Abstract
Asynchronous rotational-speed modulation of a continuous-flow left ventricular assist device (LVAD) can increase pulsatility; however, the feasibility of hemodynamic modification by asynchronous modulation of an LVAD has not been sufficiently verified. We evaluated the acute effect of an asynchronous-modulation mode under LVAD support and the accumulated effect of 6 consecutive hours of driving by the asynchronous-modulation mode on hemodynamics, including both ventricles, in a coronary microembolization-induced acute-myocardial injury sheep model. We evaluated 5-min LVAD-support hemodynamics, including biventricular parameters, by switching modes from constant-speed to asynchronous-modulation in the same animals (“acute-effect evaluation under LVAD support”). To determine the accumulated effect of a certain driving period, we evaluated hemodynamics including biventricular parameters after weaning from 6-hour (6 h) LVAD support by constant-speed or asynchronous-modulation mode (“6h-effect evaluation”). The acute-effect evaluation under LVAD support revealed that, compared to the constant-speed mode, the asynchronous-modulation mode increased vascular pulsatility but did not have significantly different effects on hemodynamics, including both ventricles. The 6 h-effect evaluation revealed that the hemodynamics did not differ significantly between the two groups except for some biventricular parameters which did not indicate negative effects of the asynchronous-modulation mode on both ventricles. The asynchronous-modulation mode could be feasible to increase vascular pulsatility without causing negative effects on hemodynamics including both ventricles. Compared to the constant-speed mode, the asynchronous-modulation mode increased pulsatility during LVAD support without negative effects on hemodynamics including both ventricles in the acute phase. Six hours of LVAD support with the asynchronous-modulation mode exerted no negative effects on hemodynamics, including both ventricles, after weaning from the LVAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The implantation of a left ventricular assist device (LVAD) has been an effective way to treat patients with end-stage heart failure. Continuous-flow LVADs have replaced pulsatile-flow LVADs, due to their superiority in size and improved durability and reliability [1, 2]. However, the continuous-flow LVADs have the drawback of diminished pulsatility, which could be related to several complications including gastrointestinal bleeding [3, 4], aortic insufficiency [5, 6], and peripheral vascular dysfunction [7, 8]. In order to address these complications and to expand the possible applications of continuous-flow LVADs, rotational-speed modulation has been investigated.
Rotational-speed modulation algorithms are generally classified into two categories: synchronous-modulation algorithms (synchronized with the native heartbeats) and asynchronous-modulation algorithms (independent of the native heartbeats). Several research groups reported that synchronous modulation can achieve phasic ventricular unloading [9,10,11], increase vascular pulsatility [9, 12, 13], and prevent aortic insufficiency by opening the aortic valve [14]. Despite these advantages of synchronous modulation, a major challenge for its clinical implementation has been the difficulty in stably sensing and tracking native heartbeats.
In contrast, asynchronous modulation has a technical advantage in that it does not require the triggering of any biological information using a sensor. There are various previous reports describing the positive effects of asynchronous modulation [9, 12, 15,16,17,18,19,20]. In summary, it was demonstrated that asynchronous modulation with a continuous-flow LVAD can improve vascular pulsatility [12, 15], provide the technologic advantage of sensorless control [9, 12], and reduce thromboembolic risk [16, 18]. However, the asynchronous modulation that improves vascular pulsatility has not yet been clinically applied, and its safety on hemodynamics including both ventricles has not been sufficiently verified. Thus, the purpose of this study was to investigate the feasibility of modifying hemodynamics by applying the asynchronous modulation. We investigated the effect of one of the asynchronous-modulation modes under LVAD support in the acute phase and the accumulated effect of 6 consecutive hours of driving followed by weaning from LVAD support on hemodynamics, including both ventricles, in comparison with constant-speed driving in an acute-myocardial injury model induced by coronary microembolization.
Materials and Methods
Animals
Ten adult sheep weighing 56–65 kg (mean ± standard deviation [SD]: 61.9 ± 2.8 kg) were used. All animals received humane care. The study was conducted in accord with the guidelines issued by Institutional Committee on Animal Experiments of National Cerebral and Cardiovascular Center (Osaka, Japan) and was approved by this committee (No. 22064).
