Abstract
The objective of the present study is to investigate if there is a potential association between the single-nucleotide polymorphisms (SNPs) of the tumor necrosis factor alpha gene (TNF-α −308G/A, rs1800629) and the susceptibility to and severity of early-onset knee osteoarthritis in the Egyptian female population. Genotype distributions and allelic frequencies of TNF-α −308G/A polymorphism were investigated in 210 knee osteoarthritis (OA) patients and 210 age-, sex-, and ethnicity-matched healthy controls (HC). Polymerase chain reaction-restricted fragment length polymorphism (PCR-RFLP) amplifications were implemented to determine TNF-α −308G/A SNP. Serum and synovial fluid levels of TNF-α, besides ESR and CRP, as laboratory markers for inflammation, were estimated for all patients and HC. Plain X-ray as well as MRI knee was done for grading of OA. Disease severity was estimated by Western Ontario and McMaster University Osteoarthritis scores. Percentages of TNF-α-G308A genotypes GG, AG, and AA were 85.7, 11.9, and 2.4% in OA patients and 54.7, 39.1, and 6.2% in controls, respectively. The frequencies of the GG genotype and G allele were significantly higher in subjects with knee OA than in HC (P = 0.04 and P < 0.001, respectively). Logistic regression analysis showed that the GG genotype and G allele are independently associated with increased risk for knee OA (odds ratio = 3.13, 95% confidence interval = 1.04–9.39, P = 0.04 for GG genotype, and odds ratio = 3.81, 95% confidence interval = 2.52–5.76, P = 0.001 for G allele). There is a close relationship between TNF-α-G308A polymorphism and individual susceptibility to and severity of early-onset knee OA in the Egyptian females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoarthritis (OA) is a degenerative joint disease with progressive loss of joint function causing physical disability and impaired quality of life in many countries [1]. Although the etiology of OA is largely unknown, it is widely believed that OA is due to an imbalance between anabolic and catabolic processes or homeostasis of cartilage metabolism [2]. Aging, trauma, hormonal, and mechanical factors are reported to contribute to the onset and progression of OA [1]. In addition, several studies have demonstrated the etiology and the pathogenesis of OA to be related to genetic background [1, 2].
Primary OA is an idiopathic phenomenon, occurring in previously intact joints, with no apparent initiating factors such as joint injury or developmental abnormalities [3]. Although, OA was often described as a non-inflammatory arthropathy, recent studies have revealed that an inflammatory process played a role in its pathogenesis [4]. Proinflammatory cytokines, particularly TNF-α and IL-1β, are now considered as important mediators in this disease, which have a major importance for cartilage destruction. They can stimulate their own production and induce chondrocytes and synovial cells to produce other cytokines, such as IL-8, IL-6, and leukocyte inhibitory factor [5].
The gene encoding TNF-α is located in the major histocompatibility complex class III region of chromosome 6 (6p21.3). Previous studies have suggested that variability in single-nucleotide polymorphisms (SNPs) in the promoter and coding regions of TNF-α gene may modulate the magnitude of the secretory response of this cytokine. There are several polymorphisms of the gene coding for TNF-α, one of which is −308G/A polymorphism [5]. This single-nucleotide conversion from guanine (G) to adenine (A) at position −308 is the most common polymorphism in general populations, and this transition has been shown to influence the expression of TNF-α [5, 6]. TNF-α plays a pivotal role in the imbalance between anabolic and catabolic processes of OA patients. It suppresses the synthesis of link proteins and type II collagen, which are major components of the extracellular matrix (ECM). Also, it stimulates chondrocytes to release matrix metalloproteinases (MMPs), which have the capacity to degrade cartilage matrix proteins [6, 7].
Synovial inflammation at the onset of OA may either be secondary to cartilage destruction or be the primary cause, with some histological features, which include the presence of mononuclear cells, indistinguishable from RA. Presence of synovitis in the mechanical models of OA makes the role of cytokines within the synovium cannot be excluded [8, 9].
