Abstract
Anti-CCP (cyclic citrullinated peptide) is considered the most useful laboratory tool in the diagnosis of rheumatoid arthritis (RA). Some authors have also found this autoantibody in patients with scleroderma (SSc). The study aimed to investigate the prevalence of anti-CCP antibodies in SSc patients from Southern Brazil and their association with clinical and serological profile of the disease. We studied 76 patients with SSc and 100 healthy volunteers for presence of anti-CCP. SSc patients charts were reviewed for clinical and laboratory data. In the SSc group, the diffuse form was present in 20.5%; 62.8% had the limited form; 14.1% had overlap with systemic lupus or polymyositis and 2.5% had SSc sine scleroderma. Anti-CCP was found in nine of 78 (11.5%) SSc patients and in one of 100 healthy volunteers (p = 0.0054). No relationship was found with arthritis, skin Rodnan m score, esophageal dysmotility, myocarditis, pulmonary hypertension and lung fibrosis. Positive association was observed with arthralgias (p = 0.02). Also, no relationship was noted with the presence of anti-centromere antibodies, anti-Scl-70, anti-RNP or rheumatoid factor. Anti-CCP are more common in SSc patients than in controls. Arthralgias but not arthritis or rheumatoid factor are more frequent in anti-CCP positive patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Scleroderma (SSc) is a connective tissue disorder that may have a broad spectrum of clinical features. Musculoskeletal involvement is seen in 24–97% of the patients [1, 2] and may present with arthritis, arthralgias, joint contractures, tenosynovitis, muscle atrophy and loss of joint function [3]. A symmetrical polyarthritis similar to rheumatoid arthritis (RA) can also be found characterizing an SSc–RA overlap syndrome [4].
Anti-CCP (cyclic citrullinated peptide), which is an antibody directed against a variety of citrullinated proteins, has been considered a useful tool in RA diagnosis and prognosis. Among the different autoantibodies described in RA patients, anti-CCP displays the strongest specificity (95–98%) with 70–80% of sensitivity [5, 6]. Anti-CCP is an antibody linked to genetic background of patients; it is related to the presence of HLA shared epitope [7].
Some authors have described anti-CCP antibodies in SSc patients. Santiago et al. [8], studying 74 SSc patients (24% of them with arthritis) found 13.9% of positivity. Ingegnoli et al. [3] investigating 75 patients with SSc (9.3% with arthritis) related positive anti-CCP in 10.6% of them. Morita et al. [9] found a lower prevalence in their 114 patients (2.6%), and all positive patients had complaints of arthralgias.
Findings of anti-CCP in patients with SSc suggest at least two possible and important implications. First, considering that anti-CCP is one of the most used tools to diagnose early RA in patients with undefined clinical picture, knowing the prevalence of this autoantibody in SSc patients is useful to avoid misdiagnosing. Secondly, it may help clarify the real nature of musculoskeletal complaints in SSc patients, as they may be caused either by joint involvement or by tightness of skin around the articular tissues.
In this study we aimed to investigate the prevalence of anti-CCP in SSc patients from Southern Brazil and the possible associations between their presence and other clinical and serological features of the disease.
Material and methods
This study has been approved by the local Committee of Ethics in Research, and the patients signed consent. A total of 78 consecutive SSc patients were included; all were seen at a single rheumatology center at University Hospital in Southern Brazil. All patients fulfilled the preliminary criteria of the American Rheumatism Association for this disease [10]. This sample was formed by the group of SSc patients seen during the 6-month period who agreed to participate in the study. In this group, 73/78 (93.6%) were females, and five of 78 (6.4%) were males, with a mean age of 48.0 ± 12.61 years. Median disease duration was 5 years. Sixteen (20.5%) had the diffuse form; 49 (62.8%) had the limited form; 11 (14.1%) had overlap with systemic lupus or polymyositis and two (2.5%) had SSc sine scleroderma. None of them presented overlap with rheumatoid arthritis. In 30/76 (39.4%) there was exposure to tobacco (current and ex-smokers). Charts were reviewed for demographic, autoantibody and clinical profile. Patient’s organ involvement was classified according to the American College of Rheumatology systemic sclerosis guidelines [11]. Pulmonary involvement was considered present if the carbon monoxide diffusing capacity was ≤ 70% of predicted value, forced vital capacity or total lung capacity was ≤ 75% of predictive value or definite chronic interstitial changes were noted on chest XR or chest CT scans [11]. Renal involvement was considered present if the serum creatinine was ≥ 1.4 mg/dl or creatinine clearance was less than 70 ml/min [11]. Muscle involvement was considered present when creatine kinase concentration was ≥ 200% of normal range or muscle strength was ≤ 4 out of 5 in proximal muscles [11]. Cardiac involvement was considered to be present when patients reported a history of congestive heart failure, cardiac arrhythmia requiring medication, pericarditis or cardiomegaly not related to causes other than SSc [11]. Joint involvement was considered present if joint tenderness count was ≥ 1; arthralgia was defined as joint pain without local inflammatory findings [11] and arthritis when inflammatory findings were found. Tenosynovitis was diagnosed by physical and/or by ultrassound examination. Pulmonary hypertension was considered when ≥ 30 mm Hg at echocardiography or right heart catheterization [12]. Skin ulcerations, digital necrosis and telangiectasias were detected by history and physical examination. Skin thickening was measured through Rodnan m score [13]. Raynaud's phenomenon was defined as pallor of fingers and/or feet after exposure to cold or stress. Esophageal involvement was considered when dilatation was seen on barium swallow or dismotility was seen at esophageal manometry [14].
