Abstract
A case of Creutzfeldt-Jakob disease (CJD) with a rare mutation of the prion protein (PrP) gene (PRNP) at codon 208 (R208H) is described. By comparison with two preceding reports, the case described here displayed two distinct biochemical and neuropathological features. Western blot analysis of brain homogenates showed, in addition to the commonly observed three bands of abnormal protease-resistant PrP isoform (PrPSc), an additional band of about 17 kDa. Neuropathological examination of the post mortem brain revealed tau pathology in the hippocampus and entorhinal cortex, as well as ballooned neurons in the cortex, hippocampus and subcortical gray matter.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
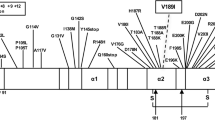
Human prion diseases are transmissible diseases that can be sporadic, acquired as in variant Creutzfeldt-Jakob disease (vCJD), and familial as in 10–15% of human prion diseases that are caused by mutations of the prion protein (PrP) gene (PRNP). Clinical and pathological changes in familial cases may be similar to or indistinguishable from sporadic (sCJD) or idiopathic CJD, as in familial CJD (fCJD) associated with the E200K mutation, or may be quite different from sCJD, as in Gerstmann-Sträussler-Scheinker syndrome (GSS) associated with the P102L mutation. In addition to the particular determining mutation causing disease, the clinical and pathological phenotype of familial prion diseases is influenced by a methionine/valine polymorphism at codon 129 of PRNP.
Here we report on a 69-year-old woman with an R208H mutation of PRNP and clinical signs typical of sCJD. In contrast to two previously reported cases with this mutation, autopsy findings in this case revealed peculiar tau protein pathology. Western blots of brain homogenates showed an unusual 17-kDa fragment of PrP in addition to the PrP banding pattern that is regularly observed in sCJD.
In sCJD, six different banding patterns of the abnormal protease-resistant PrP isoform (PrPSc) have been observed after digestion with proteinase K (PK) [14]. By and large, these six patterns can be separated in two major groups, PrPSc type 1, characterized by three bands migrating at about 30, 26 and 21 kDa, and PrPSc type 2 migrating at about 28, 24 and 19 kDa. The upper band corresponds to the diglycosylated form of PrPSc, the middle band to the monoglycosylated form and the lower band to the unglycosylated form [15].
Detailed Western blot analysis of PK-treated PrPSc has recently shown additional bands of 7–8, 11, 12, 13 and 14 kDa in various forms of human prion disease. Fragments of 7–8 kDa have only been found in GSS. Tagliavini et al. [24, 25] found a low molecular mass fragment of about 7 kDa in GSS patients with A117V, F198S and Q217R mutations of PRNP. Piccardo et al. [20] investigated brain tissue of GSS F198S patients and detected an 8-kDa fragment. In a study on seven GSS patients with the P102L mutation, Parchi et al. [17] identified a 21-kDa peptide and an additional band of 8 kDa in five cases. Two out of the seven patients showed only an 8-kDa fragment. The 8-kDa fragment correlated with the appearance of plaques. Furthermore, a 13 kDa fragment was found only in patients with GSS showing the 8- and 21-kDa fragments. Tagliavini et al. [23] investigated isolated amyloid of GSS patients with an F198S-129V mutation of PRNP, and found an additional 11-kDa fragment spanning residues 58–150. A PrPSc fragment of 11–12 kDa has recently been described in patients with iatrogenic CJD (iCJD) and some sCJD cases [22]. This protease resistant C-terminal fragment was only detectable in patients with iCJD and no PrP plaque-type deposits on histology, and in patients with MM1 and MV1 sCJD. Patients with iCJD and PrP plaque deposits, as well as patients with MV2 and VV2 sCJD and vCJD did not show this fragment. Two unglycosylated PK-resistant fragments of 12 and 13 kDa were described by Zou et al. [30] in all of 29 investigated brains, including all molecular subtypes except MV1. An unglysosylated band of 14 kDa was found in brain extracts of GSS patients with the A117V mutation of PRNP [20]. The pathogenetic or etiological significance of these small PrP fragments is unknown at present.
Clinical history
One year preceding her death this 69-year-old woman developed acute weakness and clumsiness of the left hand, dysarthria and gait disturbance. These symptoms disappeared within a few hours except for a mild clumsiness. Later her daughter noted difficulties of her mother in orientation in her own flat, which had become untidy. She also noted short-term memory deficits and general slowing of her mother. At the time of clinical presentation, 1 month later, she had mild cognitive impairment, especially short-term memory deficits and gave incoherent answers. The neurological examination showed mild left facial paresis, latent paresis of the left hand, mild dysarthria, dysdiadochokinesia and mild gait ataxia. She had arterial hypertension. She had been seriously injured in a car accident a few years before clinical admission.