Although the American College of Rheumatology criteria [10] defined the age of patients with OA as more than 50 years old, we have met many patients in our clinics who have typical clinical and radiographic manifestations of OA in spite of being younger than that age and have no any precipitating factors for OA. For this reason, and depending on the fact that TNF-α plays an important role in pathogenesis of OA [4, 7], we hypothesized that TNF-α −308G/A gene polymorphism can confer the susceptibility to as well as affect the severity of knee OA in middle-aged Egyptian females.
Methods
Participants
A total of 210 Egyptian Female patients, age 38–52 years old, complaining from bilateral knee arthritis were recruited from June 2013 to September 2015, from outpatient clinics of Rheumatology and Rehabilitation Department, Zagazig University Hospitals, Faculty of Medicine, Zagazig University, Egypt. The diagnosis of knee OA was based on the clinical, laboratory, and radiographic American College of Rheumatology criteria [10], except for the age of patients that was less than 50 years, as we intended to study the polymorphism in middle-aged individuals.
Two hundred and ten healthy volunteers, age and sex matched to the patient group, were enrolled as controls. They never had any signs or symptoms of arthritis or joint diseases (pain, swelling, tenderness, or restriction of movement). The clinical characteristics and demographic data of all enrolled subjects, including age, sex, body mass index (BMI), smoking status, bone fracture history, kneeling activity, and regular excise, were recorded, and all of established risk factors were obtained from both OA and control groups at the time of evaluation for enrollment in this study.
Exclusion criteria
We excluded from our study any patient with other etiologies causing knee diseases such as inflammatory arthritis (rheumatoid, gout, or pseudogout or autoimmune disease as systemic lupus erythematosus), previous knee injury or previous joint infection, skeletal dysplasia or developmental dysplasia, psoriasis, hemochromatosis, or any type of malignant or chronic illness.
The functional severity of the disease was determined according to the Western Ontario and McMaster University Osteoarthritis (WOMAC) Index [11]. The study was approved by the ethics review committee of our hospital, and all subjects gave a written informed consent prior to their participation in the study.
Radiographic evaluation
The radiographic severity of OA was evaluated by standard weight-bearing antero-posterior and lateral views of knee radiographs taken for all study populations. The diagnosis of OA was made according to the Kellgren–Lawrence (KL) grade classification, which is a standard radiographic five-level index, ranging from 0 to 4, used in diagnosing and staging of OA [12]. Magnetic resonance imaging (MRI) of both knees was done to confirm the presence of osteoarthritic changes in the joints in cases with early disease. MRI of the knee was performed with a 1.5-T magnet (Philips Medical System) using a knee coil. Articular cartilages were assessed in the sagittal, axial, and coronal planes using T2WI fast spin-echo images that were performed with a long repetition time (TR) (3500 to 5000 ms) and short echo time (TE) (20–30 ms). Sagittal proton density-weighted sequence was also included using TR/TE 1275/15, field of view 18 cm, slice thickness 1.5 mm, interslice gap 0 mm, matrix 512 × 512 pixels, and two acquisitions with an imaging time of 4 min.
All radiographs were evaluated by a radiologist who was completely blinded to the clinical and physical history of all patients.
Genotyping
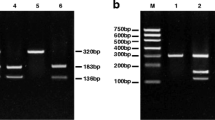
Blood samples of 5 ml were drawn from all individuals, and the buffy coat was separated by centrifugation. The DNA was extracted from the buffy coat by using a Qiagen DNA Blood Mini Kit (Qiagen, Hilden Germany) according to the enclosed instructions. The purity and concentration of the extracted DNA were tested according to a Nanodrop ND-1000 spectrophotometer. Genotyping TaqMan assays was implemented for the detection of TNF-α −308G/A single-nucleotide polymorphism (SNP). Allele identification was done by using commercially available assays from Thermo Fisher Scientifics using the primer set: forward 5′-AGGCAATAGGTTTTGAGGGCCAT-3′ and reverse 5′ TCCTCCCTGCTCCGATTCCG-3′ on an ABI 7500 real-time PCR machine (Applied Biosystems Thermo Fisher, USA) following the manufacturer’s instructions (Thermo Fisher Scientific Com. USA). To ensure the quality control of PCR results, 20% of the cases were randomly selected for repeated analyses and the results were complete contract.
