Abstract
Aortic insufficiency (AI) is a serious complication for patients on long-term support with left ventricular assist devices (LVAD). Postoperative aortic valve opening is an important predictor of AI. A system is presently available that can promote native aortic flow by reducing rotational speed (RS) for defined intervals. However, this system can cause a reduction in pump flow and lead to insufficient support. We therefore developed a novel “delayed copulse mode” to prevent AI by providing both minimal support for early systole and maximal support shortly after aortic valve opening by changing the RS in synchronization with heartbeat. To evaluate whether our drive mode could open the aortic valve while maintaining a high total flow (sum of pump flow and native aortic flow), we installed a centrifugal LVAD (EVAHEART®; Sun Medical) in seven goats each with normal hearts and acute LV dysfunction created by micro-embolization of the coronary artery. We intermittently switched the drive mode from continuous (constant RS) with 100 % bypass to delayed copulse mode with 90 % bypass. Total flow did not significantly change between the two modes. The aortic valve opened when the delayed copulse mode was activated. The delayed copulse mode allowed the aortic valve to open while maintaining a high total flow. This novel drive mode may considerably benefit patients with severe heart failure on long-term LVAD support by preventing AI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Left ventricular assist devices (LVAD) have become widely applied as a therapeutic option for patients with end-stage heart failure, and long-term LVAD support has become more important not only as a bridge to transplantation, but also as a destination therapy [1]. However, native aortic insufficiency (AI) can develop during long-term LVAD support [2–4]. Severe AI can lead to reduced forward cardiac output and increased LV preload due to recycling regurgitant blood flow from the LVAD outflow graft into the left ventricular inflow cannula, which in turn decreases the effectiveness of LVAD support and results in end-organ malperfusion. Survival rates are significantly worse for patients with, than without, AI [5]. Closure of the aortic valve after LVAD implantation is a significant predictor of AI [3–6]. Persistent closure of the aortic valve after LVAD implantation might promote reduced valve pliability and commissural fusion, consequently resulting in the occurrence or progression of AI. The aortic valve must be able to open on demand during LVAD support to prevent AI. The intermittent low-speed (ILS) mode promotes native aortic flow (AoF) by reducing rotational speed (RS) for a defined interval. However, this system is inappropriate for patients with severe heart failure because it can result in support flow that is insufficient to meet the requirements of patients. A methodology to resolve this issue is not available. Therefore, we developed a “delayed copulse mode” that can prevent AI by synchronization with the cardiac cycle in continuous-flow LVAD. The aim of this mode is to provide both minimal support during early systole to open the aortic valve and maximal support shortly after the valve has opened to maintain high pump flow (PF). Here, we compared the effects of this mode on aortic valve opening and PF with the ILS mode in animal models of normal and acute ischemic heart failure.
Materials and methods
Experimental preparation
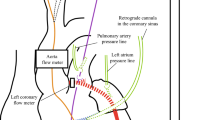
We studied seven goats (52.6 ± 4.1 kg) with normal hearts and seven (52.3 ± 4.9 kg) with acute LV dysfunction created by coronary microsphere embolization of the left anterior descending coronary artery (LAD). All animals were sedated with an intramuscular injection of ketamine (10 mg/kg). General anesthesia was induced and maintained by isoflurane inhalation (1–3 vol/100 mL in oxygen). The animals were fixed in the right lateral recumbent position, intubated and mechanically ventilated. The fifth costal bone was resected via a left thoracotomy and the heart was approached through the left thoracic space. A centrifugal LVAD (EVAHEART; Sun Medical Technology Research Corporation, Nagano, Japan) was installed [7, 8] after heparinization (300 U/kg) by inserting the inflow cannula into the left ventricular apex and suturing the outflow graft to the descending aorta. Blood flow in the ascending aorta and LVAD was measured using electromagnetic (EMF-1000: diameter, 16–18 mm; Nihon Kohden, Tokyo, Japan) and ultrasonic (TS420: 16 mm; Transonic Systems) flow meters, respectively. Pressure lines for monitoring aortic (AoP) and central venous pressure (CVP) were established from the left internal thoracic artery and the left internal thoracic veins, and a pressure line for left ventricular pressure (LVP) monitoring was inserted into the left ventricle from the anterior wall. Pacing leads for ventricular electrocardiography were sutured onto the anterior wall of the right ventricle. The vital data described above were recorded using Labchart 5 software (ADInstruments, Bella Vista NSW, Australia). We calculated the instantaneous left ventricular-aortic pressure gradient (LV-Ao PG) by subtracting AoP from LVP. We evaluated aortic valve opening by echocardiography, and measured the aortic valve area (AVA) by echocardiographic planimetry.