The cerebral MRT showed vascular lacunar lesions of the right basal ganglia, the genu of the internal capsule and periventricular white matter. The EEG initially showed diffuse slowing and progressed to triphasic and tetraphasic sharp-wave discharges as the disease progressed. The CSF showed a mild blood-CSF barrier disturbance, elevated neuron-specific enolase (74 ng/ml) and 14-3-3 proteins were detectable.
During the days after admission she presented with nocturnal confusion and agitation. The ataxia increased, sometimes the extremities were held in strange positions, and choreatiform-athetotic movements of both arms were observed. She became tetraspastic and more and more aspontaneous. Percutaneous endoscopic gastrostomy (PEG) for feeding became necessary.
A diagnosis of probable CJD was made according to the WHO diagnostic criteria (1998) [28]. She died 1 year after the onset of clinical signs. No similar diseases were reported in her family. Her mother had died at the age of 90 years. She had a urinary bladder carcinoma; no dementia was reported. Her father had died at the age of 45 years of bronchial hemorrhage. Her younger sister (67 years) has been suffering from a psychiatric disorder without dementia or neurological deficits for about 5 years. Her brother (62 years) is healthy. She has two healthy daughters aged 41 and 38. Family members refused genetic examination.
Methods
Histological examination was performed on 4-µm-thick sections of 15 regions of formalin-fixed and paraffin-embedded cerebrum, cerebellum and brainstem. Hematoxylin and eosin (H&E) as well as Gallyas silver stains were performed using standard techniques. Immunohistochemistry was performed with antibodies directed against PrP (L42, 1:100; gift from M. Groschup), phosphorylated tau (AT8, 1:200; Innogenetics, Ghent, Belgium), α-synuclein (15G7, 1:10) and αB-crystallin (1:500; Calbiochem, Nottingham, UK).
For genetic analysis DNA was extracted from blood peripheral lymphocytes according to standard procedures. The complete open reading frame of PRNP was analyzed by direct sequencing of PCR products using the automated LICOR 4200 DNA analyzer as described previously [29].
For Western blot analysis tissue from the frontal cortex and cerebellum was homogenized in 9 volumes (wt/vol) of lysis buffer (0.5% Nonidet P-40, 0.5% DOC, 10 mM EDTA, 100 mM NaCl, 100 mM TRIS, pH 7.4). After PK digestion (50 µg/ml, 1 h at 37°C), cleared homogenates were subjected to SDS-PAGE (12%) and blotted on PVDF membranes [14]. For comparison we used brain homogenates from two sCJD cases (MM1 and VV2). The monoclonal anti-PrP antibody 3F4 (1:3,000, DAKO), recognizing the epitope 109–112 of PrP, was used for immunodetection. Blots were developed using NBT-BCIP according to standard protocols. Band intensity measurement was performed using TotalLab Software (Nonlinear Dynamics, Newcastle upon Tyne, UK).
Results
Histology/immunohistochemistry
The neuropathological gross examination showed severe atrophy of the cerebrum and cerebellum and diminution of cortical thickness. Histological examination of H&E-stained sections revealed severe spongiform changes in all layers of the cerebral cortex, most severely affecting the occipital and temporal lobes. Furthermore, there was very severe neuronal degeneration as well as astrocytic gliosis in the cortex, and a rarefaction of cerebral white matter. Ballooned neurons were found mainly in the deeper layers of the frontal cortex. Marked nerve cell loss, gliosis and spongiform changes were also found in the basal ganglia and less frequently in the thalamus. The hippocampus showed only minor spongiform changes and the brainstem was nearly spared. However, astrocytic gliosis was found in various parts of the brainstem. The molecular layer of the cerebellar cortex showed severe spongiform changes. Degeneration of the granular cell layer and loss of Purkinje cells as well as mild atrophy of cerebellar white matter were noted. The dentate nucleus showed minor vacuolar change, nerve cell loss and gliosis. In addition, a remote ischemic infarct in the right putamen and a status lacunaris of the basal ganglia were found.