Laboratory markers
Blood samples were collected from all study participants. The sera were separated and stored at −40 °C. Serum levels of C-reactive protein (CRP), as well as serum and synovial TNF-α levels, were measured by sandwich ELISA using commercial human TNF-α and CRP assay kits (BioLegend, San Diego, CA, USA). The erythrocyte sedimentation rate (ESR) was determined according to the mentioned laboratory standard method. Serum rheumatoid factor (RF), anti-citrullinated protein (anti-CCP), and uric acid determinations were done to exclude other autoimmune diseases.
Statistical methods
All statistical analysis was performed with the SPSS, software version, 20 for Windows. Normality distribution of data was assessed with the Kolmogorov and Smirnoff test. The descriptive data were expressed as a percentage (%), median, and mean ± standard deviation (±SD). Demographic, laboratory, and clinical data were compared between groups by the chi-squared (X 2) test, Fisher exact test, and independent student t test or Mann-Whitney U test based on the normality distribution. Comparing the clinical and laboratory data between groups of TNF-α-G308A genotypes was done by one-way ANOVA or Kruskal-Wallis post hoc tests followed by less significance difference (LSD) according to normality distribution. Genotype and allelic frequency distributions were compared by Hardy–Weinberg equilibrium analyses, and any deviation between the observed and the expected frequencies was tested for significance using the X 2 test. The odds ratios (ORs) were calculated with 95% confidence interval (CI) by multiple logistic regression analysis to determine the association between the genotypes and the risk of OA. P < 0.05 was considered statistically significant.
Results
Our study population composed of 210 Egyptian female patients and 210 healthy female volunteers serves as controls (HC), whose ages ranged between 38 and 52 and 34 and 50 years, respectively. The disease duration for patients ranged between 6 and 12 years. There was no any previous history of heavy labor works, kneeling activity, or regular exercise for both groups. Epidemiological characteristics and laboratory markers of all subjects are demonstrated in Table 1, There was no significant statistical difference between patients and HC as regard their age, BMI (23.37 ± 2.85 and 23.17 ± 3.87 kg/m2, respectively), CRP median (range) (5 (1–20) versus 4 mg/l (1–12)), and ESR median (range) (11 (5–51) versus 10 mm/h (5–21)), respectively. Serum TNF-α level was also non-significant between patients and HC (12.30 ± 3.48 versus 11.66 ± 3.65 pg/ml), while the synovial fluid level of TNF-α was significantly higher in patients than in the control group (26.75 ± 5.78 versus 13.12 ± 3.71 pg/ml, P < 0.001).
Serum RF and anti-CCP were negative, and serum uric acid levels were within normal range in all study populations. The genotypes and allele frequency distributions of TNF-α gene polymorphisms between OA patients and healthy controls are demonstrated in Table 2. There was a significant predominance of G/G genotype (P = 0.04) and G allele (P = 0.001) in the subjects with OA than in healthy controls.
The relationship between TNF-α −308 genotypes and the radiographic severity of knee OA in the patient group showed a significant statistical correlation between G/G genotype and grade II (P = 0.042) and grade III (P = 0.001) of the Kellgren–Lawrence (KL) radiographic grading system (Table 3).
Table 4 demonstrates the relationship between TNF-α −308 genotypes and the clinical (WOMAC scores) and laboratory measures (ESR, CRP, synovial and serum levels of TNF-α) in OA patients. There was a significantly higher WOMAC pain (WPS) and WOMAC stiffness subscales (WSS) in patients carrying the G/G genotype (P < 0.05) than other groups of patients. Also, there was a significantly higher level of ESR and synovial fluid levels of TNF-α in the carriers of −308G/G genotype when compared to the −308G/A and −308A/A carriers.
Discussion
TNF-α-G308A gene polymorphism has been found to be involved in the pathogenesis of a large variety of illnesses with inflammatory and autoimmune components such as rheumatoid arthritis [13,14,15,16], psoriatic arthritis [17], juvenile idiopathic arthritis [18], systemic lupus erythematosus [19, 20], and ankylosing spondylitis [21]. Other studies have provided evidence of participation of TNF family in OA [7, 22, 23]. Moreover, it was proved that a majority of middle-aged patients with chronic idiopathic knee pain developed knee OA over 12 years [24].
