Abstract
The effect of rotary left ventricular assist devices (LVADs) on myocardial perfusion has yet to be clearly elucidated, and several studies have shown decreased coronary flow under rotary LVAD support. We have developed a novel pump controller that can change its rotational speed (RS) in synchronization with the native cardiac cycle. The aim of our study was to evaluate the effect of counterpulse mode, which increases the RS in diastole, during coronary perfusion. Experiments were performed on ten adult goats. The EVAHEART LVAD was installed by the left ventricular uptake and the descending aortic return. Ascending aortic flow, pump flow, and coronary flow of the left main trunk were monitored. Coronary flow was compared under four conditions: circuit-clamp, continuous mode (constant pump speed), counterpulse mode (increased pump speed in diastole), and copulse mode (increased pump speed in systole). There were no significant baseline changes between these groups. In counterpulse mode, coronary flow increased significantly compared with that in continuous mode. The waveform analysis clearly revealed that counterpulse mode mainly resulted in increased diastolic coronary flow. In conclusion, counterpulse mode of rotary LVADs can enhance myocardial perfusion. This novel drive mode can provide great benefits to the patients with end-stage heart failure, especially those with ischemic etiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effect of rotary left ventricular assist devices (LVADs) on myocardial perfusion has yet to be clearly elucidated [1], and several studies have shown decreased coronary flow under rotary LVAD support [2, 3]. The primary rationale for decreased myocardial perfusion is decreased left ventricular work itself due to left ventricular unloading and the associated decline in myocardial oxygen demand [3]. Decreased myocardial oxygen demand may lead to a reactive increase in coronary vascular resistance through the autoregulatory system, which is designed to eliminate excessive coronary perfusion under the well-unloaded condition [3, 4]. The mechanical characteristics of the rotary LVADs themselves also account for the diminished coronary flow. Under support of rotary LVADs with a constant rotational speed (RS), pump flow (PF) can be increased mainly in systolic phase when the pressure head [i.e., aortic pressure (AoP) − left ventricular pressure (LVP)] becomes relatively low, while PF can be decreased in diastolic phase when the pressure head becomes relatively high. A large portion of coronary flow is typically observed in diastole [2]. Thus, we have speculated that we cannot effectively increase myocardial perfusion under the assumption of a constant RS. Diminished coronary flow is not always an untoward factor in clinical practice; however it may be undesirable, especially in patients with ischemic etiology or post-coronary bypass grafting [5].
On the basis of these perspectives, we have designed a novel pump controller that can change its RS in synchronization with the native cardiac cycle. Our overall aim was to increase myocardial perfusion under rotary LVAD support by augmenting its RS only in the diastolic phase. The specific aim of the study reported here was to evaluate the effect of the counterpulse mode, which increases RS in diastole, on coronary perfusion.
Materials and methods
Experimental preparation
Experiments were performed on ten adult goats (61.3 ± 7.9 kg). After a 48-h fast and overnight withholding of drinking water, all animals were sedated with an intramuscular injection of ketamine (10 mg/kg). General anesthesia was induced and maintained by isoflurane inhalation. The animals were fixed in the right recumbent position, intubated, and mechanically ventilated. The inhaled oxygen concentration was controlled so as to obtain a target arterial oxygen pressure of 100–150 mmHg. The heart was approached via a left thoracotomy in the usual fashion, and pressure lines for AoP and central venous pressure (CVP) monitoring were established from the left internal mammalian artery and vein. The main pulmonary artery and the ascending aorta were dissected and taped, and an 18- or 20-mm electromagnetic flow probe (EMF-1000; NIHON KOHDEN, Tokyo, Japan) was placed on the ascending aorta for the monitoring of ascending aortic flow (AoF). The main trunk of the left coronary artery (LMT) was also encircled, and a 3-mm ultrasonic flow probe (HQD3FSB; Transonic System, Ithaca, NY) was attached for the monitoring of coronary flow (CoF). The hemiazygos vein was cut off, and the descending aorta was dissected. A 16-mm outflow cannula was sutured to the descending aorta, and a 16-mm ultrasonic flow probe (TS420; Transonic System) was placed around the outflow cannula for the monitoring of PF. After heparinization (300 U/kg), the left ventricular apex was punched out by a 19-mm puncher, and a 20-mm inflow cannula was inserted into the left ventricle. Both the outflow and inflow cannula were connected to the EVAHEART LVAD (Sun Medical Technology Research Corp, Nagano, Japan) [6] and the left heart bypass initiated. A 4Fr Mikro-tip catheter pressure transducer (Millar Instruments, Houston, TX) was inserted from the anterior wall of the left ventricle for LVP monitoring. A pacing lead for the ventricular electrocardiogram was sutured onto the anterior wall of the right ventricle.