The animals used in this study were maintained in accordance with the guidelines of the Committee on Animal Studies at the National Cerebral and Cardiovascular Center. This study was approved by the National Cerebral and Cardiovascular Center Animal Investigation Committee. Institutional guidelines for the care and use of laboratory animals were observed.
Making left ventricular dysfunction models
We created animal models of acute ischemic heart failure by micro-embolizing the LAD as described [9–11]. A multipurpose, 4 Fr Judkins catheter (Create Medic Co. Ltd., Yokohama, Japan) was introduced through a long sheath (4 Fr × 17 cm) into the left carotid artery towards the LAD under fluoroscopic guidance. We then injected 3.14 ± 0.29 × 104 microspheres (diameter, 75 μm; 600/kg) into the LAD. We planned to reduce and then maintain cardiac output at about 60 % of the native heart function determined before creating the model of acute ischemic heart failure. After 30 min of observation, we collected data to assure stable optimal cardiac function. We stabilized the AoP and CVP throughout the experiment to ensure that heart afterload or preload remained constant, and that heart rate also remained constant by adjusting infusion volumes and changing the depth of anesthesia. Neither vasodilators nor catecholamines were used. Ventricular arrhythmias were prevented during the experiment using 2 % lidocaine (1 mg/kg/h).
Study protocol and drive mode
We previously described a novel pump controller in which the RS changes in synchrony with the cardiac cycle [12–18]. The controller can detect R waves from the ventricular ECG and momentarily change the RS to target speed. The RS was controlled using the delayed copulse mode in each of the early systolic, ejection period and diastolic phases of the cardiac cycle (Fig. 1). During early systole, LVP must exceed AoP to allow the aortic valve to open, and some left ventricular end diastolic volume (LVEDV) is needed to increase LVP. Therefore, we adjusted the RS of diastole to avoid inducing reverse flow. We set the RS of early systole at 700 rpm (the minimum RS for this LVAD) to minimize early systole support. Thereafter, we momentarily increased the RS of the ejection period and adjusted the RS to achieve the appropriate bypass rate to maintain high support flow. We defined early systole, ejection period and diastole as 11, 22 and 67 % of the RR interval, respectively and compared the following drive modes.
Command rotational speed in delayed copulse mode. ECG electrocardiogram, RS rotational speed. Pumps were driven at early systolic RS of 700 rpm from R-wave input for 11 % of RR interval. Ejection period RS increased to target speed and was maintained for 22 % of RR interval. Diastolic RS instantly reduced to achieve diastolic PF of ~0 L/min, maintained RS for remaining 67 % of RR interval, and finally returned to initial early systolic RS of 700 rpm
The first was the intermittent delayed copulse (IDCO) mode that was intermittently activated for every 10 of 80 cardiac beats with a triggered R wave. This means that for 70 beats we ran continuous mode (constant RS) and then switched to delayed copulse mode for the next 10 beats. The bypass rate (BR) was set at around 100 and 90 % in the continuous and delayed copulse modes, respectively. The second was the ILS mode using a Jarvik 2000 (Jarvik Heart Inc., New York, NY, USA) [19] as follows. We decreased the RS from 100 % bypass to low speed (1,200 rpm) for 10 of 80 heartbeats. We calculated BR by dividing PF by total flow (TF: sum of PF and AoF) and then assessed the effects of the delayed copulse and low-speed modes upon aortic valve opening, PF, AoF, and hemodynamic parameters. All data were compared with those generated using a circuit clamp (no pump support) as a control condition (Table 2; Figs. 3, 5).