Experimental Preparation and Instrumentation
General anesthesia was induced by intramuscular ketamine hydrochloride and maintained with a combination of isoflurane inhalation and intravenous ketamine hydrochloride. After an infusion of 150 U/kg of heparin, each animal was implanted with a hydrodynamically levitated extracorporeal centrifugal pump, the BIOFLOAT NCVC (Nipro, Osaka, Japan), by a left thoracotomy. Detailed information about the BIOFLOAT NCVC, including the pump performance curve, is available in a previous report [21]. The centrifugal pump controller was modified to be able to modulate the motor speed with an arbitrary frequency and amplitude. Blood was withdrawn from the left ventricle (LV) through a 32 Fr cannula (DLP Single Stage Venous Cannulae, Medtronic, Minneapolis, MN, USA) and sent to the proximal brachiocephalic artery through an 18 Fr cannula (DLP Single Stage Venous Cannulae, Medtronic) advanced from the left carotid artery. The pump was placed outside the chest. The lengths of the withdrawing blood circuit and that of the sending blood circuits were 60 cm each. The tubes used in the circuit were made of polyvinyl chloride. Pressure lines were placed in the left internal thoracic artery, pulmonary artery, and left atrium.
Ultrasonic flow probes (Transonic Systems, Ithaca, NY, USA) were attached to the ascending aorta, left main coronary artery, and pump circuit. Conductance catheters (CA-71083-PL, CD Leycom, Hengelo, Netherlands) were advanced from a sheath in the right carotid artery into the LV, and from the pulmonary artery into the right ventricle (RV). A retrograde cannula (Senko Medical Instrument Mfg., Tokyo, Japan) was inserted into the coronary sinus through the left azygos vein. A sheath was inserted in a distal direction in the left carotid artery to maintain the cerebral perfusion. Infusions of 300 mL/h Ringer’s lactate solution were given throughout the experiments, and an infusion of 500 U/h heparin was maintained after the implantation of the LVAD. The experimental settings are illustrated in Fig. 1.
The experimental settings. The flow meters were attached to the ascending aorta, left main coronary artery, and pump circuit. Pressure lines were placed in the internal thoracic artery, superior vena cava, pulmonary artery, and the left atrium. Conductance catheters were placed in both left and right ventricles. A retrograde cannula was placed in the coronary sinus. A left ventricular assist device (BIOFLOAT NCVC) was implanted by withdrawing blood from the left ventricle and sending to the proximal brachiocephalic arteries
Creation of an Acute-Myocardial Injury Model
After establishing the experimental settings, we created the acute-myocardial injury model as performed in the previous study [22]. With the LVAD turned off, polystyrene microspheres (diameter, 90 μm; Polysciences, Warrington, PA, USA) were injected into the left anterior descending artery. The number of microspheres was defined as 600 × coronary blood flow (CBF) (mL/min) at a baseline.
Experimental Protocol
The experimental protocol is depicted in Fig. 2.
-
1.
After the experimental settings including the LVAD implantation were established, the baseline measurements were performed with the LVAD turned off for the evaluation of the initial cardiac function (Baseline).
-
2.
Acute-myocardial injury was induced by the injection of the above-described intracoronary microspheres, followed by 30-minute (min) observation. The measurements were then performed with the LVAD turned off to evaluate the initial status of the animal’s acute-myocardial injury (Acute-myocardial injury).
-
3.
The LVAD support was started with the constant-speed mode, followed by 2 h of observation to mitigate the acute hemodynamic changes due to the surgical procedures and microembolization.
-
4.
Acute-effect evaluation under LVAD support: We examined the acute hemodynamic changes caused by the different modes (constant-speed mode and asynchronous-modulation mode) by measuring hemodynamics including biventricular parameters under LVAD support by switching the modes from constant-speed mode to asynchronous modulation mode at a 5-min interval in the same animals.
-
5.
6-hour effect (6 h-effect) evaluation: After the examination of the acute effects, we assessed the effects of 6-hour (6 h) driving by measuring hemodynamics including biventricular parameters after the animals were weaned from the 6 h of LVAD support with each mode.
