Abstract
The objective was to investigate the frequency of anti-cyclic citrullinated peptides (CCP) antibodies in systemic sclerosis (SSc) and primary biliary cirrhosis (PBC), utilizing a new “third generation” anti-CCP ELISA (anti-CCP3) kit and a conventional anti-CCP2 assay. Patients with PBC, SSc, RA, and normal controls were included in the study. Serum samples were screened for autoantibodies by indirect immunofluorescence (IIF), antibodies to CCP by a second- and third-generation ELISA, antibodies to “scleroderma” antigens (CENP B, Scl-70, PM/Scl and fibrillarin—Scl-34) by a line immunoassay (LIA), and IgM RF by ELISA. The frequency of anti-CCP2 antibodies in SSc and PBC samples was 14.8% (11/74) and 6.2% (5/80), respectively, and the frequency of anti-CCP3 antibodies in SSc was 13.5% (10/74) and in PBC was 3.7% (3/80). By comparison, in the RA group the frequency of anti-CCP3 and anti-CCP2 antibodies was 79.1% (38/48) and 77% (37/48), respectively. Anti-CCP3 ELISA had a sensitivity, specificity, and positive and negative likelihood ratios (LR) of 79% (95% confidence interval [CI] = 64–89%), 93% (95% CI = 88–96%), 11.8 (95% CI = 6.8–20.3), and 0.22 (95% CI = 0.12–0.38), respectively. By comparison, the anti-CCP2 assay had a sensitivity, specificity, and positive and negative LRs of 77% (95% CI = 62–87), 90% (95% CI = 85–94), 8.3 (95% CI = 5.2–13.2), and 0.25 (95% CI = 0.15–0.42), respectively. In patients with SSc, there was an association of anti-CCP2 antibodies with the presence of arthritis, but there was no association of anti-CCP2 or anti-CCP3 with anti-CENP B, anti-Scl 70, or RF. This study confirmed the high specificity and sensitivity of both anti-CCP assays for the diagnosis of RA. The presence of anti-CCP antibodies in SSc was only correlated with the presence of arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Circulating autoantibodies are a serological hallmark of systemic rheumatic diseases and some of them have high specificity and sensitivity for diagnosis and/or prognosis. Classically, the laboratory abnormalities observed in rheumatoid arthritis (RA) patients are the presence of rheumatoid factor (RF) and an increase in acute-phase reactant proteins, but considerable interest has also focused on other autoantibodies [1–3]. More recently, antibodies directed to a variety of citrullinated peptides (CP), and in particular anti-cyclic citrullinated peptide (CCP) have been established as a specific diagnostic and prognostic tool for the diagnosis of RA (reviewed in [4–6]).
In the clinical laboratory setting, anti-CCP antibodies have been detected by enzyme-linked immunosorbent assay (ELISA) in the form of commercial kits purchased from a variety of distributors. The announcement of the first CCP ELISA kit was followed by two subsequent “generations” of anti-CCP ELISA kits, each attended by claims of improved sensitivity and specificity. For example, the “second generation” anti-CCP (CCP2) test is reported to have higher sensitivity than the first generation anti-CCP (CCP1) test (68% vs 53%), although both have similar specificity (95% vs 96%) for RA [4].
In the course of our studies of anti-CCP antibodies, we noted that sera from patients with systemic sclerosis (SSc) and some related conditions had a higher-than-expected frequency of anti-CCP. The aim of the present study was to evaluate a “third generation” anti-CCP ELISA (anti-CCP3) and compare the results to the anti-CCP2 system in SSc and PBC sera, two autoimmune conditions that can have overlapping features of an associated inflammatory arthropathy and autoimmune liver disease.