However, to the best of our knowledge, TNF-α-G308A polymorphisms have not been studied in the Egyptian populations yet, especially in middle-aged individuals with no previous history of predisposing factors for knee OA. Thus, in this study, we investigated the relationship between TNF-α-G308A gene polymorphisms and the development of early-onset knee OA in Egyptian female population.
We found a significant association between TNF-α −308G/A polymorphism and the susceptibility to primary knee OA in a middle-aged group of Egyptian females, where the distribution of G/G genotype was more frequent among OA patients than other genotypes, with a significant difference between patients and controls (P < 0.04). In addition, there was a significant increase in the G allele frequency distribution in OA patients than in HC (P < 0.001). These results indicate that the TNF-α −308G allele acts as a genetic predisposing factor for increased susceptibility to early-onset knee OA in the Egyptian females. Our results are contradictory to previous studies on Turkish [5] and Western Mexico populations [22], where the TNF-α −308 genotypes and allele frequency distributions did not show any significant statistical differences between their studied groups, failing to find any association of this SNP with susceptibility to OA. Sezgin et al. [5] documented that a possible association may be present, but has been weakened by disease heterogeneity, environmental factors, or gene-environment interactions. In addition, linkage disequilibrium is strong in this area, and it may be difficult to study the role of this SNP in isolation in the Turkish population. On the other hand, their study populations were not matched in terms of age (mainly elderly patients), sex, and BMI. Furthermore, radiographs were not taken for all controls, so they cannot exclude the presence of asymptomatic OA in them, which weakens the statistical power of their study, while in our study, all subjects were middle-aged of the same sex (females) with no any statistical difference between our study subjects as regard age or BMI. Moreover, radiographs were done in all subjects for detection and grading of OA and associating it with genotype distribution. Munoz-Valle et al. [22] explained the absence of association of the TNF-α −308G/A polymorphism with knee OA, in their study, by the different genetic backgrounds and the peculiar characteristics of the Mexican mestizo population (their study population) that due to their paternal ancestry (European 60–64%, Amerindian 21–25%, and African 15%). This genetic background leaded to low heterozygosity with difficult identification of individuals having homozygous A/A genotype for the studied polymorphisms. On the contrary, our study populations were all Egyptian females of African (Caucasian) origin that makes our results different. These differences in ethnicity and small sample size in both studies [5, 22] may be a significant cause of the discrepancy between their and our results. Additionally, the wide range of age in a Mexican population (31–86 years for OA and 22–67 for HC), despite including middle-aged individuals, as in our study, helped in getting false-negative results as well.
However, we partially agree with other studies that observed that rs1800629 (TNF-α −308) single-nucleotide polymorphism (SNP) in the TNF-α gene increased the risk of OA in Korean [7] and Han Chinese populations [23], who found to have a significantly higher risk for OA in carriers of the TNF-α −308A allele not the G allele. Ji et al. [23] explained their discrepant results by small sample sizes in the studies of Sezgin et al. [5] and Munoz-Valle et al. [22] (OA patients/HC were only 151/84 and 50/100), respectively. Thus, it was difficult for them to detect the genotype AA of TNF-α −308, whose percentage is low and may limit the statistical power to detect any existing association, leading to the potential of false-negative results. However, in our study, we have recruited a relatively large number of patients, comparable to that of Ji et al. [23] and Han et al. [7], that lower the incidence of false results in our study. On the other hand, ethnic differences (Asian versus Caucasian, in our study) may also help in these partially discrepant results. Our study populations were all females, while other previous studies included both sexes. Thus, we provide evidence that TNF-α −308 SNP is associated with early-onset knee OA in Caucasian females, which is consistent with the previous concept that gene associations with OA are dependent on gender and ethnicity [25].