Disease severity was measured according to Medsger et al. [15]. As control group we studied sera from 100 healthy individuals from the same geographic area (82.0% female, 18.0% male, mean age 47.6 years, range 23–81 years).
After the consent, 5 ml venous blood was collected from each patient and also from controls. Samples were centrifuged and serum separated, aliquoted and stored at −80°C until used. Serum levels of anti-CCP were measured using the QUANTA LiteTM CCP3 IgG ELISA kit (INOVA Diagnostics, Inc., San Diego, CA) according to the manufacturer’s instructions. The cutoff point was set to 20 units/ml.
Categorical variables were presented as frequency (percent) and analyzed by contingency tables using Fisher’s exact testing, chi-square, unpaired t-test and Mann Whitney, as indicated. Statistical significance was accepted when p < 0.05. The statistical analysis was undertaken using the software Graph Pad Prism version 4.0.
Results
In the studied SSc population, we found clinical and serological data as shown in Table 1. Disease severity measured according to Medsger scale was done in 52 patients and varied from 2 to 16 (mean of 5.52 ± 3.01).
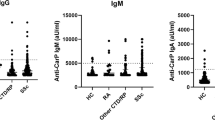
In the group with SSc, nine of 78 (11.5%) were anti-CCP positive with titers from 20.1 to 235 U/ml (mean 72.2 ± 78.6 U/ml; 95% CI of mean from 11.71 to 132.7 U/ml); in the healthy control group, only one of 100 (1%) was positive for anti-CCP (p = 0.0054). When comparing the groups of SSc patients with positive anti-CCP (n = 9) and those negative for anti-CCP (n = 69) according to clinical and laboratory data, we found that only patients with arthralgia presented a significant higher prevalence of anti-CCP (p = 0.026). No significant differences were found in demographic data, tobacco exposure, skin thickness, Raynaud presence, digital necrosis, telangiectasias, ischemic ulcers, arthritis, tenosynovitis, esophageal dysmotility, myocarditis, pulmonary hypertension, lung fibrosis, disease severity or presence of ANA, anti-Scl-70, anti-centromere, anti-Ro, Anti-La, RNP or RF.
All nine anti-CCP positive patients were submitted to hand radiographs. Four (44.4%) of them were normal; calcinosis was observed in two, acroosteolysis in two and two had digit amputations. None of them presented erosion or narrowing of articular space. Two of anti-CCP positive patients had overlap with systemic lupus.
Discussion
Actually, anti-CCP is one of the most studied autoantibodies in rheumatology. They may appear years before clinical onset of arthritis and are considered highly sensible and specific for RA. In this decade, anti-CCP has evolved from being a diagnostic tool to a biological marker which allows understanding of the pathogenetic features of RA [2]. Also, it has been observed that anti-CCP positive RA occurs mainly in patients with shared-epitope alleles and characterizes more aggressive disease with higher radiological degree of joint destruction [3, 4]. Therefore, it could be possible to use this biomarker in other rheumatic diseases such as SSc, since early identification of patients with erosive arthritis is important for the correct treatment. In this situation, an adequate therapy might prevent joint damage and worsening of quality of life.
Some patients may develop a true overlap between scleroderma and rheumatoid arthritis (SSc–RA) that can be considered a distinct genetic, clinical and serological entity. This is a rather uncommon situation. Szücs et al. [16] found this overlap in 4.6% of their 477 SSc patients followed up for 16 years. It has also been described that longstanding RA patients may develop scleroderma features and its specific autoantibodies during disease development [17]. As arthritis and joint contractures are present in RA and SSc, it may be difficult to delimit to which disease the symptoms belong. Usually radiographic joint destruction is less severe in SSc than in RA [18]. None of our patients could be considered as belonging to the SSc–RA overlap group although arthritis was verified in 38% of the sample.
The prevalence of anti-CCP found in the present investigation is similar to that of Santiago et al. [8] and Ingegnoli et al. [3] and higher than that of Morita et al. [9]. Our findings suggest that this positivity could not be attributed to overlap features of these two diseases, neither to an erosive disease even though others have found that anti-CCP are more common in SSc patients with arthritis and marginal erosion [3]. Santiago et al. [8] have also noted this dissociation in their cohort of 74 SSc patients. These authors found a positive association of anti-CCP-2, but not CCP-3 with arthritis. Nevertheless, in our sample this autoantibody was more common in those with arthralgias. Morita et al. [9] also related a positive association of anti-CCP and arthralgia; however, they too found association with lung fibrosis, a fact not observed in our study.