Materials and methods
Patients and Sera
Seventy-four SSc patients were part of the Canadian Scleroderma Consortium Study that was established by 15 Canadian rheumatologists who reviewed the SSc patients yearly and entered clinical and laboratory data onto standardized forms. Patients also completed questionnaires and several standardized instruments such as the Health Assessment Questionnaire (HAQ) and the Medical Outcomes Study Short Form 36 (SF-36). The SSc dataset included a history of and evaluation for the presence of arthritis (including polyarthritis) and co-morbid illnesses such as RA. The diagnosis in 74 PBC patients was established according to published clinical parameters and histological features of liver biopsies [7, 8], and sera were obtained from the Health Sciences Research Institute in Yokohama, Japan [9, 10]. Sera from 48 RA patients were diagnosed according to the American College of Rheumatology criteria [11]. Normal blood donor sera (NHS) were included as comparative controls. The collection of sera and their use for serological studies had the approval of the ethics review board of each participating center.
Autoantibodies (AA) to nuclear and cytoplasmic components
AA to nuclear and cytoplasmic components were screened by indirect immunofluorescence (IIF) on HEp-2 substrate (ImmunoConcepts, Sacramento, CA) as previously described [12]. Antibodies to mitochondria (M2, pyruvate dehydrogenase complex) and gp210, a nuclear envelope autoantigen, were detected by ELISA (INOVA Diagnostics, San Diego, CA).
ELISA for anti-CCP antibodies
All serum samples were tested for the presence of anti-CCP2 (INOVA Diagnostics) and anti-CCP 3 (INOVA Diagnostics) antibodies by ELISA following the procedures recommended by the manufacturer. Values <20 U were considered negative; between 20 and 39 U “weak”, 40 to 59 U “moderate”, and ≥60 U “strong” positive. Another ELISA for anti-CCP2 antibodies from a different manufacturer (Axis-Shield, Norton, MA) was also performed, with the protocol and cutoffs as recommended by the manufacturer.
Addressable Laser Bead Immunoassay (ALBIA)
Citrullinated peptides were synthesized and covalently coupled to microspheres (Luminex Corporation, Austin, TX) using techniques previously described [13, 14]. The diluted serum samples were added to microtiter plates followed by the phycoerythrin-conjugated anti-human IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) followed by semi-quantitative determination of these antibodies using a Luminex 100™ dual laser flow cytometer (Luminex Corporation). Results were expressed as mean fluorescence units.
Line Immunoassay (LIA)
Samples from SSc patients were tested with a commercial LIA kit (Mikrogen, Neuried, Germany) developed to detect antibodies to an array of scleroderma-related antigens (CENP B, Scl-70, PM/Scl, and fibrillarin-Scl-34) following the protocol as recommended by the manufacturer.
IgM rheumatoid factor (ELISA)
An ELISA kit (Quantalite; INOVA Diagnostics) was used to determine the presence of IgM rheumatoid factor (RF). As suggested by the manufacturer, the samples were classified as positive when >6 U reactivity was observed.
Statistical analysis
The Statistical Package for the Social Sciences (SSPS) for Windows (version 14.0) program was used for analysis of the data. Some results were expressed as mean ± standard deviation. The sensitivity, specificity, positive and negative likelihood ratios (LR) of the ELISA assays were calculated and the results presented in 95% confidence interval (CI). The Kolmogorov–Smirnov test was used to determine the normality of the variable. The Wilcoxon signed ranks test was used to compare the means. The association between qualitative variables was evaluated by Chi-square corrected (Yates) or exact Fisher test, when indicated, considering p < 0.05 as of statistical significance.
Results
A total of 242 sera were included in the study. The cohort of 74 SSc patients included 66 (89%) women and 8 (11%) men with a mean age of 58 ± 13 years. Twenty-nine (39%) had diffuse SSc, 40 (54%) had limited SSc, and 5 (7%) were unclassified. Clinical characteristics of these patients are shown in Table 1. Although 18/74 (24%) had arthritis by history, only three had a concurrent diagnosis of, or fulfilled the criteria for, RA. The Japanese PBC cohort was comprised of 74 (92.5%) women and 6 (7.5%) men with a mean age of 62 ± 10 years. Seventy-four (92.5%) had anti-mitochondria M2 antibodies and 16 (20.3%) had antibodies to the nuclear envelope antigen gp210, a marker for rapid disease progression and a poor outcome in PBC [15]. Three (3.8%) of the Japanese PBC patients had a concurrent diagnosis of RA. The comparative study groups included sera from 48 RA patients and 40 NHS.