After the animals had been prepared as described above, we waited for at least 30 min to obtain a hemodynamically stable condition. All experimental protocols were approved by the Animal Research Committee of the National Cerebral and Cardiovascular Center Research Institute and conducted according to its guidelines under the care of a veterinarian.
Experimental protocol
During data collection, a target preload was set at 10 mmHg by mean CVP, and the infusion volume was regulated to maintain this target. The target heart rate and systolic pressure were 60–90 bpm and 80–120 mmHg, respectively. If these parameters deviated greatly from the target value, we adjusted the concentration of inhaled isoflurane to keep them stabilized. We did not use any vasopressor agents during data collection. Only continuous infusion of 2% lidocaine (1 mg/kg/h) was given during the experiment to prevent ventricular arrhythmia.
We compared the amount of the coronary flow under four conditions, namely, circuit-clamp (i.e., no pump support), continuous mode (constant pump speed), counterpulse mode (increased pump speed in diastole), and copulse mode (increased pump speed in systole). The bypass rate was calculated by dividing the PF by the sum of PF and AoF. In the continuous, counterpulse, and copulse conditions, bypass rates were adjusted to 50% according to the guideline that is described in the following section (Drive mode). In other words, the mean PF was almost the same in these three conditions, and we just changed pump support phase and analyzed its effect on coronary circulation. Between each condition setting and data collection, we waited at least 5 min to obtain stable hemodynamics. Pressure and flow data were collected by 1,000 Hz in Labchart5 (ADInstruments, Sidney, Australia). After the data were collected, coronary waveforms were integrated by time, and diastolic and systolic coronary flows were calculated. The 3-mm flow probe could not be attached on the LMT in three of the ten animals, and it was placed instead on the left anterior descending artery (LAD); the amount of the LAD flow was increased by half and compared to that of the LMT [7]. The animals were sacrificed at the end of the experiment. The hearts were excised and trimmed and the weight of the left ventricle measured. The coronary flow was normalized per 200-g weight of the left ventricle.
Drive mode
Our group has developed a novel pump controller that can change its RS. This controller detects the R wave from the ventricular electrocardiogram and can instantly increase (or decrease) its RS to the target speed after a configured delay time from the R wave. The duration time of the target speed is also configurable. In this experiment, the duration of the systolic phase was defined as the first 33% of the RR interval; the remaining 67% of the systolic phase was defined as the diastolic phase. The actual duration of the native diastole phase always varies between animals. If the diastolic phase was set shorter than the native diastole phase, we observed a certain amount of backward flow; thus, it was necessary to set the former to be longer than the native diastole phase. However, if the diastolic phase was set to be too long, the pump controller could not maintain the plateau phase of the systolic rotational speed properly in the tachycardiac condition. Ultimately, we set the diastolic phase at 67% of the RR interval. For example at a native heart rate of 90 bpm, the RR interval is about 670 ms, with 33% being 220 ms. Therefore, we set the pump for a target systolic RS of 220 ms from the R wave and then instantly increased (or decreased) the RS to the target diastolic RS. We maintained diastolic RS for the remaining 450 ms, which is about 67% of the 670 ms, or the RR interval, and finally returned the RS to the initial systolic RS.