Statistics
All numerical data are shown as averages ± standard deviation (SD). Groups were compared using a repeated-measures analysis of variance followed by Tukey’s multiple comparison test. All analyses were two-sided, and a p value <0.05 was considered statistically significant. All data were analyzed using PASW Statistics ver. 20 (IBM SPSS).
Results
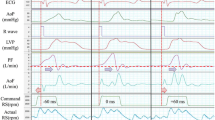
Table 1 shows the average RS in the continuous and delayed copulse modes. The RS required to achieve 100 % bypass in continuous mode was around 1,800 and 1,650 rpm in the animal models with a normal heart and in those with acute ischemic heart failure, respectively. The RS of the ejection period needed to achieve 90 % bypass in delayed copulse mode was ~2,100 and 2,000 rpm in the animals with a normal heart and in the models, respectively. The RS of diastolic phase required to avoid reverse flow in delayed copulse mode was 1,700 and 1,450 rpm in the animal models with a normal heart and with acute ischemic heart failure, respectively. Figure 2 shows typical waveforms of pressure and flow data when continuous mode with 100 % bypass was switched to either the delayed copulse or the low-speed mode in the models of acute ischemic heart failure. The AoF increased and the PF slightly decreased in delayed copulse mode. The AoF increased and the PF significantly decreased along with the RS reduction in the low-speed mode. Pulse pressure and the instantaneous maximum LV-Ao PG were increased in both the delayed copulse and low-speed modes when activated.
Sample waveforms of pressure and flow data. AoF ascending aortic flow, AoP aortic pressure, ECG electrocardiogram, IDCO intermittent delayed copulse, ILS intermittent low speed, LV-Ao left ventricular-aortic pressure gradient, LVP left ventricular pressure, PF pump flow, RS rotational speed. Waveforms typical at the portion switched from continuous mode with 100 % bypass to either delayed copulse or low-speed (1,200 rpm) mode in animal models of acute ischemic heart failure. AoF increased and PF slightly decreased in delayed copulse mode. AoF increased and PF significantly decreased along with reduction of RS in low-speed mode. Pulse pressure and maximal LV-Ao PG were instantaneously increased in both delayed copulse and low-speed modes when activated
Table 2 shows numerical hemodynamic data. Changes in heart rate and mean AoP did not significantly differ between continuous and either delayed copulse or low-speed modes. Pulse pressure significantly increased in both delayed copulse and low-speed modes, compared with the continuous mode (p < 0.05). Left ventricular end systolic pressure (LVESP) and mean LVP tended to be higher in both delayed copulse and low-speed modes, than in the continuous mode. Instantaneous maximum LV-Ao PG was obviously increased in both delayed copulse and low-speed modes (p < 0.05). Left ventricular end diastolic pressure (LVEDP) significantly differed between the delayed copulse and low-speed modes in the models of acute heart failure. Delayed copulse mode activation improved pulsatility and LVEDP did not significantly change.
Figure 3a, b show the 80-beat average of TF in circuit-clamp, continuous, IDCO, and ILS modes. The TF in IDCO mode tended to be larger than that in the ILS mode, but the difference did not reach significance. Figures 3c, d show a 10-beat average of TF when the delayed copulse and the low-speed modes are activated, and in circuit-clamp, continuous mode. When the drive mode was switched from continuous to low-speed mode, PF significantly decreased, but the native heart could not keep up with it, and thus TF decreased (p < 0.01). The decreased ratio of TF was higher in the models (37.8 ± 8.8 %) than in animals with a normal heart (31.4 ± 10.3 %). On the other hand, TF in delayed copulse mode did not significantly change in animals with a normal heart and in the models of acute ischemic heart disease.