The combined frequency of anti-CCP3 antibodies in the PBC and SSc patients was 8.4%; 13.5% (10/74) in SSc and 3.7% (3/80) in PBC. As illustrated in Fig. 1, only a few of these samples had high titers of CCP3 antibodies. The overall frequency of anti-CCP2 antibodies was 10.3%: 14.8% (11/74) in SSc and 6.2% (5/80) in PBC (Fig. 2). The mean units of reactivity of anti-CCP3 antibodies was not statistically different from anti-CCP2 (p = 0.563). Four samples were positive for both anti-CCP2 and anti-CCP3, 12 were positive for anti-CCP2 but negative for anti-CCP3 and nine only reacted with anti-CCP3.
As it was considered that the positive results might be a feature of the ELISA assay platform (i.e., nonspecific binding to microtiter plates used in ELISA), 13 selected samples from the RA or PBC cohorts were then tested for reactivity to citrullinated peptides in the ALBIA platform (Table 2). There was concordance in the results of CCP3, CCP2, and ALBIA for three positive samples and five negative samples. In addition, one sample negative for both CCP2 and CCP3 showed a low positive reaction in ALBIA. Furthermore, two samples were positive only for CCP3, one for CCP2 alone, and one positive for CCP2.
In the RA group, the frequency of anti-CCP3 and anti-CCP2 antibodies was 79.1% (38/48) and 77% (37/48), respectively. In the sera from NHS, anti-CCP3 and anti-CCP2 antibodies were identified in 0/40 (0%) and 2/40 (5%), respectively. The results of anti-CCP3 and anti-CCP2 antibodies in the four groups studied are shown in Figs. 1 and 2.
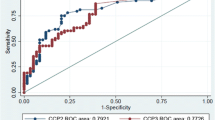
When the performance of the two anti-CCP ELISA systems was calculated comparing the RA group with the remaining three groups (non-RA), it was found that anti-CCP3 ELISA had a sensitivity, specificity, and positive and negative likelihood ratios (LRs) of 79% [95% confidence interval (CI) = 64–89%], 93% (95% CI = 88–96%), 11.8 (95% CI = 6.8–20.3), and 0.22 (95% CI = 0.12–0.38), respectively. By comparison, the anti-CCP2 assay had a sensitivity, specificity, and positive and negative LRs of 77% (95% CI = 62–87), 90% (95% CI = 85–94), 8.3 (95% CI = 5.2–13.2), and 0.25 (95% CI = 0.15–0.42), respectively.
We also determined the relationship, in both SSC and PBC, between anti-CCP antibodies and either the anti-nuclear antibody staining pattern or the presence of other specific autoantibodies. The frequency of AA as determined by IIF on HEp-2000 substrates in SSc and PBC was 97.2 and 98.6%, respectively. Various combinations of staining patterns were commonly observed in a single sample, but when separated into individual patterns, the most common pattern in SSc was nuclear speckled (64.8%; 48/74), followed by centromere 31/74 (41.8%) and nucleolar 25/74 (33.7%). Other patterns included nuclear homogeneous, NSP1 [16], SS-A/Ro [17], mitochondria, and a diffuse cytoplasmic pattern. In PBC samples, the most common pattern was mitochondrial or cytoplasmic speckled, observed in 70/80 (87.5%), followed by nuclear speckled 68/80 (85%) and cytoplasmic dots 37/80 (46.2%). Other patterns included nuclear envelope 34/80 (42.5%), centromere 20/80 (25%), and nuclear dots 9/80 (11.2%).
The frequency of antibodies to CENP-B, Scl 70 (topoisomerase I), PM/Scl, and fibrillarin in SSc as determined by LIA was 36/74 (48.6%), 22/74 (29.7%), 2/74 (2.7%) and 0/74, respectively. In the 80 PBC samples, there were 21 with anti-CENP B antibodies (26.2%), but none of the other SSc-related autoantibodies were detected by the LIA. The frequency of IgM RF as determined by ELISA was 35% (28/80) in PBC and 18.9% (14/74) in the SSc groups.
