Abstract
In genetic prion diseases (gPrD), five genetic variants (E200K, V210I, V180I, P102L, and D178N) are responsible for about 85% of cases. The R208H is one of the several additional rare mutations and to date, only 16 cases carrying this mutation have been reported worldwide. To describe the phenotypic features of 5 affected patients belonging to apparently unrelated Sardinian (Italian) families with R208H gPrD, and provide evidence for a possible founder effect are the aims of this study. The R208H PRNP mutation has a much higher relative frequency in Sardinia than elsewhere in Italy (72% vs. 4.4% of gCJD cases). Our cohort shared similar phenotypic features to the previously described patients with R208H-129M haplotype with most patients showing the classical Creutzfeldt-Jakob disease (CJD) phenotype. The analysis of 10 controls and 5 patients by NGS sequencing identified 4 haplotypes, 3 associated with the wild type variant, and one (H1) shared by all patients carrying the 208His variant. This is the first report of a regional cluster for R208H mutation in gPrD and the first report of the presence of a common ancestor for this Sardinian R208H cluster, confirming the probable consequences of genetic isolation process even for rare diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prion diseases (PrDs) are neurodegenerative disorders caused by a transmissible abnormal conformer of the prion protein (PrPSc), also known as prion, which stand for proteinaceous infectious particle [1]. Typically, human PrDs include sporadic forms (sPrD), with sporadic Creutzfeldt-Jakob disease (sCJD) and sporadic fatal insomnia; genetic forms (gPrD), with genetic CJD (gCJD), fatal familial insomnia, and Gerstmann-Sträussler-Scheinker; and acquired (aPrD) forms, including iatrogenic and variant CJD. SPrD account for 85–90%, gSPrD for 10 to 15%, and aPrD for < 1% of human PrD, respectively.
GPrDs are caused by mutations in the PRNP gene encoding for the prion protein and mapping to chromosome 20p12-pt [2]. PRNP variants include point mutations leading to amino acid substitution, premature stop codon, or insertion of octapeptide repeats in the N-terminal region of the gene. To date, more than 60 PRNP variants have been identified, but five of them (E200K, V210I, V180I, P102L, and D178N) account for about 85% of gPrD (data from nine major surveillance centers worldwide) [3]. Some variants rely on regional clusters due to a proven (E200K mutation clusters among Slovakians, Sephardic Jews from Libya and Calabria region in Italy, D178N in Spain, Germany, and Italy) or likely founder effect (the V180I mutation in Japan, and V210I in Italy) [4]. In contrast, other less common mutations have been only found in single or few isolated individuals usually in the absence of a positive family history for the disease [5, 6].
The R208H substitution in the PRNP gene has been to date described in 16 cases from European countries, Japan, and China [3]. Here, we describe five new patients with R208H gCJD belonging to five apparently unrelated families from Sardinia, the second-largest island in Italy. Clinical and genetic characterization of this cohort revealed an unusually high prevalence of R208H mutation in Sardinia and a probable founder effect.
Methods
Patients
The present study patients were admitted to the neurology departments of AOU Policlinico Hospital and AO Brotzu Hospital in Cagliari and referred to the Italian Registry of CJD at the Istituto Superiore di Sanità, Rome, Italy (ISS) from 2006 to 2016. Demographic, clinical, and instrumental data were obtained by review of hospital records. CSF samples of the patients were analyzed for 14-3-3 protein by Western blot at the ISS Laboratory and stored at − 80 °C. The detection of the pathological form of PrPSc by real-time quaking-induced conversion (RT-QuIC) [7] was performed retrospectively for the purpose of this study, since it has been only recently included in the diagnostic criteria for CJD [8]. The direct complete sequencing of the PRNP gene and the neuropathological assessment were performed at the moment of diagnosis according to published protocol [9, 10]. All subjects’ or CJD patients’ next of kin provided informed written consent. The diagnosis of probable or definite gCJD was made according to internationally recognized diagnostic criteria from the National UK CJD Research & Surveillance Unit Protocol [11].
Ten anonymous control DNA samples from the Sardinian population were included in the study.
Epidemiologic data on CJD in Italy and Sardinia, from 2006 to 2016, were obtained from the Italian Registry of CJD, as summarized in Table 1. In the selected time period, 50 cases of CJD (including our own patients) were diagnosed in Sardinia and 1473 in Italy.
NGS sequencing and gametic phase reconstruction
Molecular and genetic studies were performed at the Genetica Medica Laboratory, R. Binaghi Hospital, Cagliari.