Rheumatoid factor was positive in 30% of our patients. It is known that in RA a link between anti-CCP and RF positivity has been found [19]. In our patients with SSc, this could not be observed, and the same dissociation was described by Santiago et al. [8]. This fact suggests that the formation of these autoantibodies may not be a uniform process and that finding an anti-CCP in SSc patients has a different meaning than finding it in RA patients.
Concluding, we demonstrated that Brazilian SSc patients have a higher prevalence of anti-CCP than healthy individuals and that the presence of this autoantibody is associated with the presence of arthralgia; however, it is not linked to arthritis, presence of RF or radiological erosions. Furthermore, no association was found of anti-CCP with systemic features of this disease or with its severity or other specific autoantibodies.
References
Misra R, Darton K, Jewkes RF, Black CM, Maini RN (1995) Arthritis in scleroderma. Br J Rheumatol 34:831–837
La Montagna G, Solano A, Capurro V, Malesci D, Valentini G (2005) The arthropathy of systemic sclerosis: a 12 month prospective clinical and imaging study. Skeletal Radiol 34:35–41
Ingegnoli F, Galbiati V, Zeni S et al (2007) Use of antibodies recognizing cyclic citrullinated peptide in the differential diagnosis of joint involvement in systemic sclerosis. Clin Rheumatol 26:510–514
Blocka KN, Basset LW, Furst DE, Clements PJ, Paulus HE (1981) The arthropathy of advanced progressive systemic sclerosis. A radiographic survey. Arthritis Rheum 24:874–884
Ioan-Facsinay A, Willemze A, Robinson DB, Peschken CA, Markland J, van der Woude D et al (2008) Marked differences in fine specificity and isotype usage of the anti-citrullinated protein antibody in health and disease. Arthritis Rheum 58:3000–3008
Nishimura K, Sugiyama D, Kogata Y, Tsuji G, Nakazawa T, Kawano S et al (2007) Meta-analysis: diagnostic accuracy of anti-cyclic citrullinated peptide antibody and rheumatoid factor for rheumatoid arthritis. Ann Intern Med 146:797–808
Cui J, Taylor KE, Destefano AL, Criswell LA, Izmailova ES, Parker A et al (2009) Genome-wide association study of determinants of anti-cyclic citrullinated peptide antibody titer in adults with rheumatoid arthritis. J Mol Med 15:136–143
Santiago M, Baron M, Miyachi K et al (2008) A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti-CCP assay (CCP-3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin Rheumatol 27:77–83
Moryta Y, Muro K, Sugiura K, Tomita Y (2008) Anti citrullinated peptide antibody in systemic sclerosis. Clin Exp Rheumatol 26:542–547
Subcommitee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Committee (1980) Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum 23:581–590
Furst DE, Clements PJ, Wong WK, Mayes MD, Wigley F, White BE et al (2001) Effects of the American College of Rheumatology systemic sclerosis trial guidelines on the nature of systemic sclerosis patients entering a clinical trial. Rheumatol 40:615–622
Denton CP, Black CM (2003) Pulmonary hypertension in systemic sclerosis. Rheuma Dis Clin N Am 29:335–349
Akenson A, Fiori G, Krieg T, van den Hoogen FHJ, Seibold JR (2003) The assessment of the patient with systemic sclerosis. The assessment of skin, joint, tendon and muscle involvement. Clin Exp Rheumatol 21(3 Suppl 29):S5–S8
Bassotti G, Battaglia E, Debernardi V, Germani U, Quiriconi F, Dughera L et al (1997) Esophageal dysfunction in scleroderma. Arthritis Rheum 40:2252–2259
Medsger TA Jr, Silman AJ, Steen VD et al (1999) A disease severity scale for systemic sclerosis: development and testing. J Rheumatol 26:2159–2167
Szücs G, Szekanecz Z, Zilahi E et al (2007) Systemic sclerosis-rheumatoid arthritis overlap syndrome: a unique combination of features suggests a distinct genetics serological and clinical entity. Rheumatology 46:989–993
Zimmermann C, Steiner G, Skriner K, Hassfeld W, Petera P, Smoles JS (1998) The concurrence of rheumatoid arthritis and limited systemic sclerosis. Arthritis Rheum 41:1936–1945
Rodnan GP (1962) The nature of joint involvement in progressive systemic sclerosis (diffuse scleroderma). Ann Inter Med 56:422–439
Goeldner I, Skare TL, de Messias Reason IT, Nisihara RM, Silva MB, Utiyama SR (2010) Anti-cyclic citrullinated peptide antibodies and rheumatoid factor in rheumatoid arthritis patients and relatives from Brazil. Rheumatology 49:1590–1593
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Polimeni, M., Feniman, D., Skare, T.S. et al. Anti-cyclic citrullinated peptide antibodies in scleroderma patients. Clin Rheumatol 31, 877–880 (2012). https://doi.org/10.1007/s10067-011-1930-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1930-z