Using Gallyas silver staining, we found a small number of neurofibrillary tangles (NFT) and numerous threads in the CA1 region of the hippocampus and external layer of the entorhinal cortex. In the alveus we noted some argyrophilic inclusions in oligodendrocytes, interpreted as coiled bodies (Fig. 1a). There were no argyrophilic inclusions in the neocortex, amygdala, basal ganglia or cerebellum.
Histological section of the CA1 region and alveus showing argyrophilic threads (arrows) as well as argyrophilic inclusions in oligodendrocytes (coiled bodies) (arrowhead). Gallyas silver stain. b Immunohistochemistry with antibody L42 shows synaptic PrP staining of all layers of the cerebral cortex with a predilection for the deep cortical layers. c Immunohistochemistry with antibody AT8. There are numerous AT8-positive tiny granules in the CA1 region and as a felt-like network, most dominant in the alveus. The small box shows the tiny granules. d Immunohistochemistry with antibody against αB-crystallin, showing a ballooned neuron in the cortex of the cingulate gyrus (PrP prion protein)
Immunohistochemistry with an antibody against the human PrP revealed synaptic staining of all layers of the cerebral and cerebellar cortex, with a predilection for the deep cortical layers of the cerebrum and the deep and middle cortical layer of the entorhinal and transentorhinal cortex (Fig. 1b). Synaptic staining was also present in the hippocampus, amygdala and less pronounced in the basal ganglia, thalamus and tectum. A coarse staining pattern of PrPSc was found in the thalamus and granular layer of the cerebellum. Histological sections from the pituitary gland and ganglion Gasseri were negative for PrP.
Immunostaining with an antibody against hyperphosphorylated tau protein (AT8) revealed a few NFT and neurons with stained cytoplasm (pre-tangles) in the CA1 region. There and in the external layer of the entorhinal cortex we found a small number of AT8-positive inclusions in oligodendrocytes and astrocytes. In addition, there were numerous AT8-positive, L42-negative tiny granules in the CA1 region, in the entorhinal cortex, present as a felt-like network most conspicuous in the alveus (Fig. 1c) and less obvious in the medulla oblongata and pons. There were no AT8-positive inclusions in the cerebellum. Numerous αB-crystallin-immunoreactive ballooned neurons were found in the cortex (layer VI) of the cingulate gyrus (Fig. 1d), smaller numbers in the frontal and temporal lobe, subiculum, entorhinal and transentorhinal cortex and only a few in the hippocampus, amygdala, putamen and claustrum. These ballooned neurons did not show argyrophilic staining.
Genetics
Genetic analysis revealed a single point mutation at codon 208, consisting in a guanine to adenine transition, which results in an amino acid change from arginine to histidine. Homozygosity for methionine was found at codon 129 of PRNP.
Western blot analysis
Protein homogenates for Western blot analyses were prepared from the frontal lobe and cerebellum. A typical PrPSc pattern type 1 was found [15]. In addition, there was a fragment migrating at 17 kDa, detected with the antibody 3F4 (Fig. 2: lanes 3 and 4). This fragment was more abundant in the cerebellum than in the frontal lobe. We used band intensity measurements to determine the percentage of the three glycoforms. Dilution series of recombinant human PrP were used for calibration. The results for frontal and cerebellar diglycosylated, monoglycosylated, unglycosylated 21 kDa and unglycosylated 17 kDa bands were 16% and 22.6%; 34.9% and 38.4%; 42.5% and 32.5%; 6.3% and 6.4%, respectively. Deglycosylation of the homogenates prior to PK treatment resulted in two immunoreactive bands of 21 and 17 kDa (data not shown).
Western blot analysis of PrP after proteinase K treatment using mAb 3F4. Lane 1: PrPSc type 1 from a sporadic CJD case; lane 2: PrPSc type 2 from a sporadic CJD case; lane 3: frontal cortex of case presented here; lane 4: cerebellar cortex of case presented here. As seen in lanes 3 and 4, there is an additional band migrating at 17 kDa (PrPSc protease-resistant PrP isoform)
Discussion
The R208H mutation of PRNP has been reported in two previous cases of a 60-year-old man and 58-year-old woman, both of whom were methionine homozygotes at codon 129 of PRNP. Both presented first with anorexia, weight loss and cognitive decline followed by hallucinations, agitation and ataxia. Both had a disease duration of 7 months. The histological examination revealed extensive spongiform degeneration and astrocytic gliosis mainly in the cerebral cortex. Immunostaining for PrPSc showed a synaptic pattern, and no tau pathology was reported. The Western blot analysis revealed PrPSc type 1 in both cases [2, 13]. Thus, the two previously reported cases were very similar clinically and histopathologically to sCJD in codon 129 methionine homozygous patients with PrPSc type 1 (sCJD-MM1) [18]. A negative family history was reported for the 60-year-old male [13]. An asymptomatic relative with the mutation at codon 208 was revealed by genetic examination of the family. He was under 50 years of age. The family history of the 58-year-old female was negative [2].