Levels of TNF-α were previously proved to be elevated in the synovial fluid, synovial membrane, subchondral bone, and cartilage of OA joints [26], which conforms well with our results denoting significantly elevated levels of TNF-α in the synovial fluid of OA patients than in controls as well as in G/G genotype carriers than other groups, which is consistent with that of Munoz-Valle et al. [22], who found that sTNF-α levels increased in G/G versus G/A genotype carriers and the G allele of TNF-α −308 polymorphism was associated with high mRNA and soluble expression in knee OA patients, although it is not a marker of susceptibility to OA disease in Western Mexico [22]. The results confirm the role of the G allele in increasing the secretion of TNF-α, even if not influencing the disease susceptibility.
The presence of active inflammation in osteoarthritic joints was previously reported [5, 8, 9]. Additionally, it is suggested that there is an association between joint inflammation and the radiographic progression in OA [4, 27, 28]. Our results demonstrated significant elevations of ESR in the group of patients carrying G/G genotype who have higher levels of serum and synovial TNF-α and significantly associated with grades II and III of KL radiographic grading system of OA. These results provide evidence that TNF-α −308G/G genotype is a susceptibility and severity gene in OA, increasing the secretion of TNF-α, which has a major role in the process of joint inflammation, cartilage degradation, and subchondral bone affection in OA joints as previously reported [6, 7, 29].
As previously documented [23], we found a significant association between higher WOMAC stiffness and pain subscales and the G/G genotype carriers than G/A and A/A carriers, confirming the effect of the studied polymorphism on the functional severity of OA in Egyptian female patients. Therefore, we can confirm that TNF-α, as a proinflammatory cytokine [5], plays an important role in the pathophysiology of OA.
Conclusion
TNF-α −308G/A gene polymorphism has a potential relationship with susceptibility to and severity of early-onset knee OA in the Egyptian female population. G/G genotype and G allele could be considered as genetic markers for susceptibility and radiographic as well as functional severity of knee OA, which was explained by the higher expression of TNF-α in the −308G allele carriers than in the −308A carriers.
References
Chen S, Zhou Y, Li J, et al (2012) The effect of bradykinin B2 receptor polymorphisms on the susceptibility and severity of osteoarthritis in a Chinese cohort. J Biomed Biotechnol; Article ID 597637, 5 pages
Tawonsawatruk T, Changthong T, Pingsuthiwong S et al (2011) A genetic association study between growth differentiation factor 5 (GDF 5) polymorphism and knee osteoarthritis in Thai population. J Orthop Surg Res 6:47
Liu J, Cai W, Zhang H et al (2013) Rs143383 in the growth differentiation factor 5 (GDF5) gene significantly associated with osteoarthritis (OA)—a comprehensive meta-analysis. Int J Med Sci 10(3):312–319
Pelletier JP, Martel-Pelletier J, Abramson SB (2001) Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 44(6):1237–1247
Sezgin M, Barlas İÖ, Ankara HÇ et al (2008) Tumor necrosis factor α -308G/A gene polymorphism: lack of association with knee osteoarthritis in a Turkish population. Clin Exp Rheumatol 26:763–768
Kou S, Wu Y (2014) Meta-analysis of tumor necrosis factor alpha −308 polymorphism and knee osteoarthritis risk. Musculoskelet Disord 15:373
Han L, Song JH, Yoon JH et al (2012) TNF-α and TNF-β polymorphisms are associated with susceptibility to osteoarthritis in a Korean population. Korean J Pathol 46:30–37
Walker ER, Boyd RD, Wu DD et al (1991) Morphologic and morphometric changes in synovial membrane associated with mechanically induced osteoarthrosis. Arthritis Rheum 34:515–524
Shafiaa S, Shaha ZA, Sofib FA (2016) TNF-Α, IL-1β and IL-6 cytokine gene expression in synovial fluid of rheumatoid arthritis and osteoarthritis patients and their relationship with gene polymorphisms. Rheumatology 6:1
Altman R, Asch E, Bloch D (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum 29(8):1039–1052