Specific libraries were prepared for each individual included in the study. These were produced using a long-read PCR protocol (LR-PCR) and NGS library prepared using the Nextera library preparation kit (Illumina). For this purpose, 10 ng of high molecular weight DNA were amplified with specific primers described in Table 2. These produced a fragment of 7055 bp which includes the exon 2 of the PRNP gene and the regions flanking it. Table 2 shows the primer sequences and the genomic positions referring to the hg19 release. The LR-PCRs were purified using the AMPure paramagnetic beads (Beckman Coulter s.r.l. Milan, Italy), and 1 ng of amplification product was fragmented for library preparation. Each library was labeled using two index sequences (Nextera Index kit A). The libraries were normalized to the concentration of 4 nM and verified for the correct composition of fragments in a fragment analyzer system (Agilent techn. Santa Clara, USA). The libraries were then pooled and loaded onto a MiSeq Reagent Kits v3 (Illumina Inc. Milan, Italy) flow cell/cardrige system of an Illumiana MiSeq sequencer. The FastQ files produced by the MiSeq sequencer were analyzed using the MiSeq Reporter package (Illumina Inc. Milan, Italy) in order to obtain the VCF and BAM output files containing the identified variants. These were inspected with the Variant Studio 3 (Illumina) and IGV (Integrative Genomics Viewer-Broad Institute) software. The gametic phase and therefore the structure of the haplotypes was reconstructed by inspecting the BAM files containing the reads. The variants were considered in phase when present in the same read.
Results
Patients
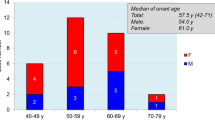
All clinical, genetic, neuroimaging, and laboratory features of the 5 study patients are described in Table 3. There were 3 men and 2 women, with a mean age at symptom onset of 63 years old (range 54–73 years old). All cases were apparently unrelated, and their family history was unremarkable for CJD or other neurodegenerative diseases. Clinical prodromes (including depression and slight memory impairment) in the year preceding clinical onset were reported by 2/5 patients. The most common symptoms at onset were ataxia (4/5 cases), cognitive impairment (3/5 cases), and language disturbances (aphasia in 3/5 cases and dysarthria in 2/5 cases). Further signs/symptoms that developed over the disease course were visual symptoms (1 patient), pyramidal or extrapyramidal motor signs (3 patients), myoclonus (4 patients), and akinetic mutism (2 patients). (Table 3).
On laboratory and instrumental investigations, 3 patients showed typical periodic and triphasic sharp waves at EEG, and 2 patients had a positive brain MRI (it is worth noting that 2/3 patients who showed a normal brain MRI underwent a low-field 0.5 T MRI). All patients had positive 14-3.3 protein in CSF and a positive prion RT-QuIC CSF assay. Autopsy was performed in 3/5 patients: all of them showed mild to moderate spongiform change, neuronal loss, and gliosis mainly involving the cerebral cortex, striatum, thalamus, and cerebellum as in the typical, most common sCJD subtype linked to homozygosity for methionine (MM) or methionine/valine heterozygosity (MV) at codon 129 and PrPSc type 1 [9]. (Table 3).
The R208H mutation was found associated with MM in 4 patients, and with MV in one case at codon 129. R208H-129M haplotype was found in all patients.
According to internationally recognized diagnostic criteria [11], 3 of our patients were diagnosed as definite gCJD, while 2 patients satisfied the criteria for probable gCJD.
Genetic and molecular testing
NGS sequencing of the region was conducted in the five patients described in this study and in the 10 control samples. The presence of the NM_000311.3 variant: c.623G > A (NP_000302.1: p. Arg208His) in heterozygosity was confirmed in all patients (Table 4).
In the region of more than 7 Kb of the PRNP gene analyzed, there were 684 SNPs (Genome Aggregation Database-gnomAD). Of these, almost all consisted of rare variants, whereas 7 SNPs in the European population showed a percentage frequency greater than 20% (Fig.1). These SNPs resulted in 14 different haplotypes in the European population of which one showed a frequency > 50% and 5 others a frequency between 3 and 18%.
In all the analyzed individuals, we determined the gametic phase with certainty and therefore the structure of the haplotypes. Overall, in the 5 patients and 10 controls, we identified the presence of 4 haplotypes (Tab. 3). Of these, the haplotype indicated as H1 was present in all patients carrying the (A) 208His variant, while the other haplotypes (H1WT, H2WT, and H3WT) were all associated with the wild type (G) variant. Interestingly, variant (A) 208His was always associated with the variant A 129M. These data show a common founder for all Sardinian patients carrying the 208His variant.
Discussion
The 5 patients herein reported from Sardinia, Italy, were apparently unrelated to each other up to 2 generations. Nevertheless, genetic analysis revealed that all patients were positive for the R208H mutation and exhibited a large risk haplotype across the R208H locus. The latter observation indicated that they were part of a larger kindred and probably shared a common ancestor.