-
i.
The baseline measurements were initially performed with the constant-speed mode (6 h-driving baseline). The 10 sheep were then divided into two groups: a constant-speed group (n = 5) and an asynchronous-modulation group (n = 5).
-
ii.
Each driving mode was administered to each group of sheep and maintained for 6 h. After that, the sheep of each group were weaned from the LVADs by clamping the circuit of LVAD and 30 min later, the measurements were performed with the LVAD turned off (6 h-effect evaluation).
-
i.
The experimental protocol. Acute-effect evaluation under LVAD support: we examined the acute hemodynamic changes caused by the constant-speed and asynchronous-modulation modes by measuring the hemodynamic and biventricular parameters under LVAD support by switching the modes at a 5-min interval in the same animals. 6 h-effect evaluation: we assessed the effects of 6-h driving by measuring the hemodynamics including biventricular parameters after the animals were weaned from 6 h of LVAD support in each mode. LVAD left ventricular assist device
Rotational-Speed Modulation Algorithm
In the asynchronous-modulation mode, the rotational speed of the LVAD was modulated periodically, independent of the native heartbeats. We designed a rotational-speed modulation algorithm as follows: first, we maximized the amplitude of the rotational speed to achieve high pulsatility. Because the design speed of the BIOFLOAT NCVC was between 3000 and 7000 rpm, we decided to use this amplitude.
The next step was to create one cycle. The rotational speed could be varied at 15,000 rpm/s; therefore, it took approximately 500 ms (8000/15,000) for the rotational speed to increase from 3000 to 7000 rpm and decrease from 7000 to 3000 rpm. With reference to a physiological cardiac cycle in which the diastole is longer than the systole, the rotational speed was increased by one-third of the pump speed cycle and decreased by two-thirds, that is, one cycle was 500 × 3 = 1500 ms or 40 cycles per min. The LVAD was able to appropriately follow the command rotational speed.
For the constant-speed mode, the rotational speed was set at 3750 rpm, which was equivalent to the mean rotational speed of the asynchronous-modulation mode. These settings are indicated in Fig. 3.
A The asynchronous rotational speed modulation algorithm. The rotational speed was increased by one-third of the pump speed cycle and decreased by two-thirds at 40 cycles per min from 3000 to 7000 rpm. For the constant-speed mode, the rotational speed was set at 3750 rpm, which was equivalent to the mean rotational speed of the asynchronous-modulation mode. B Actual and command rotational speeds of the LVAD. This indicates that the LVAD was able to appropriately follow the command rotational speed. LVAD left ventricular assist device
Systemic Hemodynamics and Pressure-Volume Loop Analyses of Left and Right Ventricles
The hemodynamics including heart rate (HR), arterial blood pressure (ABP), central venous pressure (CVP), pulmonary artery pressure (PAP), left atrial pressure (LAP), CBF, aortic blood flow (AoF), and pump blood flow (PF) were recorded. Pressure-volume loops of both the right and left ventricles were measured and analyzed to calculate the dP/dt max, the dP/dt min, the ventricular end-diastolic pressure and volume (EDP and EDV), the time constant of isovolumic relaxation (Tau), and stroke work (SW). The arterial pulse pressure (ΔP) was calculated as described [23]. All of these data were recorded and analyzed via Labchart 8 (ADInstruments, Dunedin, New Zealand).
We collected all of the above-described data at the baseline, the acute-myocardial injury, the acute-effect evaluation under LVAD support, the 6 h-driving baseline, and the 6 h-effect evaluation. The values of PF, LV EDP, LV EDV were also collected every hour during the 6 h of LVAD support for an evaluation of the degree of LV unloading. All of the data were defined as the average values over a 1-min period at each time point. The right ventricular (RV) EDV at the 6 h-effect evaluation was normalized to that at the 6 h-driving baseline.