Tuzun EH, Eker L, Aytar A et al (2005) Acceptability, reliability, validity and responsiveness of the Turkish version of WOMAC osteoarthritis index. Osteoarthr Cartil 13:28–33
Kellgren JK, Lawrence JS (1957) Radiological assessment of osteoarthritis. Ann Rheum Dis 16:494–501
Maxwell JR, Potter C, Hyrich KL et al (2008) Association of the tumor necrosis factor-308 variant with differential response to anti-TNF agents in the treatment of rheumatoid arthritis. Hum Mol Genet 17(22):3532–3538
Nemec P, Pavkova-Goldbergova M, Stouracova M et al (2007) Polymorphism in the tumor necrosis factor-alpha gene promoter is associated with severity of rheumatoid arthritis in the Czech population. Clin Rheumatol 27:59–65
Cvetkovic JT, Wallberg-Jonsson S, Stegmayr B et al (2002) Susceptibility for and clinical manifestations of rheumatoid arthritis are associated with polymorphisms of the TNFalpha, IL-1beta, and IL-1Ra genes. J Rheumatol 29:212–219
Rezaieyazdi Z, Afshari JT, Sandooghi M et al (2007) Tumour necrosis factor a -308 promoter polymorphism in patients with rheumatoid arthritis. Rheumatol Int 28(2):189–191
Höhler T, Grossmann S, Stradmannbellinghausen B et al (2002) Differential association of polymorphisms in the TNF alpha region with psoriatic arthritis but not psoriasis. Ann Rheum Dis 61:213–218
Ozen S, Alikasifoglu M, Bakkaloglu A et al (2002) Tumour necrosis factor alpha G-->A −238 and G-->A −308 polymorphisms in juvenile idiopathic arthritis. Rheumatology (Oxford) 4:223–227
Ahmed HH, Taha FM, Darweesh HE et al (2014) Association between TNF promoter −308 G>A and LTA 252 A>G polymorphisms and systemic lupus erythematosus. Mol Biol Rep 41(4):2029–2036
Rood MI, Van Krugten MV, Zanelli E et al (2000) TNF-308A and HLA-DR3 alleles contribute independently to susceptibility to systemic lupus erythematosus. Arthritis Rheum 43:129–134
Rudwaleit M, Siegert S, Yin Z et al (2001) Low T cell production of TNF alpha and IFN gamma in ankylosing spondylitis: its relation to HLA-B27 and influence of the TNF-308 gene polymorphism. Ann Rheum Dis 60:36–42
Munoz-Valle JF, Oregon-Romero E, Rangel-Villalobos H et al (2014) High expression of TNF alpha is associated with −308 and −238 TNF alpha polymorphisms in knee osteoarthritis. Clin Exp Med 14(1):61–67
Ji B, Shi J, Cheng X, Zhou J et al (2013) Association analysis of two candidate polymorphisms in the tumour necrosis factor-α gene with osteoarthritis in a Chinese population. Int Orthop 37:2061–2063
Thorstensson CA, Andersson MLE, Jönsson H, Saxne T et al (2009) Natural course of knee osteoarthritis in middle-age subjects with knee pain: 12-year follow-up using clinical and radiographic criteria. Ann Rheum Dis 68:1890–1893
Honsawek S, Deepaisarnsakul B, Tanavalee A, Yuktanandana P (2011) Association of the IL-6 -174G/C gene polymorphism with knee osteoarthritis in a Thai population. Genet Mol Res 10(3):1674–1680
Kapoor M, Martel-Pelletier J, Lajeunesse D et al (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7(1):33–42
Hoff P, Buttgereit F, Burmester GR et al (2013) Osteoarthritis synovial fluid activates pro-inflammatory cytokines in primary human chondrocytes. Int Orthop 37(1):145–151
Stannus O, Jones G, Cicuttini F, Parameswaran V et al (2010) Circulating levels of IL-6 and TNF-a are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil 18(11):1441–1447
Westacott CI, Barakat AF, Wood L, Perry MJ et al (2000) Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthr Cartil 8(3):213–221
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the ethics review committee of our hospital, and all subjects gave a written informed consent prior to their participation in the study.
Disclosures
None.
Funding
There was no external funding source for this study.
Rights and permissions
About this article
Cite this article
Abdel Galil, S.M., Ezzeldin, N., Fawzy, F. et al. The single-nucleotide polymorphism (SNP) of tumor necrosis factor α −308G/A gene is associated with early-onset primary knee osteoarthritis in an Egyptian female population. Clin Rheumatol 36, 2525–2530 (2017). https://doi.org/10.1007/s10067-017-3727-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3727-1