To date, we are aware of a total of 16 patients carrying the R208H mutation reported worldwide, [3] even though detailed information was available only for 11 cases [12,13,14,15,16,17,18,19,20,21,22]. These 11 patients showed different codon 129 genotypes: 6 patients were MM [12,13,14,15,16, 20], 4 patients were VV [17, 18, 21, 22], and 1 patient was MV [19]. It is worth noting that the R208H VV cases showed different clinical/laboratory features in comparison to the R208H MM and R208H MV cases, i.e., longer disease duration (12.5 months), higher frequency of psychiatric symptoms at onset, and a reduced sensitivity of 14-3-3 (6 positive out of 9 tested) and RT-QuIC (1 positive out of 2 tested) assays. (Table 3). Considering codon 129 haplotype, in our cohort no patient was VV, 4 patients were MM, and one patient carried MV heterozygosity. This is the second report of a patient with MV c129 gCJD with PRNP R208H mutation. Overall, the clinical features showed by our patients were similar to those of patients with the same PRNP R208H-129M haplotype reported in the literature (Table 3) and to sCJD patients [23].
While E200K represents by far the most common PRNP mutation worldwide, in Italy, the gCJD cases carrying the V210 mutation outnumbered those carrying E200K, representing 42% of all gCJD cases recorded in the period 2006–2016. In contrast, the R208H mutation accounted for only 12/275 (4.4%) of the gCJD identified in Italy in the same period (including the 5 cases herein described, three patients from the literature, and 4 unpublished cases recorded by the ISS registry). When the analysis is limited to Sardinia, however, of the 7 gCJD cases identified in 2006–2016 period, 5 (71%) carried the R208H mutation, one (14.5%) the V210I mutation, and one (14.5%) the E200K mutation. Sardinia, with a population of 1.7 million, is the second-largest Italian island, located 120 miles west of the main Italian coastline. Despite numerous invasions over the centuries, many studies indicate that Sardinians are phylogenetically distinct from other European populations, including mainland Italians [24, 25]. Thus, the greater frequency of the R208H mutation in Sardinia probably reflects genetic isolation. The genetic analysis revealed that our 5 patients all shared a large risk haplotype across the R208H locus, an observation indicating that they had a common ancestor and were part of a larger kindred. This founder effect is comparable with that described for the E200K mutation clusters worldwide [26] and it is the first report for this effect for R208H. Interestingly, despite an uneven geographical distribution of gCJD due to the V210I mutation has been observed in Italy, with an excess of cases in Campania and Apulia, a common ancestor for these patients has not been formally demonstrated yet. [4, 5]
In conclusion, we reported the first regional familial cluster of patients carrying the R208H-129M haplotype worldwide. We also expanded the knowledge about the origin of mutations in the PRNP gene, identifying a common ancestor and showing that our patients, both the previously reported R208H patients and the sCJD patients, shared similar phenotypic features. The future perspectives based on the present results include the study of other R208H patients in Italy to determine whether they also share the same common ancestor of the Sardinian cluster and to evaluate the frequency of R208H mutation in the entire Sardinian population, in order to identify the potential risk and protective factors for the development of the disease.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. If needed, further data that support the findings of this study are available from the corresponding author, [MM], upon reasonable request.
References
Prusiner SB (1982) Novel proteinaceous infectious particles cause scrapie. Science 216:136–144
Takada LT, Kim MO, Cleveland RW, Wong K, Forner SA, Gala II, Fong JC, Geschwind MD (2017) Genetic prion disease: experience of a rapidly progressive dementia center in the United States and a review of the literature. Am J Med Genet B Neuropsychiatr Genet 174:36–69
Ladogana A. and Gabor G. Kovacs (2018) Handbook of clinical neurology, Vol. 153 (3rd series) Human Prion Diseases. M. Pocchiari and J. Manson, Editors
Ladogana A, Puopolo M, Poleggi A, Almonti S, Mellina V, Equestre M, Pocchiari M (2005) High incidence of genetic human transmissible spongiform encephalopathies in Italy. Neurology 64:1592–1597
Minikel EV, Sonia M, Vallabh SM, Lek M et al (2016) Quantifying penetrance in a dominant disease gene using large population control cohorts. Sci Transl Med 8(322):322ra9. https://doi.org/10.1126/scitranslmed Aad 5169