In the SSc cohort there was no association between the presence of anti-CCP3 antibodies and CENP-B (p = 0.40) or a centromere IIF staining pattern (p = 0.58), Scl-70 (p = 0.62) as detected by LIA, IgM RF as detected by ELISA, or the presence of arthritis (as determined by self-reported diagnosis or physician assessment) (p = 0.4). However, in the SSc cohort, anti-CCP2 was associated with arthritis (Chi-square corrected—Yates, p = 0.03), although not with IgM RF. In addition, anti-CCP3 was not significantly associated with anti-CCP2 (p = 0.3), although both were positive for the serum with the highest level of CCP reactivity (Figs. 1 and 2).
In the PBC cohort, there was no association between the centromere IIF pattern of staining and the presence of anti-CCP3 antibodies (p = 0.48), or between anti-CENP B by LIA and anti-CCP3 antibodies by ELISA (p = 0.60). Also, there was no association between anti-CCP3 and anti-gp210 antibodies (p = 0.10). Likewise, there was no association between anti-CCP2 and CENP B by LIA (p = 0.2), centromere pattern by IIF (p = 0.2) or anti-gp210 (p = 0.6). In addition, there was no association between the presence of either anti-CCP2 or anti-CCP3 and the concomitant diagnosis of RA (p = 0.8), and there was no association between the presence of IgM RF and RA (p = 0.2), anti-CCP3 (p = 0.70), or anti-CCP2 (p = 0.055).
Discussion
As part of an investigation of different autoantibodies in SSc and PBC sera, we observed a higher-than-expected frequency of anti-CCP antibodies. To examine the possibility that the presence of anti-CCP antibodies was attributed to autoantibodies that cross-reacted with other known autoantibodies, the samples were tested for antibodies directed against a variety of well-documented autoantibodies that are characteristic for each of these diseases. Our data indicated that there was no association between the presence of anti-CCP3 and these other antibodies. Therefore, the attribution of the anti-CCP reactivity in SSc and PBC sera to autoantibodies that bind common autoantigens seems unlikely. In addition, the possibility that this reactivity is a nonspecific feature of the ELISA platform seems unlikely because reactivity was generally corroborated in an ALBIA.
Studies of the prevalence of anti-CCP in SSc have been infrequently reported in the literature. A recent study demonstrated the presence of anti-CCP2 antibodies in 8/75 (10.6%) SSc serum samples [18], a finding that approximates the frequency of 14.8% obtained in the present study. In that study, the authors found a statistically significant association between the presence on anti-CCP2 and arthritis and erosions on hand and wrist radiographs. These clinical findings are somewhat similar to what we observed in the present study, although we did not perform routine radiographs of joints in all patients
Since another study [18] suggested that antibodies to CCP2 in SSc patients may be a useful biomarker to identify patients with clinical features of RA and an overlap RA/SSc syndrome, we sought to determine if there was a correlation of this autoantibody with IgM RF. In the present study, there were 18 patients with arthritis in the entire SSc cohort and only three of these had a diagnosis of RA/SSc overlap, but even in these three patients, there was no association of anti-CCP with RA, although a statistically significant association of anti-CCP2 but not anti-CCP3 with arthritis was noted. Further, it was noted that 19% of the SSc sera had RF, but there was no statistically significant association of RF with antibodies to CCP2, CCP3, or the presence of arthritis.
Although the presence of autoantibodies such as anti-mitochondria and ANA in PBC are well documented (reviewed in [19]), there are only a few reports of the presence of anti-CCP in this condition. In a study published a decade ago, it was observed that PBC sera did not react with keratin [20], an antigen that has been shown to have citrullinated peptide domains. In another study of anti-CCP2 in type 1 autoimmune hepatitis (AIH-1) and other chronic liver diseases, the frequency of anti-CCP2 in AIH-1 was 9% (12/133) and 4% (2/49) in PBC sera [21]. These findings are in keeping with the present study where we found that the frequency of anti-CCP2 in the sera of 80 Japanese PBC patients was 6.2%.