Diastolic and systolic RS were adjusted according to the following guideline. A target bypass rate was set at 50% and RS was changed between 700 and 2500 rpm. For example, in counterpulse mode, we first set both the systolic and diastolic RS at 700 rpm. Just after R wave input, the pump was driven in 700 rpm for 33% of the RR interval, or “configured systolic phase”. The RS was then increased instantly to the target diastolic RS. In our study, the target diastolic RS was adjusted to achieve a bypass rate of 50%. The bypass rate was calculated in real time and displayed on a monitor. We manually controlled diastolic RS until the bypass rate became 50%. This diastolic RS was then kept for 67% of the RR interval and finally returned to 700 rpm. On occasion we were unable to achieve a bypass rate of 50% even if we increased the diastolic RS to 2500 rpm. In this case, we also increased the systolic RS from 700 rpm. In copulse mode, we did the reverse
Statistics
All numerical data are shown here as the average ± standard deviation (SD). The statistical difference between the circuit-clamp and continuous mode conditions was determined by the two-sided paired t test. The continuous and copulse modes were compared by repeated analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. In Dunnett’s test, the continuous mode was regarded as the baseline condition. All analyses were performed by two-sided test, and a p value < 0.05 was considered to be statistically significant. We used PASW Statistics ver. 18 (SPSS, Chicago, IL) for all statistical analyses.
Results
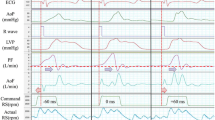
Figure 1 shows sample waveforms of the pressure and flow data for all four conditions. The RS, shown at the bottom of the figure, was controlled according to the guideline described in the Drive mode section. In continuous mode, the RS was around 1500–1800 rpm, and in the counterpulse and copulse modes, the maximum and minimum RS varied between 2000 and 2500 rpm and 700 and 1000 rpm, respectively. The diastolic PF and AoP were higher in the counterpulse mode than in the continuous mode. In contrast, in the copulse mode, the AoP decreased and the PF became negative in diastolic phase, indicating that a certain amount of retrograde flow can exist in a pump-circuit.
Pressure and flow waveforms in the four modes tested. ECG Electrocardiogram, AoP aortic pressure, CVP central venous pressure, LVP left ventricular pressure, PF pump flow, AoF ascending aortic flow, CoF coronary flow, RS rotational speed. The diastolic pump flow and aortic pressure were higher in counterpulse mode than in continuous mode. In copulse mode, mean aortic pressure was lower
Flow and pressure numerical data are shown in Table 1. Continuous mode was compared with (A) clamp first, and then the counterpulse and copulse modes were compared with the continuous mode as a baseline. There was no significant change in CVP and bypass rates in the continuous, counterpulse, and copulse modes, but AoP was significantly decreased in the copulse mode. End-diastolic pressure (EDP) and end-systolic pressure (ESP) are also shown in Table 1. EDP was significantly increased in copulse mode compared with continuous mode.
Figure 2 shows changes in the amount of coronary flow in the four modes. Continuous mode significantly decreased mean, diastolic, and systolic coronary flow compared with the clamp condition. Counterpulse mode significantly increased mean and diastolic coronary flow. In copulse mode, the systolic coronary flow was increased; however, diastolic flow was decreased and the mean coronary flow remained unchanged.
Changes in coronary flow between the continuous mode (black) and clamp condition with (green) was compared by the paired t test, and those between the counterpulse (blue) and copulse (red) modes were compared with the continuous mode by repeated analysis of variance followed by the Dunnett method. In the counterpulse mode, mean and diastolic coronary flow were significantly increased. In the copulse mode, systolic coronary flow was higher; however, diastolic coronary flow was lower and the mean coronary flow remained unchanged
Coronary flow patterns are shown in Fig. 3. In the clamp, continuous, and counterpulse modes, CF was mainly observed in the diastolic phase. In the continuous mode, CR gradually decreased towards the next R wave, while in counterpulse mode, the peak CF was maintained until the next R wave relative to the continuous condition. In copulse mode, the systolic CF increased, while the waveform itself was totally different from that of the other three conditions.