Rodríguez-Martínez AB, Alfonso-Sánchez MA, Peña JA, Sánchez-Valle R, Zerr I, Capellari S, Calero M, Zarranz JJ, de Pancorbo MM (2008 May) Molecular evidence of founder effects of fatal familial insomnia through SNP haplotypes around the D178N mutation. Neurogenetics. 9(2):109–118. https://doi.org/10.1007/s10048-008-0120-x
McGuire LI, Poleggi A, Poggiolini I et al (2016) Cerebrospinal fluid real-time quaking-induced conversion is a robust and reliable test for sporadic creutzfeldt-jakob disease: an international study. Ann Neurol 80(1):160–165. https://doi.org/10.1002/ana.24679
Mackenzie G, Will R (2017) Creutzfeldt-Jakob disease: recent developments. F1000Res 6:2053
Poleggi A, van der Lee S, Capellari S et al (2018) Age at onset of genetic (E200K) and sporadic Creutzfeldt-Jakob diseases is modulated by the CYP4X1 gene. J Neurol Neurosurg Psychiatry 89:1243–1249
Lattanzio F, Abu-Rumeileh S, Franceschini A et al (2017) Prion-specific and surrogate CSF biomarkers in creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Abeta42 levels. Acta Neuropathol 133(4):559–578
National UK CJD Research & surveillance unit protocol: https://www.cjd.ed.ac.uk/sites/default/files/criteria.pdf
Mastrianni JA, Iannicola C, Myers RM et al (1996) Mutation in prion protein gene at codon 208 in familial Creutzfeldt-Jakob disease. Neurology 47:1305
Roeber S, Krebs B, Neumann EM et al (2005) Creutzfeldt-Jakob disease in a patient with an R208H mutation of the prion protein gene (PRNP) and a 17-kDa prion protein fragment. Acta Neuropathol 109:443–448. https://doi.org/10.1007/s00401-004-0978-0
Nozaki I, Hamaguchi T, Sanjo N et al (2010) Prospective 10-year surveillance of human prion diseases in Japan. Brain 133:3043–3057
Chen C, Shi Q, Tian C et al (2011) The first Chinese case of Creutzfeldt-Jakob disease patient with R208H mutation in PRNP. Prion 5(3):232–234
Shi Q, Zhou W, Chen C et al (2015) The features of genetic prion diseases based on Chinese surveillance program. PLoS One 10(10):e0139552
Basset-Leobon C, Uro-Coste E, Peoc’h K, Haik S, Sazdovitch V, Rigal M, Andreoletti O, Hauw JJ, Delisle MB (2006) Familial Creutzfeldt-Jakob disease with an R208H-129V haplotype and Kuru plaques. Arch Neurol 63:449–452
Matej R, Kovacs GG, Johanidesova S et al (2012) Genetic Creutzfeldt–Jakob disease with R208H mutation presenting as progressive supranuclear palsy. Mov Disord 27(4):476–479. https://doi.org/10.1002/mds.24002
Mitrova E, Belay G, Slivarichova-Zakova D, Stelzer M (2015) The first case of genetic CJD with the rare mutation R208H, Met/Val heterozygous at codon 129 of the prion protein gene. Clin Med J 1(3):101–105
Capellari S, Cardone F, Notar S et al (2005) Creutzfeldt–Jakob disease associated with the R208H mutation in the prion protein gene. Neurology 64:905–907
Tiple D, Poleggi A, Mellina V et al (2019) Clinicopathological features of the rare form of Creutzfeldt-Jakob disease in R208H-V129V PRNP carrier. Acta Neuropathol Commun 7:47. https://doi.org/10.1186/s40478-019-0699-1
Vita MG, Gaudino S, Di Giuda D et al (2013) R208H-129VV haplotype in the prion protein gene: phenotype and neuroimaging of a patient with genetic Creutzfeldt-Jakob disease. J Neurol 260:2650–2652. https://doi.org/10.1007/s00415-013-7078-9
Appleby BS, Appleby KK, Crain BJ, Onyike CU, Wallin MT, Rabins PV (2009) Characteristics of established and proposed sporadic Creutzfeldt-Jakob disease variants. Arch Neurol 66(2):208–215. https://doi.org/10.1001/archneurol.2008.533
Piazza A, Mayr WR, Contu L et al (1985) Genetic and population structure of four Sardinian villages. Ann Hum Genet 49(pt 1):47–63
Contu D, Morelli L, Santoni F et al (2008) Y-chromosome based evidence for pre-neolithic origin of the genetically homogeneous but diverse Sardinian population: inference for association scans. PLoS One 3(1):e1430
Lee HS, Sambuughin N, Cervenakova L, Chapman J, Pocchiari M, Litvak S, Qi HY, Budka H, del Ser T, Furukawa H, Brown P, Gajdusek DC, Long JC, Korczyn AD, Goldfarb LG (1999 Apr) Ancestral origins and worldwide distribution of the PRNP 200K mutation causing familial Creutzfeldt-Jakob disease. Am J Hum Genet 64(4):1063–1070
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Melis, M., Molari, A., Floris, G. et al. Genetic Creutzfeldt-Jakob disease in Sardinia: a case series linked to the PRNP R208H mutation due to a single founder effect. Neurogenetics 21, 251–257 (2020). https://doi.org/10.1007/s10048-020-00618-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-020-00618-1