Echocardiography and Myocardial Oxygen Consumption
Epicardial echocardiography (Vivid E9, GE Healthcare, Horten, Norway) was performed with the chest open. Left ventricular ejection fraction (LVEF; using the modified Simpson technique) and fractional area change (FAC) of the RV were measured. The end-diastolic diameters (EDD) of both ventricles were measured with an apical four-chamber view at the level of the tricuspid and mitral annuli. The EDD ratio was defined as the RV EDD/LV EDD. The left ventricular myocardial oxygen consumption (LVvO2) was calculated as described [22] by using paired blood samples from the carotid artery and the coronary sinus. The measurements of echocardiographic data and LVvO2 were performed at the 6 h-driving baseline and the 6 h-effect evaluation.
Statistical Analyses
The data are presented as the mean ± SD. We used a paired t test to compare the data obtained at the baseline with those at the acute-myocardial injury. We also used a paired t test to compare the acute-effect evaluation data under LVAD support between the two driving modes. An unpaired t test was used to compare the 6 h-driving baseline data and the 6 h-effect evaluation data between the two groups. The time-course data during the 6 h of LVAD support were analyzed by a two-way analysis of variance (ANOVA) for repeated measures. Statistical significance was set at p < 0.05.
Results
Waveforms of Pressure and Flow
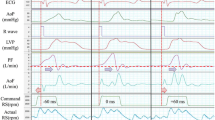
Figure 4 provides two types of representative waveforms of hemodynamic parameters in the baseline, acute-myocardial injury, and acute-effect evaluation (constant-speed and asynchronous-modulation mode). The waveforms of ABP, PF, and AoF in the asynchronous-modulation mode had larger amplitudes than those in the constant-speed mode. No reversal flow through the pump was observed in any of the experiments.
Representative waveforms of hemodynamic parameters in the baseline, acute-myocardial injury, and acute-effect evaluation (constant-speed and asynchronous-modulation mode). A and B each show hemodynamic waveforms of the same animals. ABP arterial blood pressure, AoF aortic blood flow, CBF coronary blood flow, CVP central venous pressure, LAP left atrial pressure, LVAD left ventricular assist device, PAP pulmonary artery pressure, PF pump blood flow
Hemodynamics Before the LVAD Support
Table 1 summarizes the data at the baseline and acute-myocardial injury. The hemodynamic data revealed that the HR, ABP, PAP, and LAP changed significantly from the baseline to the acute-myocardial injury. LV parameters including the dP/dt min, EDP, EDV, and Tau and RV parameters including the EDP, EDV, and Tau changed significantly from the baseline to the acute-myocardial injury. These findings indicate that a significant deterioration of hemodynamics was induced within 30 min after microembolization.
Acute-Effect Evaluation Under LVAD Support
We evaluated acute hemodynamic changes caused by the two driving modes by comparing hemodynamics including biventricular parameters under LVAD support in the same animals. Table 2 shows the results of this examination. The ΔP was significantly higher in the asynchronous-modulation mode compared to the constant-speed mode (16.9 ± 5.2 vs. 12.4 ± 6.2 mmHg, p = 0.012). None of the other parameters differed significantly between the two modes. These findings indicate that the asynchronous-modulation mode increased the pulsatility in the 5-min assessment but did not affect both ventricles differently from the constant-speed mode.
6 h-Effect Evaluation
In the 6 h-effect evaluation, we investigated how hemodynamics including both ventricles were affected by a certain period of driving with the two modes by comparing hemodynamics including biventricular parameters after weaning from 6 h of LVAD support. Table 3 provides the data at the 6 h-driving baseline. None of the parameters, including body weight and the number of microspheres, differed significantly between the two groups.
Figure 5 depicts the time-course data during the 6 h of LVAD support. The PF, LV EDP, and LV EDV did not differ significantly between the two groups during the LVAD support. Table 4 summarizes the results of the 6 h-effect evaluation. Hemodynamic parameters, including HR, ABP, ΔP, CVP, PAP, LAP, CBF, and AoF did not differ significantly between the two groups, whereas HR, ABP, and AoF tended to be higher in the asynchronous-modulation group. In the echocardiographic evaluation, LVEF and FAC did not differ significantly between the two groups, whereas the EDD ratio was significantly lower in the asynchronous-modulation group than in the constant-speed group (0.89 ± 0.09 vs. 1.08 ± 0.06, p = 0.003). The LVvO2 showed no significant differences between the two groups.