Coronary waveforms. In the clamp, continuous, and counterpulse modes, coronary flow is mainly observed in diastolic phase. In the continuous mode, coronary flow gradually decreased towards the next R wave, while in the counterpulse mode, peak coronary flow was maintained until the next R wave relative to the continuous condition. In copulse mode, systolic coronary flow was increased, however, diastolic coronary flow was clearly decreased
Discussion
We have evaluated the effect of a novel drive mode of rotary LVADs on coronary circulation and determined that the counterpulse mode can enhance myocardial perfusion. There is an ongoing discussion on the effect of the synchronous mode of pulsatile LVADs [8]. However, in rotary LVADs, it has become structurally possible to unload the left ventricle in diastolic phase with concomitant diastolic PF augmentation; an operation had been virtually impossible in pulsatile LVADs with mechanical valves. Thus, any discussion on the mechanism of counterpulsation mode in rotary LVADs must be completely independent of that in pulsatile LVADs. To the best of our knowledge, our study is the first to report on a drive mode of rotary LVADs that can change its RS in synchronization with the native heart cycle. In this context, we believe that our findings are unique and valuable.
Our perspectives are based on a few reports that suggest a negative effect of rotary LVADs on coronary circulation [2–5]. A rational explanation of the decreased CF under rotary LVAD support is the coronary autoregulatory system. In effectively unloaded normal hearts, coronary vascular resistance may increase so as to reduce any excessive CF relative to the oxygen demands [9]. Our results show that CR was significantly reduced in the continuous mode compared with the clamp condition (Table 1). Our experimental protocol aimed as keeping bypass rates constant at 50% and just altering the support phase of LVADs by changing the systolic and diastolic RS. Under the same half bypass support, the counterpulse mode produced the best myocardial perfusion. According to the phase analysis (Table 1), diastolic CF was significantly increased in the counterpulse mode.
The positive effect of counterpulsation on coronary perfusion has already been established in intra-aortic balloon pumping or other counterpulse devices [10, 11]. The determinants of CF are heart rate, diastolic pressure, and coronary vascular resistance [9]. Table 1 shows that there was no deviation in the heart rate. However, diastolic augmentation was evidently obtained in the counterpulse mode (Fig. 1), which may have contributed to enhanced coronary perfusion. Among these three determinants, coronary vascular resistance is regulated by various factors and is affected not only by the LVP, but also by cardiac work, myocardial oxygen consumption, and metabolic changes through neural and hormonal regulation [9].
A positive association between elevated EDP and subendocardial ischemia has been reported; therefore, a lower EDP can be considered to be desirable for better myocardial perfusion [12]. In terms of LVP, there was no significant change in mean LVP, EDP, and ESP between the counterpulse and continuous modes. However, there seemed to be a decreasing tendency in EDP by counterpulsation, so this mode may have exerted a certain influence on enhanced coronary perfusion. The reason for the absence of any significant change in EDP between the continuous and counterpulse modes and the clamp mode can be speculated as follows. Normally, it would appear to be natural that pump support may decrease EDP by volume unloading; however, in the current experimental setting, EDP was not decreased by the continuous and counterpulse modes relative to the clamp mode. As shown in Fig. 1, a small amount of retrograde flow was observed in the continuous and counterpulse modes, possibly because of the low circuit resistance of the EVAHEART LVAD system. The EVAHEART LVAD system has a large cross-sectional area through the whole blood pathway; consequently, circuit resistance is very low [13]. This may cause a certain amount of retrograde flow with low rotational speed, possibly explaining why we did not obtain EDP decrease in the continuous and counterpulse modes. In other words, it can create a large amount of forward flow with high rotational speed, which is ideal for creating flow change in one cardiac cycle, which is what we attempted to achieve in this study.