As in the SSc cohort study, we hypothesized that the presence of anti-CCP antibodies might be caused by cross-reacting PBC autoantibodies. However, we could not confirm any serological correlations with the common PBC-related autoantigens. In particular our attention was focused on anti-CENP because these antibodies are seen in both SSc and PBC [22], but such a correlation could not be established. Finally, it has been recently reported that anti-gp210 antibodies are associated with a severe and rapidly progressive form of PBC [15], but no correlation of CCP antibodies with this serological marker was observed.
Of note, it was previously reported that anti-CCP2 antibodies were not found in hepatitis C sera, even in patients with articular involvement [23]. In another study of hepatitis C sera, it was concluded that the production of anti-citrullinated peptides was not exclusively dependent on liver tissue damage [24]. Unfortunately, we did not have access to detailed clinical data on the PBC patients, but it was noted that although three had a concurrent diagnosis of RA, none had anti-CCP. Curiously, the PBC sera were not entirely concordant for the two anti-CCP assays, suggesting that the production of antibodies to citrullinated peptides is not a uniform process and its detection depends on the specific peptide used in the assay.
Although the present study has confirmed the higher specificity of anti-CCP antibodies for the diagnosis of RA, as described by others they are also found in other autoimmune disorders such as PBC, SSc, SLE, and SjS [25]. These observations suggest that caution is needed in establishing the diagnosis of early RA based exclusively on the presence of these antibodies, as arthritis is a common feature of other systemic autoimmune rheumatic diseases. On the other hand, it is possible that long-term follow-up of the SSc and PBC patients with anti-CCP antibodies may reveal the eventual development of inflammatory joint disease and a diagnosis of RA.
Abbreviations
- ALBIA:
-
Addressable laser bead immunoassay
- CCP:
-
Cyclic citrullinated peptide
- ELISA:
-
Enzyme linked immunoassay
- PBC:
-
Primary biliary cirrhosis
- RA:
-
Rheumatiod arthritis
- SPSS:
-
Statistical package for the Social Sciences
- SSc:
-
Systemic sclerosis
- SSN:
-
Serum sample number
- U:
-
Units
References
Paulus HE, Wiesner J, Bulpitt KJ, et al (2002) Autoantibodies in early seropositive rheumatoid arthritis, before and during disease modifying antirheumatic drug treatment. J Rheumatol 29:2513–2520
Goldbach-Mansky R, Lee J, McCoy A, et al (2000) Rheumatoid arthritis associated autoantibodies in patients with synovitis of recent onset. Arthritis Res 2:236–243
Nell VPK, Machold KP, Stamm TA, et al (2005) Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis 64:1731–1736
Avouac J, Gossec L, Dougados M (2006) Diagnostic and predictive value of anti-cyclic citrullinated protein antibodies in rheumatoid arthritis: a systematic literature review. Ann Rheum Dis 65:845–851
Zendman AJ, Van Venrooij WJ, Pruijn GJ (2006) Use and significance of anti-CCP autoantibodies in rheumatoid arthritis. Rheumatology (Oxford) 45:20–25
Menard HA (2007) Anti-CCP versus anti-Sa antibodies for the diagnosis of RA. Nat Clin Pract Rheumatol 3:76–77
Scheuer PJ (1967) Primary biliary cirrhosis. Proc R Soc Med 60:1257–1260
Scheuer PJ (1988) Ludwig Symposium on biliary disorders—part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc 73:179–183
Miyachi K, Hirano Y, Horigome T, et al (2004) Autoantibodies from primary biliary cirrhosis patients with anti-p95c antibodies bind to recombinant p97/VCP and inhibit in vitro nuclear envelope assembly. Clin Exp Immunol 136:568–573
Miyachi K, Hankins RW, Matsushima H, et al (2003) Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmun 20:247–254