Regarding the LV parameters, no significant differences were observed except for dP/dt max which was significantly higher in the asynchronous-modulation group than in the constant-speed group (919.2 ± 124.6 vs. 688.4 ± 168.3 mmHg/s, p = 0.039).
Regarding the RV parameters, no significant differences were observed except for the EDP, normalized EDV, and Tau, which were significantly lower in the asynchronous-modulation group than in the constant-speed group (EDP: 5.8 ± 2.3 vs. 10.8 ± 3.1 mmHg, p = 0.021; normalized EDV: 1.07 ± 0.09 vs. 1.24 ± 0.12, p = 0.033; and Tau: 61.1 ± 15.9 vs. 117.0 ± 33.1 ms, p = 0.009).
Discussion
We evaluated the effect of the asynchronous modulation on hemodynamics, including both ventricles, in a sheep model of acute-myocardial injury in order to investigate the feasibility of modifying hemodynamics by applying the asynchronous modulation. The acute-effect evaluation under LVAD support revealed that, compared to the constant-speed mode, the asynchronous-modulation mode increased vascular pulsatility but did not have a significantly different influence on hemodynamics, including both ventricles. The 6 h-effect evaluation revealed that the hemodynamic parameters did not differ significantly between the two groups, except for some other parameters including echocardiography: EDD ratio, LV parameters: LV dP/dt max, and RV parameters: EDP, normalized EDV, and Tau, which did not indicate negative effects on both ventricles.
Thus, the asynchronous-modulation which could provide vascular pulsatility is expected to be a feasible driving method for continuous-flow LVADs. It has been reported that the use of a synchronous co-pulse mode (increasing the rotational speed in the systole of native heartbeats) increases the vascular pulsatility and LV load, whereas the use of a synchronous counter-pulse mode (increasing the rotational speed in the diastole) decreases the LV load and vascular pulsatility [9,10,11]. These studies suggest that the synchronous-modulation presents a trade-off between LV unloading and pulsatility. However, the asynchronous-modulation mode used in the present study could increase vascular pulsatility, as observed in other studies [9, 12], whereas no negative effect on hemodynamics, including both ventricles, was observed.
Six hours of driving in the asynchronous-modulation mode caused no significant changes in the native LV parameters, except for the LV dP/dt max. It has been reported that pulsatile-flow LVADs were more effective for myocardial recovery than continuous-flow LVADs [24, 25], but it is not yet known whether this was due to the different degrees of LV unloading and/or the different patterns of LV unloading. Other studies demonstrated that when the PF changes, independent of the native heartbeats, an LV unloading pattern changes at each heartbeat [26, 27]. As observed in those studies, we speculate that the unloading pattern of the asynchronous-modulation mode in the present study was different at each heartbeat. We estimated the degree of LV unloading by measuring the LV EDP and LV EDV as the average values over 1 min, and based on this, the degree of LV unloading during 6 h of driving did not differ significantly between the two groups. We thus postulate that the difference in LV dP/dt max in the 6 h-effect evaluation between the two groups might be related to the difference in the unloading patterns between the two modes during 6 h of driving. However, considering that the LV parameters at the 6 h-effect evaluation other than LV dP/dt max did not differ significantly between the two groups, further studies are needed to assess the reliability and mechanism of the possible positive effects on native LV function.