The case reported here shows a number of similarities and significant differences when compared to the previous two cases. The unilateral signs at onset, such as clumsiness and weakness of the left hand and dysarthria, may be related to an infarct or may have been a first unilateral sign of CJD [6, 18]. Because of nearly complete recovery of these initial signs and since cerebral MRI revealed an additional older lacunar lesion in the right genu of the internal capsule, we prefer to interpret the unilateral signs as an ischemic event rather than as signs of CJD. The clinical course with presentation of cognitive decline and gait ataxia shows features typical of sCJD-MM1. Anorexia and weight loss as reported in the preceding cases were absent in our patient. The age at onset fits in the group of sCJD-MM1 (65.5 years). The disease duration of 1 year was longer than the average for the group of sCJD-MM1, which is about 3.9 months (1–18 months) [18].
Concerning the histological features, the distribution of spongiform changes in our case corresponds to the lesion profile of sCJD-MM1 [6, 18]. Immunohistochemistry showed a synaptic PrPSc pattern with a laminar predilection for the deep cortical layers, which is not typical of sCJD-MM1, and was not described in the two other cases. In addition, we noted tau-positive and argyrophilic inclusions as well as ballooned neurons. The combination and distribution pattern of NFT, argyrophilic threads and ballooned neurons in our case were not characteristic for a particular neurodegenerative disease with tau pathology [1]. We found small AT8-positive granules mainly in the CA1 region and alveus, as well as argyrophilic inclusions in the alveus. In addition, a small number of αB-crystallin-positive ballooned neurons were found in the amygdala. However, since there were no argyrophilic grains in the amygdala, a diagnosis of AGD was rejected [26]. Ballooned neurons found in other brain regions of our case may be related to CJD, as they have been described as a neuropathological aspect of sCJD-VV1, but notably not in sCJD-MM1 [10, 21]. It is difficult to state with certainty whether any of the clinical signs are directly related to tau pathology rather than CJD in this case.
There are reports dealing with the “coincidence” of sCJD and tau pathology, which is mostly interpreted as an incidental phenomenon in aged patients [8, 11]. On the other hand, the combination of tau pathology (NFT, neuropil threads) and GSS is a major feature of the neuropathological phenotype in patients with mutations F198S [4, 7, 9] and Q217R [9] as well as some with the A117V [27] and P105L mutations of PRNP [12]. Biochemical analysis of PK- and peptide-N-glycosidase F (PNGase F)-treated brain homogenates of GSS patients with the F198S mutation has shown a unique PrP profile of three prominent unglycosylated PrP fragments of 27–29, 18–19 and 8 kDa [19], which is different from the PrP pattern in Western blot analysis of GSS patients with the P102L mutation, by far the most common mutation linked to GSS, which shows two unglycosylated bands of 21 and 8 kDa after PK and PNGase F treatment [17].
The Western blot analysis of PK-treated brain samples of our case revealed a typical PrPSc type 1 pattern. In addition, a shorter band of 17 kDa was detected. Deglycosylation experiments with our samples revealed that the 17-kDa fragment is unglycosylated. By measuring the glycosylation ratio, we found a pattern that differs from the ratios described by Parchi et al. [15] and Furukawa et al. [5] in sCJD and fCJD with the V210I and the E200K mutations. The monoglycosylated band is the strongest band in sCJD and fCJD with the V210I and E200K mutations. In addition, there were slight differences in the glycosylation patterns in the two mutations (V210I vs E200K) [3, 16]. In our case the unglycosylated band of 21 kDa was the strongest followed by the mono- and diglycosylated bands. The finding of an additional 17-kDa PrP fragment is remarkable, because it was absent in two other cases with the same R208H mutation but no tau pathology. It remains to be shown whether the additional PrP band is related to tau protein pathology.