Arnett FC, Edworthy SM, Bloch DA, et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Fritzler MJ, Miller BJ (1995) Detection of autoantibodies to SS-A/Ro by indirect immunofluorescence using a transfected and overexpressed human 60 kD Ro autoantigen in HEp-2 cells. J Clin Lab Anal 9:218–224
Selak S, Woodman RC, Fritzler MJ (2000) Autoantibodies to early endosome antigen (EEA1) produce a staining pattern resembling cytoplasmic anti-neutrophil cytoplasmic antibodies (C-ANCA). Clin Exp Immunol 122:493–498
Eystathioy T, Chan EKL, Yang Z, et al (2003) Clinical and serological associations of autoantibodies to a novel cytoplasmic autoantigen, GW182 and GW bodies. J Mol Med 81:811–818
Nakamura M, Kondo H, Mori T, et al (2007) Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology 45:118–127
Fritzler MJ, Valencia DW, McCarty GA (1984) Speckled pattern antinuclear antibodies resembling anticentromere antibodies. Arthritis Rheum 27:92–96
Fritzler MJ, Hanson C, Miller J, Eystathioy T (2002) Specificity of autoantibodies to SS-A/Ro on a transfected and overexpressed human 60 kDa Ro autoantigen substrate. J Clin Lab Anal 16:103–108
Ingegnoli F, Galbiati V, Zeni S, et al (2006) Use of antibodies recognizing cyclic citrullinated peptide in the differential diagnosis of joint involvement in systemic sclerosis. Clin Rheumatol 26:510–514
Fritzler MJ, Manns MP (2002) Anti-mitochondrial antibodies. Clin Appl Immunol Rev 3:87–113
Fusconi M, Berti CC, Monti G, et al (1996) Antikeratin antibodies (AKA) negativity in primary biliary cirrhosis (PBC): confirmation of their specificity in the diagnosis of rheumatoid arthritis (RA). Clin Rheumatol 15:617–618
Fusconi M, Vannini A, Dall’Aglio AC, et al (2005) Anti-cyclic citrullinated peptide antibodies in type 1 autoimmune hepatitis. Aliment Pharmacol Ther 22:951–955
Makinen D, Fritzler MJ, Davis P, Sherlock S (1983) Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum 26:914–917
Bombardieri M, Alessandri C, Labbadia G, et al (2004) Role of anti-cyclic citrullinated peptide antibodies in discriminating patients with rheumatoid arthritis from patients with chronic hepatitis C infection-associated polyarticular involvement. Arthritis Res Ther 6:R137–R141
Wener MH, Hutchinson K, Morishima C, Gretch DR (2004) Absence of antibodies to cyclic citrullinated peptide in sera of patients with hepatitis C virus infection and cryoglobulinemia. Arthritis Rheum 50:2305–2308
Matsui T, Shimada K, Ozawa N, et al (2006) Diagnostic utility of anti-cyclic citrullinated peptide antibodies for very early rheumatoid arthritis. J Rheumatol 33:2390–2397
Acknowledgments
The authors acknowledge the technical assistance of Jane Zhang and Mark Fritzler. This study was supported by grants #38034 and #10884 from the Canadian Institutes of Health Research. MJF holds the Arthritis Society Research Chair at the University of Calgary, and funds from this endowment supported the sabbatical leave of MS. MS also received a scholarship from CAPES—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. Diagnostic kits for CCP3, gp210, and rheumatoid factor were generous gifts of INOVA Diagnostics, San Diego, CA. HEp-2 kits were a gift of ImmunoConcepts Inc., Sacramento, CA.
Conflict of Interest Statement
Mittermayer Santiago: none
Murray Baron: none
Kiyomitsu Miyachi: none
Canadian Scleroderma Research Group: none
Marvin Fritzler: is a paid consultant of ImmunoConcepts Inc. (Sacramento, CA)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santiago, M., Baron, M., Miyachi, K. et al. A comparison of the frequency of antibodies to cyclic citrullinated peptides using a third generation anti-CCP assay (CCP3) in systemic sclerosis, primary biliary cirrhosis and rheumatoid arthritis. Clin Rheumatol 27, 77–83 (2008). https://doi.org/10.1007/s10067-007-0656-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0656-4