a, b 80-beat average of total flow (TF) data in circuit-clamp (control condition), continuous, intermittent delayed copulse (IDCO) and intermittent low-speed (ILS) modes. TF, sum of pump (blue) and native aortic (red) flow. Continuous, IDCO and ILS modes did not significantly differ in normal animals (a) and in models of acute heart failure (b). Total flow in IDCO mode tended to be larger than in the ILS mode, but the two modes did not significantly differ between these two modes in both groups of animals. Figure 3c, d 10-beat average of total flow (TF) data in circuit-clamp (control condition), continuous, delayed copulse and low-speed (1,200 rpm) modes. TF sum of pump (blue) and native aortic (red) flow. During mode switch, TF did not significantly change between continuous and the delayed copulse modes in normal animals (c) and in models of acute heart failure (d). After mode switch, TF significantly decreased between continuous and low-speed modes in both groups of animals. Delayed copulse and low-speed modes also significantly differed in both groups of animals
Figure 4 shows echocardiography in the short axis view of the aortic valve in models of acute ischemic heart failure. The aortic valve did not open in the continuous mode with 100 % bypass. Valve opening was better in the delayed copulse, than in the low-speed mode.
Echocardiography in short axis view of aortic valve in animal models of acute ischemic heart failure (a, b and c, continuous, delayed and low-speed (1,200 rpm) modes, respectively), Dotted line maximal aortic valve area in each mode. Aortic valve did not open in continuous mode (a), but opened more efficiently in delayed copulse, than in low-speed mode (b, c)
Figure 5 shows changes in AVA after the mode switch. The AVA significantly increased after switching the drive mode in both the delayed copulse and low-speed modes in the models (p < 0.01). In animal models of acute heart failure, the AVA in the delayed copulse mode was larger than in the low-speed mode (p < 0.01). These results indicated that the aortic valve opened in delayed copulse mode while high TF was maintained. However, TF decreased in the low-speed mode after the aortic valve opened.
Aortic valve area (AVA) in circuit-clamp (control condition), continuous, delayed copulse and low-speed (1,200 rpm) modes. Asterisk significant difference (p < 0.01). After switching, AVA significantly increased in both delayed copulse and low-speed modes (a, b) and these two modes significantly differed (b) in animal models of acute heart failure (p < 0.01)
Discussion
The REMATCH trial demonstrated that long-term mechanical cardiac support is clinically effective as destination therapy [20], and thus LVADs have become increasingly popular as long-term therapy for patients with end-stage heart failure. However, prolonged LVAD support remarkably alters cardiac and vascular physiology and function. The development of AI during long-term LVAD is regarded as the most important complication, because survival is significantly worse for patients with, than without, AI due to insufficient LVAD support and end-organ mal-perfusion [5].
Less frequent aortic valve opening significantly correlates with more severe AI [3–6], and Hatano et al. [4] reported that the incidence of AI is higher in patients with a continuous-flow than with a pulsatile-flow LVAD. Because almost all pulsatile devices function in asynchrony with the native cardiac cycle, the loading condition of the LV changes with each heartbeat. Therefore, the aortic valve is more likely to open in patients with pulsatile- than with continuous-flow LVADs. Among preoperative clinical parameters, a lower LV ejection fraction is an independent risk factor for the development of AI [4, 5]. Poor LV contractile function might contribute to inefficient aortic valve opening. Toda et al. [5] reported that preoperative functional mitral regurgitation (MR) is related to AI progression. This is because severe MR could lead to the aortic valve opening less frequently due to regurgitant blood flowing into the left atrium during LVAD support.
