of Systemic Sclerosis: A Longitudinal EUSTAR Study. PLoS One 11:e0163894. https://doi.org/10.1371/journal.pone.0163894
Tonelli AR, Plana JC, Heresi GA, Dweik RA (2012) Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest 141:1457–1465. https://doi.org/10.1378/chest.11-1903
Smith V, Riccieri V, Pizzorni C, Decuman S, Deschepper E, Bonroy C, Sulli A, Piette Y, De Keyser F, Cutolo M (2013) Nailfold capillaroscopy for prediction of novel future severe organ involvement in systemic sclerosis. J Rheumatol 40:2023–2028. https://doi.org/10.3899/jrheum.130528
Repa A, Avgoustidis N, Kougkas N, Bertsias G, Zafiriou M, Sidiropoulos P (2019) Nailfold videocapillaroscopy as a candidate biomarker for organ involvement and prognosis in patients with systemic sclerosis. Mediterr J Rheumatol 30:48–50. https://doi.org/10.31138/mjr.30.1.48
Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR (2015) Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 65:531–539. https://doi.org/10.1161/HYPERTENSIONAHA.114.04737
Georgiopoulos G, Tsioufis C, Kalos T, Magkas N, Roussos D, Chrysohoou C, Sarri G, Syrmali K, Georgakopoulos P, Tousoulis D (2019) Serum uric acid is independently associated with diastolic dysfunction in apparently healthy subjects with essential hypertension. Curr Vasc Pharmacol 17:99–106. https://doi.org/10.2174/1570161116666171226124959
Lee HJ, Kim HL, Lim WH, Seo JB, Kim SH, Zo JH, Kim MA (2019) Subclinical alterations in left ventricular structure and function according to obesity and metabolic health status. PLoS One 14:e0222118. https://doi.org/10.1371/journal.pone.0222118
Widya RL, de Mutsert R, den Heijer M, le Cessie S, Rosendaal FR, Jukema JW, Smit JW, de Roos A, Lamb HJ; NEO Study Group (2016) Association between hepatic triglyceride content and left ventricular diastolic function in a population-based cohort: the Netherlands epidemiology of obesity study. Radiology 279:443–450. https://doi.org/10.1148/radiol.2015150035
Ayalon N, Gopal DM, Mooney DM, Simonetti JS, Grossman JR, Dwivedi A, Donohue C, Perez AJ, Downing J, Gokce N, Miller EJ, Liang CS, Apovian CM, Colucci WS, Ho JE (2014) Preclinical left ventricular diastolic dysfunction in metabolic syndrome. Am J Cardiol 114:838–842. https://doi.org/10.1016/j.amjcard.2014.06.013
McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; ESC Committee for Practice Guidelines (2012) ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developedin collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 33:1787–1847. https://doi.org/10.1093/eurheartj/ehs104
Acknowledgements
We thank CRDC multi-center co-authors as above for assistance with cases collections
Funding
This study was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-2–008).
Author information
Authors and Affiliations
Contributions
Min Hui and Jiaxin Zhou: Data curation, Writing-Original draft preparation.
Liyun Zhang and Xinwang Duan: Supervision.
Mengtao Li and Qian Wang: Visualization, Investigation.
Jiuliang Zhao: Conceptualization, Methodology, Software.
Yong Hou: Software, Validation.
Dong Xu and Xiaofeng Zeng: Writing- Reviewing and Editing.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by Chinese Rheumatism Data Center (CRDC) with the number S-478. Informed consent was obtained.
Consent for publication
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hui, M., Zhou, J., Zhang, L. et al. Prevalence and risk factors for left ventricular diastolic dysfunction in systemic sclerosis: a multi-center study of CRDC cohort in China. Clin Rheumatol 40, 4589–4596 (2021). https://doi.org/10.1007/s10067-021-05804-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05804-6