References
Braak H, Braak E (1995) Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging 16:271–278
Capellari S, Ladogana A, Volpi G, Ronocaro F, Sita D, Baruzzi A, Pocchiari M, Parchi P (2001) First report of the R208H-129MM haplotype in the prion protein gene in a European subject with CJD. Neurol Sci 22:109
Cardone F, Liu QG, Petraroli R, Ladogana A, D’Alessandro M, Arpino C, Di Bari M, Macchi G, Pocchiari M (1999) Prion protein glycotype analysis in familial and sporadic Creutzfeldt-Jakob disease patients. Brain Res Bull 49:429–433
Farlow MR, Yee RD, Dlouhy SR, Conneally PM, Azzarelli B, Ghetti B (1989) Gerstmann-Straussler-Scheinker disease. I. Extending the clinical spectrum. Neurology 39:1446–1452
Furukawa H, Doh-ura K, Kikuchi H, Tateishi J, Iwaki T (1998) A comparative study of abnormal prion protein isoforms between Gerstmann-Straussler-Scheinker syndrome and Creutzfeldt-Jakob disease. J Neurol Sci 158:71–75
Gambetti P, Kong Q, Zou W, Parchi P, Chen SG (2003) Sporadic and familial CJD: classification and characterisation. Br Med Bull 66:213–239
Ghetti B, Tagliavini F, Masters CL, Beyreuther K, Giaccone G, Verga L, Farlow MR, Conneally PM, Dlouhy SR, Azzarelli B (1989) Gerstmann-Straussler-Scheinker disease. II. Neurofibrillary tangles and plaques with PrP-amyloid coexist in an affected family. Neurology 39:1453–1461
Hainfellner JA, Wanschitz J, Jellinger K, Liberski PP, Gullotta F, Budka H (1998) Coexistence of Alzheimer-type neuropathology in Creutzfeldt-Jakob disease. Acta Neuropathol 96:116–122
Hsiao K, Dlouhy SR, Farlow MR, Cass C, Da Costa M, Conneally PM, Hodes ME, Ghetti B, Prusiner SB (1992) Mutant prion proteins in Gerstmann-Straussler-Scheinker disease with neurofibrillary tangles. Nat Genet 1:68–71
Kato S, Hirano A, Umahara T, Llena JF, Herz F, Ohama E (1992) Ultrastructural and immunohistochemical studies on ballooned cortical neurons in Creutzfeldt-Jakob disease: expression of alpha B-crystallin, ubiquitin and stress-response protein 27. Acta Neuropathol 84:443–448
Kawashima T, Doh-ura K, Iwaki T (1999) Argyrophilic grains in late-onset Creutzfeldt-Jakob diseased brain. Pathol Int 49:369–373
Kitamoto T, Amano N, Terao Y, Nakazato Y, Isshiki T, Mizutani T, Tateishi J (1993) A new inherited prion disease (PrP-P105L mutation) showing spastic paraparesis. Ann Neurol 34: 808–813
Mastrianni JA, Iannicola C, Myers RM, DeArmond S, Prusiner SB (1996) Mutation of the prion protein gene at codon 208 in familial Creutzfeldt-Jakob disease. Neurology 47:1305–1312
Notari S, Capellari S, Giese A, Westner I, Baruzzi A, Ghetti B, Gambetti P, Kretzschmar HA, Parchi P (2004) Effects of different experimental conditions on the PrPSc core generated by protease digestion: implications for strain typing and molecular classification of CJD. J Biol Chem 279:16797–16804
Parchi P, Castellani R, Capellari S, Ghetti B, Young K, Chen SG, Farlow M, Dickson DW, Sima AA, Trojanowski JQ, Petersen RB, Gambetti P (1996) Molecular basis of phenotypic variability in sporadic Creutzfeldt-Jakob disease. Ann Neurol 39:767–778
Parchi P, Capellari S, Chen SG, Petersen RB, Gambetti P, Kopp N, Brown P, Kitamoto T, Tateishi J, Giese A, Kretzschmar H (1997) Typing prion isoforms. Nature 386:232–234
Parchi P, Chen SG, Brown P, Zou W, Capellari S, Budka H, Hainfellner J, Reyes PF, Golden GT, Hauw JJ, Gajdusek DC, Gambetti P (1998) Different patterns of truncated prion protein fragments correlate with distinct phenotypes in P102L Gerstmann-Straussler-Scheinker disease. Proc Natl Acad Sci USA 95:8322–8327
Parchi P, Giese A, Capellari S, Brown P, Schulz-Schaeffer W, Windl O, Zerr I, Budka H, Kopp N, Piccardo P, Poser S, Rojiani A, Streichemberger N, Julien J, Vital C, Ghetti B, Gambetti P, Kretzschmar H (1999) Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann Neurol 46:224–233