The loss of pulsatility caused by continuous-flow LVADs seems to closely correlate with AI development. A healthy aortic valve has a significantly redundant coaptation surface. The coaptation area of the normal aortic valve is directly related to the diameter of the aortic root, which is sensitive to root pressure [21]. We speculated that the same hemodynamic alterations induced by continuous-flow LVAD, loss of pulsatility and persistent elevation of aortic root pressure, cause aortic root dilation. Pressure in the ascending aorta is persistently higher than LVP in such patients and native aortic valves consequently do not open.
Mudd et al. [6] found evidence that a commissural fusion in eight of nine patients with continuous-flow LVAD correlated with decreased valve opening and an increasing prevalence of AI. Letsou et al. [22] identified some degree of commissural fusion in 51.5 % of patients on LVAD support, and a histopathological examination of areas of fusion revealed loose fibrous tissue between commissures. Pak et al. [3] identified significantly larger aortic root circumferences in patients with, than without AI.
From the above, we speculate that the main causes of AI are persistent aortic valve closure and elevated aortic root pressure. These can lead to commissural fusion, reduced valve pliability, and aortic root dilation, and consequently result in the occurrence or development of AI during long-term LVAD support. To open the aortic valve and improve pulsatility, pump flow should be reduced to promote the native AoF. However, this can reduce support flow and result in insufficient support to meet physiological requirements. Our delayed copulse mode can resolve these issues.
Our results showed that the delayed copulse mode could open the aortic valve and improve pulsatility while maintaining high bypass flow. The delayed copulse mode is characterized by minimal support of the early systolic phase and maximal support soon after the aortic valve has opened. Therefore, this unique drive mode can open the aortic valve while maintaining high bypass flow. The delayed copulse mode could open the aortic valve without reducing the TF not only in the normal heart but also in the animal models of acute heart failure. These findings indicate that the delayed copulse mode will be effective for patients with low cardiac output. Furthermore, because LVEDP did not significantly change between continuous mode and delayed copulse modes, the latter might be able to maintain adequate LV unloading.
We demonstrated that the 80-beat average of TF in the ILS mode tended to be smaller than that in the continuous mode, and that the two modes did not significantly differ (Fig. 3a, b). However, TF significantly decreased when the low-speed mode was activated (Fig. 3c, d). This tendency was evident in animal models of acute heart failure with LV dysfunction compared with animals with a normal heart. We speculate that preprogrammed intermittent reduction of pump speed can maintain TF for long periods, but this also has a risk of not providing sufficient support to meet physiological requirements at slow pump speeds. From this perspective, we believe that the delayed copulse mode carried an extremely small risk of insufficient support that could result in transient ischemic attacks.
The EVAHEART is an implantable centrifugal blood pump with a flat pressure-flow curve that can provide a significantly high PF rate. This feature can provide higher flow during systole and lower flow during diastole, thus providing pulsatile high-flow, which can solve the current clinical problems with the continuous-flow LVAD [7, 8]. With respect to AI, higher flow during systole might interfere with aortic valve opening. In contrast, high pulsatility might prevent AI development because of a lower pressure effect during diastole. Therefore, we speculate that the EVAHEART confers both an advantage and a disadvantage against AI. The concept of the delayed copulse mode is to overcome the disadvantage by opening the aortic valve and to enhance the advantage by improving its pulsatility.
The delayed copulse mode has some possible negative aspects. First, it might elevate hemolysis levels due to high shear stress caused by a momentary increase in the RS. The effects on blood should be evaluated using hemolysis tests in vitro and by long-term studies of animal models of chronic heart failure. The long-term effects on device durability should be similarly evaluated. The delayed copulse mode might increase regurgitant blood flow during early diastole in patients with extant mild AI, because the pressure effect caused by an increase of the RS during the ejection period can be slightly delayed. However, even if patients have mild AI before LVAD implantation, the likelihood that LV preload increases in the delayed copulse mode is hardly conceivable. This is because we adjusted the RS of diastole to avoid inducing reverse flow in delayed copulse mode, and thus regurgitant blood flow can be reduced during the mid- and late-diastole as compared with continuous mode.