The results of our acute-effect evaluation under LVAD support revealed that the effect of the asynchronous-modulation mode on the RV was not significantly different from that of the constant-speed mode over a short term (5 min). This finding is consistent with a report that an acute modulation of the pump speed of a continuous-flow LVAD only minimally affected RV function [28]. Bouwmeester et al. conducted a wave-intensity analysis and reported that asynchronous modulation resulted in no observable changes in RV function in the acute phase [29], which also agrees with our finding. However, these previous studies did not investigate the accumulated effects of asynchronous modulation on the RV caused by a certain period of driving followed by weaning from LVAD support. We investigated the accumulated effect of asynchronous modulation, and it was shown that 6 h of driving with the asynchronous-modulation mode caused no significant changes to the native RV parameters, except for possible positive changes in EDP, normalized EDV, and Tau. Thus, 6 h of driving with the asynchronous-modulation mode may positively modify RV function, although it is limited within the range of speculation because of the limitation of this study involving animal models with acute-myocardial injury and evaluation procedures in which reliability and reproducibility were not necessarily guaranteed. We speculate that a reduction of the degree of septal shift due to asynchronous modulation under LVAD support might be related to RV function. It has been reported that the ventricular septum has a significant impact on RV function [30, 31], and in LVAD patients the leftward shift of the ventricular septum, aggravated by high rotational speed, causes a deterioration of RV function [32,33,34,35,36]. We thus hypothesize that the mechanism of the effect on the RV may involve the following factor: the asynchronous-modulation group had periods of low rotational speed in two-thirds of the pump cycle, which could decrease the degree of septal shift overall. We did not examine the degree of septal shift in this study, and further investigation is necessary to test our hypothesis.
Our results suggested that the asynchronous-modulation could be feasible to increase vascular pulsatility without causing negative effects on hemodynamics including both ventricles, while it could be expected to have some positive effects on native heart. Further studies are necessary to evaluate the effects of asynchronous modulation by mimicking the clinical scenario of patients with LVADs using an optimal modulation algorithm. The findings from this study can provide important reference material for future chronic experiments on the asynchronous modulation of LVADs using a chronic heart failure model.
Study Limitations
There are several study limitations to address. The model used in this study was an acute-myocardial injury model without LV remodeling, which is observed in the chronic heart failure model.
It is also unclear whether various modulation algorithms other than the one used here have the same effects on pulsatility and hemodynamics, including both ventricles. Furthermore, we cannot conclude that the modulation algorithm used in this study was optimal. The influences of the pump cycle rate, the rotational speed amplitude, and the timing and duration of increasing the rotational speed remain to be explored in future research.
Several factors affected pulse transmission in the asynchronous-modulation mode. First, the outflow cannula used was smaller than a typical LVAD outflow graft. An outflow cannula was inserted through the carotid artery to minimize surgical invasiveness. Second, the LVAD circuit lengths were longer than those of implantable LVADs. Third, the LVAD circuit consisted of polyvinyl chloride tubes; therefore, compliance might differ in clinical settings. However, all these factors affecting pulse transmission were considered equivalent in all the experiments in this study.
Conductance catheter data have definite limitations in terms of accuracy and reproducibility, particularly for measurement of the RV.
Conclusions
The asynchronous-modulation could be feasible to increase vascular pulsatility without causing negative effects on hemodynamics including both ventricles. Compared to the constant-speed mode, the asynchronous-modulation mode increased pulsatility during LVAD support without negative effects on hemodynamics including both ventricles in the acute phase. Six hours of LVAD support with the asynchronous-modulation mode exerted no negative effects on hemodynamics, including both ventricles, after weaning from the LVAD.
References
Mancini, D., and P. C. Colombo. Left ventricular assist devices: a rapidly evolving alternative to transplant. J. Am. Coll. Cardiol. 65:2542–2555, 2015.
Purohit, S. N., W. K. Cornwell 3rd., J. D. Pal, J. Lindenfeld, and A. V. Ambardekar. Living Without a pulse: the vascular implications of continuous-flow left ventricular assist devices. Circ. Heart Fail.11:e004670, 2018.
Muthiah, K., D. Conno, K. Ly, et al. Longitudinal changes in hemostatic parameters and reduced pulsatility contribute to non-surgical bleeding in patients with centrifugal continuous-flow left ventricular assist devices. J. Heart Lung Transplant. 35:743–751, 2016.
Kataria, R., and U. P. Jorde. Gastrointestinal bleeding during continuous-flow left ventricular assist device support: state of the field. Cardiol. Rev. 27:8–13, 2019.
Cowger, J., F. D. Pagani, J. W. Haft, M. A. Romano, K. D. Aaronson, and T. J. Kolias. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ. Heart Fail. 3:668–674, 2010.