We also evaluated copulse mode as well as counterpulse mode. However in copulse mode, there was no obvious benefit on coronary circulation (Fig. 2), possibly because of diastolic pump backward flow. In copulse mode, systolic RS did increase, while diastolic RS decreased. In diastole, the pressure head becomes relatively high, and a relatively low RS in diastole will create a certain amount of intracircuit retrograde flow. In fact, in diastolic phase, the PF in copulse mode became negative and the diastolic pressure clearly decreased (Fig. 1). The EDP also increased, possibly due to the same reason (Table 1). Because of these factors that negatively affect coronary circulation [9], we speculate that mean CR remained unchanged in copulse mode, even if the systolic CF significantly increased (Table 1). As shown in Fig. 3, the coronary waveform in copulse mode is totally different that in the other three conditions. One explanation for the observed systolic coronary flow increase in copulse mode, despite an apparent decrease in systolic AoP (Fig. 1), may be the following. Normally in systolic phase, the coronary vascular bed may collapse by active ventricular contraction. However, in copulse mode, we increased the RS in systolic phase, which may have prevented the collapse of the coronary vascular bed in systole by decreasing ventricular wall tension. In addition, among the four experimental settings, the total flow (sum of the aortic flow and PF) in copulse mode was also relatively decreased, and the retrograde flow mentioned above in copulse mode may have caused this total flow decrease.
Our study has several limitations. First, it was performed on normal hearts under half bypass; experiments on ischemic hearts under full bypass may have provided different results [14–17]. Second, we did not refer to cardiac work. The amount of coronary flow may depend partially on native heart work, and any discussion on coronary flow should be linked with pressure volume area or myocardial oxygen consumption. The cardiac work in all four experimental modes may not be exactly the same. For example, diastolic volume unloading can be obtained in the counterpulse mode, while in the copulse mode, volume reloading can be seen due to diastolic reverse PF (Fig. 1). Third, we selected the descending aorta as a perfusion site, not the ascending aorta, which was more difficult to expose through left thoracotomy. However, this choice may have caused stagnant flow in the aortic root and may have affected the amount of coronary flow. Finally, we should developing the controller itself. In the current system, we can freely configure the delay time from the R wave; however, we have to change it manually when the heart rate changes dramatically. In the near future, this regulation should be programmed and performed automatically. The system can function properly at a heart rate under 100 bpm, but it should be improved to function in tachycardiac condition. We defined diastolic phase as 67% of the RR interval, which is not physiologically correct. We ought to create a more precise diastole detection system from T waves or AoP waveforms, similar to that found in the current intra-aortic balloon pump.
Most of these limitations will be resolved by future improvements in the current controller, and we believe in the utility of this novel system. For example, this device can be applied to ischemic cardiomyopathy with rotary LVAD support. In the chronic phase of this etiology, a constant rotation speed can decrease coronary flow by left ventricular unloading, which may adversely aggravate the myocardium itself. The counterpulse mode of rotary LVADs can enhance myocardial perfusion and may be beneficial to the native heart. Also in the acute postoperative phase of coronary bypass graftings, the system can increase graft flow by elevating diastolic pressure, which may lead to an improvement in graft patency.
Conclusion
The counterpulse mode of rotary LVADs can enhance myocardial perfusion. This novel drive mode can provide great benefits to patients with end-stage heart failure, especially those with ischemic etiology.
References
Thalmann M, Schima H, Wieselthaler G, Wolner E. Physiology of continuous blood flow in recipients of rotary cardiac assist devices. J Heart Lung Transplant. 2005;24:237–45.
Ootaki Y, Kamohara K, Akiyama M, Zahr F, Kopcak MW Jr, Dessoffy R, Fukamachi K. Phasic coronary blood flow pattern during a continuous flow left ventricular assist support. Eur J Cardiothorac Surg. 2005;28:711–6.