The present study has several limitations. Our results are based on animals with a normal heart and an animal model of acute ischemic heart failure. Thus, to determine whether or not the same results would apply to chronic heart failure in the clinical setting is difficult. We have not yet examined the effectiveness of the long-term application of the delayed copulse mode, or its effects on pathological changes of the aortic valve. We have started to evaluate the effects of delayed copulse mode on the native heart in animal models of chronic heart failure. We aim to assess the effects of the delayed copulse mode on hemodynamics, pathological changes in the aortic valve and device durability in a larger study of animal models of chronic heart failure. We defined early systole, the ejection period, and diastole as 11, 22, and 67 % of the RR interval, respectively. However, these parameters might widely differ among individuals or according to the status of the native heart. If we prolong early systole, the aortic valve will be easier to open, but maintaining high support flow will become more difficult. Therefore, the appropriate interval of each phase needs to be determined according to the status of the native heart in the clinical setting. In addition, a method of actual adjustment of the RS of the ejection period and diastolic phase in the clinical setting needs to be established. Monitoring PF during pump support is difficult with the current system and BR needs to be estimated in the clinical setting by echocardiography. The delayed copulse mode was intermittently activated for every 10 out of 80 cardiac beats with a triggered R wave. However, it is not possible to determine whether this intermittent interval is optimal for preventing AI during long-term LVAD support from experiments on animals with acute conditions. Delayed copulse mode should perhaps be activated throughout sleep. However, the optimal method for preventing AI remains unclear.
Conclusions
Our delayed copulse mode allowed aortic valve opening while maintaining high pump flow in goats with normal and acute ischemic hearts. This novel drive mode might confer considerable benefits upon patients with chronic heart failure on long-term LVAD support by preventing AI. Further investigation is currently underway in models of chronic heart failure.
References
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51.
Cowger J, Pagani FD, Haft JW, Romano MA, Aaronson KD, Kolias TJ. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3:668–74.
Pak SW, Uriel N, Takayama H, Cappleman S, Song R, Colombo PC, Charles S, Mancini D, Gillam L, Naka Y, Jorde UP. Prevalence of de novo aortic insufficiency during long-term support with left ventricular assist devices. J Heart Lung Transplant. 2010;29:1172–6.
Hatano M, Kinugawa K, Shiga T, Kato N, Endo M, Hisagi M, Nishimura T, Yao A, Hirata Y, Kyo S, Ono M, Nagai R. Less frequent opening of the aortic valve and a continuous flow pump are risk factors for postoperative onset of aortic insufficiency in patients with a left ventricular assist device. Circ J. 2011;75:1147–55.
Toda K, Fujita T, Domae K, Shimahara Y, Kobayashi J, Nakatani T. Late aortic insufficiency related to poor prognosis during left ventricular assist device support. Ann Thorac Surg. 2011;92:929–34.
Mudd JO, Cuda JD, Halushka M, Soderlund KA, Conte JV, Russell SD. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J Heart Lung Transplant. 2008;27:1269–74.
Yamazaki K, Kihara S, Akimoto T, Tagusari O, Kawai A, Umezu M, Tomioka J, Kormos RL, Griffith BP, Kurosawa H. EVAHEART: an implantable centrifugal blood pump for long-term circulatory support. Jpn J Thorac Cardiovasc Surg. 2002;50:461–5.
Yamazaki K, Saito S, Kihara S, Tagusari O, Kurosawa H. Completely pulsatile high flow circulatory support with a constant-speed centrifugal blood pump: mechanisms and early clinical observations. Gen Thorac Cardiovasc Surg. 2007;55:158–62.
Klocke R, Tian W, Kuhlmann MT, Nikol S. Surgical animal model of heart failure related to coronary heart disease. Cardiovasc Res. 2007;74:29–38.
Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–71.
Gill RM, Jones BD, Corbly AK, Wang J, Braz JC, Sandusky GE, Shen W. Cardiac diastolic dysfunction in conscious dogs with heart failure induced by chronic coronary microembolization. Am J Physiol Heart Circ Physiol. 2006;291:3154–8.