Piccardo P, Seiler C, Dlouhy SR, Young K, Farlow MR, Prelli F, Frangione B, Bugiani O, Tagliavini F, Ghetti B (1996) Proteinase-K-resistant prion protein isoforms in Gerstmann-Straussler-Scheinker disease (Indiana kindred). J Neuropathol Exp Neurol 55:1157–1163
Piccardo P, Liepnieks JJ, William A, Dlouhy SR, Farlow MR, Young K, Nochlin D, Bird TD, Nixon RR, Ball MJ, DeCarli C, Bugiani O, Tagliavini F, Benson MD, Ghetti B (2001) Prion proteins with different conformations accumulate in Gerstmann-Straussler-Scheinker disease caused by A117V and F198S mutations. Am J Pathol 158:2201–2207
Pietrini V, Danieli D, Bevilacqua P, Lechi A (1993) Panencephalopathic type of Creutzfeldt-Jakob disease with neuropathologic features similar to Pick’s disease. Clin Neuropathol 12:1–6
Satoh K, Muramoto T, Tanaka T, Kitamoto N, Ironside JW, Nagashima K, Yamada M, Sato T, Mohri S, Kitamoto T (2003) Association of an 11–12 kDa protease-resistant prion protein fragment with subtypes of dura graft-associated Creutzfeldt-Jakob disease and other prion diseases. J Gen Virol 84:2885–2893
Tagliavini F, Prelli F, Ghiso J, Bugiani O, Serban D, Prusiner SB, Farlow MR, Ghetti B, Frangione B (1991) Amyloid protein of Gerstmann-Straussler-Scheinker disease (Indiana kindred) is an 11 kd fragment of prion protein with an N-terminal glycine at codon 58. EMBO J 10:513–519
Tagliavini F, Prelli F, Porro M, Rossi G, Giaccone G, Farlow MR, Dlouhy SR, Ghetti B, Bugiani O, Frangione B (1994) Amyloid fibrils in Gerstmann-Straussler-Scheinker disease (Indiana and Swedish kindreds) express only PrP peptides encoded by the mutant allele. Cell 79:695–703
Tagliavini F, Lievens PM, Tranchant C, Warter JM, Mohr M, Giaccone G, Perini F, Rossi G, Salmona M, Piccardo P, Ghetti B, Beavis RC, Bugiani O, Frangione B, Prelli F (2001) A 7-kDa prion protein (PrP) fragment, an integral component of the PrP region required for infectivity, is the major amyloid protein in Gerstmann-Straussler-Scheinker disease A117V. J Biol Chem 276:6009–6015
Tolnay M, Monsch AU, Staehelin HB, Probst A (1999) [Argyrophilic grain disease: differentiation from Alzheimer disease]. Pathologe 20:159–168
Tranchant C, Sergeant N, Wattez A, Mohr M, Warter JM, Delacourte A (1997) Neurofibrillary tangles in Gerstmann-Straussler-Scheinker syndrome with the A117V prion gene mutation. J Neurol Neurosurg Psychiatry 63:240–246
WHO (1998) Human transmissible spongiform encephalopathies. Weekly Epidemiol Rec 47:361–365
Windl O, Giese A, Schulz-Schaeffer W, Zerr I, Skworc K, Arendt S, Oberdieck C, Bodemer M, Poser S, Kretzschmar HA (1999) Molecular genetics of human prion diseases in Germany. Hum Genet 105:244–252
Zou WQ, Capellari S, Parchi P, Sy MS, Gambetti P, Chen SG (2003) Identification of novel proteinase K-resistant C-terminal fragments of PrP in Creutzfeldt-Jakob disease. J Biol Chem 278:40429–40436
Acknowledgements
This study was supported by a grant from the Federal Ministry of Health (Bundesgesundheitsministerium) and the European Commission (TSELAB QLK 2- CT-2002–81523). We thank Dr. M. Groschup for providing the antibody L42 and E. Staniszewski, A. Henn and G. Kwiatkowski for expert technical assistance in genetic analysis, histological processing and Western blot analysis. We thank Dr. Hillig (general practitioner) and Dr. Schaefer (Institute for Human Genetics Frankfurt a.M.) for providing information on the family history.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roeber, S., Krebs, B., Neumann, M. et al. Creutzfeldt-Jakob disease in a patient with an R208H mutation of the prion protein gene (PRNP) and a 17-kDa prion protein fragment. Acta Neuropathol 109, 443–448 (2005). https://doi.org/10.1007/s00401-004-0978-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-004-0978-0