Mudd, J. O., J. D. Cuda, M. Halushka, K. A. Soderlund, J. V. Conte, and S. D. Russell. Fusion of aortic valve commissures in patients supported by a continuous axial flow left ventricular assist device. J. Heart Lung Transplant. 27:1269–1274, 2008.
Witman, M. A., R. S. Garten, J. R. Gifford, et al. Further peripheral vascular dysfunction in heart failure patients with a continuous-flow left ventricular assist device: the role of pulsatility. JACC Heart Fail. 3:703–711, 2015.
Amir, O., B. Radovancevic, R. M. Delgado 3rd., et al. Peripheral vascular reactivity in patients with pulsatile vs. axial flow left ventricular assist device support. J. Heart Lung Transplant. 25:391–394, 2006.
Soucy, K. G., G. A. Giridharan, Y. Choi, et al. Rotary pump speed modulation for generating pulsatile flow and phasic left ventricular volume unloading in a bovine model of chronic ischemic heart failure. J. Heart Lung Transplant. 34:122–131, 2015.
Umeki, A., T. Nishimura, M. Ando, et al. Alteration of LV end-diastolic volume by controlling the power of the continuous-flow LVAD, so it is synchronized with cardiac beat: development of a native heart load control system (NHLCS). J. Artif. Organs. 15:128–133, 2012.
Arakawa, M., T. Nishimura, Y. Takewa, et al. Alternation of left ventricular load by a continuous-flow left ventricular assist device with a native heart load control system in a chronic heart failure model. J. Thorac. Cardiovasc. Surg. 148:698–704, 2014.
Ising, M. S., M. A. Sobieski, M. S. Slaughter, S. C. Koenig, and G. A. Giridharan. Feasibility of pump speed modulation for restoring vascular pulsatility with rotary blood pumps. ASAIO J. 61:526–532, 2015.
Naito, N., T. Nishimura, K. Iizuka, et al. Rotational speed modulation used with continuous-flow left ventricular assist device provides good pulsatility. Interact. Cardiovasc. Thorac. Surg. 26:119–123, 2018.
Kishimoto, Y., Y. Takewa, M. Arakawa, et al. Development of a novel drive mode to prevent aortic insufficiency during continuous-flow LVAD support by synchronizing rotational speed with heartbeat. J. Artif. Organs. 16:129–137, 2013.
Cox, L. G., S. Loerakker, M. C. Rutten, B. A. de Mol, and F. N. van de Vosse. A mathematical model to evaluate control strategies for mechanical circulatory support. Artif. Organs. 33:593–603, 2009.
Chassagne, F., M. Miramontes, V. K. Chivukula, et al. In vitro investigation of the effect of left ventricular assist device speed and pulsatility mode on intraventricular hemodynamics. Ann. Biomed. Eng. 49:1318–1332, 2021.
Chen, Z., S. K. Jena, G. A. Giridharan, et al. Shear stress and blood trauma under constant and pulse-modulated speed CF-VAD operations: CFD analysis of the HVAD. Med. Biol. Eng. Comput. 57:807–818, 2019.
Ortiz, S., V. Vu, R. Montes, and K. May-Newman. Left ventricular flow dynamics with the HeartMate3 left ventricular assist device: effect of inflow cannula position and speed modulation. ASAIO J. 67:1301–1311, 2021.
Tuzun, E., K. Chorpenning, M. Q. Liu, et al. The effects of continuous and intermittent reduced speed modes on renal and intestinal perfusion in an ovine model. ASAIO J. 60:19–24, 2014.
Vandenberghe, S., P. Segers, J. F. Antaki, B. Meyns, and P. R. Verdonck. Hemodynamic modes of ventricular assist with a rotary blood pump: continuous, pulsatile, and failure. ASAIO J. 51:711–718, 2005.
Shimamura, J., T. Mizuno, Y. Takewa, et al. Miniaturized centrifugal ventricular assist device for bridge to decision: preclinical chronic study in a bovine model. Artif. Organs. 43:821–827, 2019.
Tanaka, S., T. Nishinaka, A. Umeki, et al. Coronary microembolization sheep model by adjusting the number of microspheres based on coronary blood flow. Artif. Organs. 47:138–147, 2022.