Tuzun E, Eya K, Chee HK, Conger JL, Bruno NK, Frazier OH, Kadipasaoglu KA. Myocardial hemodynamics, physiology, and perfusion with an axial flow left ventricular assist device in the calf. ASAIO J. 2004;50:47–53.
Noda H, Takano H, Taenaka Y, Kinoshita M, Tatsumi E, Yagura A, Sekii H, Sasaki E, Akutsu T. Regulation of coronary circulation during left ventricular assist. ASAIO Trans. 1989;35:445–7.
Xydas S, Rosen RS, Pinney S, Hickey KT, Wasserman H, Mancini DM, Naka Y, Oz MC, Bergmann SR, Maybaum S. Reduced myocardial blood flow during left ventricular assist device support: a possible cause of premature bypass graft closure. J Heart Lung Transplant. 2005;24:1976–9.
Yamazaki K, Kihara S, Akimoto T, Tagusari O, Kawai A, Umezu M, Tomioka J, Kormos RL, Griffith BP, Kurosawa H. EVAHEART: an implantable centrifugal blood pump for long-term circulatory support. Jpn J Thorac Cardiovasc Surg. 2002;50:461–5.
Grundeman PF, Borst C, van Herwaarden JA, Verlaan CW, Jansen EW. Vertical displacement of the beating heart by the octopus tissue stabilizer: influence on coronary flow. Ann Thorac Surg. 1998;65:1348–52.
Nakamura T, Hayashi K, Seki J, Nakatani T, Noda H, Takano H, Akutsu T. Effect of drive mode of left ventricular assist device on the left ventricular mechanics. Artif Organs. 1988;12:56–66.
Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205.
Weber KT, Janicki JS. Intraaortic balloon counterpulsation. A review of physiological principles, clinical results, and device safety. Ann Thorac Surg. 1974;17:602–36.
Shimizu T, Kyo S, Imanaka K, Nakaoka K, Nishimura E, Okumura T, Ishii M, Hisagi M, Nishimura T, Motomura N, Ono M, Takamoto S. A novel external counterpulsation system for coronary artery disease and heart failure: pilot studies and initial clinical experiences. J Artif Organs. 2010;13:161–9.
Elhabyan AK, Reyes BJ, Hallak O, Broce M, Rosencrance JG, Lucas BD, Fazal H. Subendocardial ischemia without coronary artery disease: is elevated left ventricular end diastolic pressure the culprit? Curr Med Res Opin. 2004;20:773–7.
Yamazaki K, Saito S, Kihara S, Tagusari O, Kurosawa H. Completely pulsatile high flow circulatory support with a constant-speed centrifugal blood pump: mechanisms and early clinical observations. Gen Thorac Cardiovasc Surg. 2007;55:158–62.
Nakata K, Shiono M, Orime Y, Hata M, Sezai A, Saitoh T, Sezai Y. Effect of pulsatile and nonpulsatile assist on heart and kidney microcirculation with cardiogenic shock. Artif Organs. 1996;20:681–4.
Voitl P, Vollkron M, Bergmeister H, Wieselthaler G, Schima H. Coronary hemodynamics and myocardial oxygen consumption during support with rotary blood pumps. Artif Organs. 2009;33:77–80.
Smalling RW, Cassidy DB, Barrett R, Lachterman B, Felli P, Amirian J. Improved regional myocardial blood flow, left ventricular unloading, and infarct salvage using an axial-flow, transvalvular left ventricular assist device. A comparison with intra-aortic balloon counterpulsation and reperfusion alone in a canine infarction model. Circulation. 1992;85:1152–9.
Hata M, Shiono M, Orime Y, Nakata K, Sezai A, Yamada H, Saito T, Sezai Y. Coronary microcirculation during left heart bypass with a centrifugal pump. Artif Organs. 1996;20:678–80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ando, M., Takewa, Y., Nishimura, T. et al. A novel counterpulsation mode of rotary left ventricular assist devices can enhance myocardial perfusion. J Artif Organs 14, 185–191 (2011). https://doi.org/10.1007/s10047-011-0573-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-011-0573-9