Ando M, Takewa Y, Nishimura T, Yamazaki K, Kyo S, Ono M, Tsukiya T, Mizuno T, Taenaka Y, Tatsumi E. A novel counterpulsation mode of rotary left ventricular assist devices can enhance myocardial perfusion. J Artif Organs. 2011;14:185–91.
Ando M, Takewa Y, Nishimura T, Yamazaki K, Kyo S, Ono M, Tsukiya T, Mizuno T, Taenaka Y, Tatsumi E. Coronary vascular resistance increases under full bypass support of centrifugal pumps-relation between myocardial perfusion and ventricular workload during pump support. Artif Organs. 2012;36:105–10.
Ando M, Nishimura T, Takewa Y, Yamazaki K, Kyo S, Ono M, Tsukiya T, Mizuno T, Taenaka Y, Tatsumi E. Electrocardiogram-synchronized rotational speed change mode in rotary pumps could improve pulsatility. Artif Organs. 2011;35:941–7.
Ando M, Nishimura T, Takewa Y, Kyo S, Ono M, Taenaka Y, Tatsumi E. Creating an ideal “off-test mode” for rotary left ventricular assist devices: establishing a safe and appropriate weaning protocol after myocardial recovery. J Thorac Cardiovasc Surg. 2012;143:1176–82.
Ando M, Nishimura T, Takewa Y, Ogawa D, Yamazaki K, Kashiwa K, Kyo S, Ono M, Taenaka Y, Tatsumi E. A novel counterpulse drive mode of continuous-flow left ventricular assist devices can minimize intracircuit backward flow during pump weaning. J Artif Organs. 2011;14:74–9.
Ando M, Nishimura T, Takewa Y, Ogawa D, Yamazaki K, Kashiwa K, Kyo S, Ono M, Taenaka Y, Tatsumi E. What is the ideal off-test trial for continuous-flow ventricular-assist-device explantation? Intracircuit back-flow analysis in a mock circulation model. J Artif Organs. 2011;14:70–3.
Umeki A, Nishimura T, Ando M, Takewa Y, Yamazaki K, Kyo S, Ono M, Tsukiya T, Mizuno T, Taenaka Y, Tatsumi E. Alteration of LV end-diastolic volume by controlling the power of the continuous-flow LVAD, so it is synchronized with cardiac beat: development of a native heart load control system (NHLCS). J Artif Organs. 2012;15:128–33.
Tuzun E, Gregoric ID, Conger JL, Golden K, Jarvik R, Frazier OH, Kadipasaoglu KA. The effect of intermittent low speed mode upon aortic valve opening in calves supported with a Jarvik 2000 axial flow device. ASAIO J. 2005;51:139–43.
Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH era: implications for patient selection. Circulation. 2007;116:497–505.
Swanson M, Clark RE. Dimensions and geometric relationships of the human aortic valve as a function of pressure. Circ Res. 1974;35:871–82.
Letsou GV, Connelly JH, Delgado RM 3rd, Myers TJ, Gregoric ID, Smart FW, Frazier OH. Is native aortic valve commissural fusion in patients with long-term left ventricular assist devices associated with clinically important aortic insufficiency? J Heart Lung Transplant. 2006;25:395–9.
Acknowledgments
The present study was supported by a Grant-in-Aid for Scientific Research A (No. 21249073 and No. 21249076) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and an Intramural Research Fund (22-3-3) for Cardiovascular Diseases of the National Cerebral and Cardiovascular Center.
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kishimoto, Y., Takewa, Y., Arakawa, M. et al. Development of a novel drive mode to prevent aortic insufficiency during continuous-flow LVAD support by synchronizing rotational speed with heartbeat. J Artif Organs 16, 129–137 (2013). https://doi.org/10.1007/s10047-012-0685-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-012-0685-x