Soucy, K. G., S. C. Koenig, G. A. Giridharan, M. A. Sobieski, and M. S. Slaughter. Defining pulsatility during continuous-flow ventricular assist device support. J. Heart Lung Transplant. 32:581–587, 2013.
Krabatsch, T., M. Schweiger, M. Dandel, et al. Is bridge to recovery more likely with pulsatile left ventricular assist devices than with nonpulsatile-flow systems? Ann. Thorac. Surg. 91:1335–1340, 2011.
Kato, T. S., A. Chokshi, P. Singh, et al. Effects of continuous-flow versus pulsatile-flow left ventricular assist devices on myocardial unloading and remodeling. Circ. Heart Fail. 4:546–553, 2011.
Amacher, R., A. Weber, H. Brinks, et al. Control of ventricular unloading using an electrocardiogram-synchronized Thoratec paracorporeal ventricular assist device. J. Thorac. Cardiovasc. Surg. 146:710–717, 2013.
Amacher, R., G. Ochsner, and M. Schmid Daners. Synchronized pulsatile speed control of turbodynamic left ventricular assist devices: review and prospects. Artif. Organs. 38:867–875, 2014.
Tran, T., A. Muralidhar, K. Hunter, et al. Right ventricular function and cardiopulmonary performance among patients with heart failure supported by durable mechanical circulatory support devices. J. Heart Lung Transplant. 40:128–137, 2021.
Bouwmeester, J. C., J. Park, J. Valdovinos, and P. Bonde. Wave intensity analysis of right ventricular function during pulsed operation of rotary left ventricular assist devices. ASAIO J. 65:465–472, 2019.
Kaul, S. The interventricular septum in health and disease. Am. Heart J. 112:568–581, 1986.
Klima, U. P., M. Y. Lee, J. L. Guerrero, P. J. Laraia, R. A. Levine, and G. J. Vlahakes. Determinants of maximal right ventricular function: role of septal shift. J. Thorac. Cardiovasc. Surg. 123:72–80, 2022.
Moon, M. R., A. F. Bolger, A. DeAnda, et al. Septal function during left ventricular unloading. Circulation. 95:1320–1327, 1997.
Houston, B. A., K. B. Shah, M. R. Mehra, and R. J. Tedford. A new “twist” on right heart failure with left ventricular assist systems. J. Heart Lung Transplant. 36:701–707, 2017.
Topilsky, Y., J. K. Oh, D. K. Shah, et al. Echocardiographic predictors of adverse outcomes after continuous left ventricular assist device implantation. JACC Cardiovasc Imaging. 4:211–222, 2011.
Sack, K. L., Y. Dabiri, T. Franz, et al. Investigating the role of interventricular interdependence in development of right heart dysfunction during LVAD support: a patient-specific methods-based approach. Front Physiol. 9:520, 2018.
Anne Dual, S., A. Nayak, Y. Hu, et al. Does size matter for female continuous-flow LVAD recipients? A translational approach to a decade long question. ASAIO J. 68:21–27, 2022.
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP21K08853 and Nipro Corporation. We would like to thank KN International and Editage for English proofreading.
Author information
Authors and Affiliations
Contributions
ST, TN, AU, TT, TM, and MO substantially contributed to the study’s conception and design. ST, TN, AU, TM, SI, TM, and TT contributed to the data acquisition. All authors contributed to the data analysis and interpretation. ST drafted the manuscript, and all authors revised it. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Tomohiro Nishinaka reports collaborative research agreements with Nipro Corporation and Senko Medical Instrument Mfg. Co., Ltd. The other authors have no conflicts of interest to declare.
Additional information
Associate Editor Ender A. Finol oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tanaka, S., Nishinaka, T., Umeki, A. et al. Hemodynamic Evaluation of Asynchronous Speed Modulation of a Continuous-Flow Left Ventricular Assist Device in an Acute-Myocardial Injury Sheep Model. Ann Biomed Eng 52, 364–375 (2024). https://doi.org/10.1007/s10439-023-03383-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-023-03383-y