Abstract
Knowledge about the hydrochemical conditions of deep groundwater is crucial for the design and operation of geothermal facilities. In this study, the hydrochemical heterogeneity of the groundwaters in the Malm aquifer, Germany, is assessed, and reasons for the extraordinarily high H2S concentrations in the central part of the Bavarian Molasse Basin are proposed. Samples were taken at 16 sites, for a total of 37 individual wells, to analyze cations, anions, gas loading and composition. The hydrochemical characteristics of the Malm groundwater in the center of the Molasse Basin are rather heterogeneous. Although the groundwater in the central basin is dominated by meteoric waters, there is a significant infiltration of saline water from higher strata. Care has to be taken in the interpretation of data from geothermal sites, as effects of chemical stimulation of the boreholes may not be fully removed before the final analyses. The distribution of H2S in the gas phase is correlated to the gas loading of the water which increases in the central basin. Temperatures, isotopic data and the sulfur mass balance indicate that H2S in the central basin is related to thermochemical sulfate reduction (south of Munich) and bacterial sulfate reduction (north of Munich).
Résumé
La connaissance de l’état hydrochimique des eaux souterraines profondes est cruciale pour la conception et l’exploitation des installations géothermales. Dans la présente étude, l’hétérogénéité hydrochimique des eaux de l’aquifère du Malm (Allemagne) est évaluée et les causes des concentrations extraordinairement élevées de H2S dans la partie centrale du Bassin Molassique Bavarois exposées. Des échantillons ont été prélevés sur 16 sites, dans au total 37 puits privés, en vue d’une analyse des cations, des anions, de la pression et composition du gaz. Les caractéristiques hydrochimiques de la nappe du Malm dans le centre du Bassin Molassique sont plutôt hétérogènes. Bien que les eaux souterraines de cette partie du bassin soient sous la dépendance des eaux météoriques, il y a une infiltration significative d’eaux salées depuis les couches surincombantes. Du soin doit être apporté à l’interprétation des données sur les sites géothermaux car les effets de la stimulation chimique des forages peuvent ne pas avoir été totalement éliminés avant la réalisation des analyses finales. La distribution du H2S dans la phase gazeuse est corrélée à la pression des gaz de l’eau, qui croît dans la partie centrale du bassin. Les températures, les données isotopiques et l’équilibre massique des sulfures indiquent que le H2S y est lié à la réduction des sulfates par voie thermochimique (Sud de Munich) et par voie bactérienne (Nord de Munich).
Resumen
El conocimiento acerca de las condiciones hidroquímicas del agua subterránea profunda es crucial para el diseño y la operación de las instalaciones geotermales. En este estudio, se evalúa la heterogeneidad hidroquímica del agua subterránea en el acuífero Malm, Alemania, y se proponer las razones de las extraordinariamente altas concentraciones de H2S en la parte central de la cuenca de Bavarian Molasse. Para analizar cationes, aniones, composición y carga de gas se tomaron muestras en 16 sitios, en un total de 37 pozos individuales. Las características hidroquímicas del agua subterránea del Malm en el centro de la cuenca Molasse son bastante heterogéneas. Aunque el agua subterránea en la cuenca central está dominada por aguas meteóricas, hay una significativa infiltración de agua salina desde estratos más altos. Se debe tener cuidado en la interpretación de los datos de los sitios geotermales, ya que los efectos de estimulación química de las perforaciones no pueden ser totalmente removidos antes del análisis final. Se correlacionó la distribución de H2S en la fase gaseosa con la carga de gas del agua que se incrementa en la cuenca central. Las temperaturas, los datos isotópicos y el balance de masa de sulfuro indican que el H2S en la cuenca central está relacionada a la reducción termoquímica del sulfato (sur de Munich) y a reducción bacterial de sulfato (norte de Munich).
Resumo
O conhecimento das condições hidroquímicas das águas subterrâneas profundas é crucial para o projeto e operação de instalações geotérmicas. Neste estudo, a heterogeneidade hidroquímica das águas subterrâneas do aquífero Malm, na Alemanha, é avaliado, e são propostas razões para as concentrações extremamente elevadas de H2S na parte central da Bacia de Molasso da Baviera. As amostras foram colhidas em 16 locais, para um total de 37 furos individuais, para analisar catiões, aniões, a quantidade de gás e a sua composição. As caraterísticas hidroquímicas da água subterrânea do Malm, no centro da Bacia de Molasso, são bastante heterogéneas. Embora a água subterrânea na bacia central seja dominada pelas águas meteóricas, existe uma infiltração significativa de água salina a partir de camadas superiores. E necessário ter cuidado na interpretação dos dados provenientes de sítios geotérmicos, visto que os efeitos da estimulação química dos furos não podem ser completamente removidos antes das análises finais. A distribuição de H2S na fase gasosa está correlacionada com a carga de gás da água, que aumenta na bacia central. Temperaturas, dados isotópicos e o balanço de massa de enxofre indicam que o H2S na bacia central está relacionado com a redução termoquímica do sulfato (a sul de Munique) e com a redução bateriana de sulfato (a norte de Munique).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background
Geothermal exploration of the Malm aquifer in the Bavarian Molasse Basin is very active with a focus on the area around Munich where nine geothermal projects are already operating successfully and a dozen new projects are in progress. The geothermal wells extend into the Malm aquifer which is located between 1,500 and 5,500 m below ground surface. Typical production rates of the geothermal wells are in the range of 30–150 L/s and the production temperatures reach up to 160 °C. There was no a priori information about the hydrochemical conditions as the geothermal wells were, and still are, the first sampling points in this area. Nevertheless, the hydrochemical composition of the Malm groundwater is as important as the hydraulic conditions, because of precipitation and corrosion issues, and safety concerns arising from the presence of significant gas concentrations; therefore a screening and monitoring program was launched with financial support from the German Federal Ministry for the Environment, Nature Conservation and Nuclear Safety. Within the scope of this study, five geothermal projects with nine wells in the central basin underwent detailed hydrochemical and isotopic analysis and gas phase characterization.
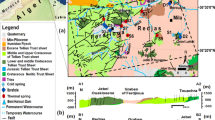
The Bavarian Molasse Basin is a sedimentary foreland basin located in South Germany (see marked area in Fig. 1). One hundred and fifty million years ago in the Late Jurassic a large sea-level rise flooded the southern region of Germany. In this marine environment, different types of carbonates were deposited. Compact and layered carbonates were formed in lagoons and flat settings, whereas reef or compacted limestones were built of reef debris. At the end of the Jurassic, the sea level fell and the carbonate sediments where partly exposed at the land surface during the Cretaceous leading to karstification. In the Tertiary, the Molasse Basin was formed as a result of the Alpine orogenesis and the eroded debris was deposited there. The western part of the Molasse Basin is dominated by terrestrial sedimentation, whereas the eastern part has a mainly marine character (Bayer. Geol. Landesamt 1996).
Overview of the study area (regions redrawn from Birner et al. 2011)
From the northern margin to the central basin at the edge of the Alps, the basin depth reaches up to 6,000 m. The main target for geothermal exploration in the Bavarian Molasse Basin is the upper Malm formation. It is a carbonate-karstified deep groundwater aquifer which dips to the south and attains a vertical thickness up to 600 m in the Southern Franconian Alb and beneath the Southern Molasse sediments (Prestel et al. 1991; see schematic cross-section in Fig. 2).
The composition of the Malm thermal water in the Molasse Basin changes from the margin regions to the central basin. The groundwaters in the recharge regions at the margin of the basin are dominated by young meteoric water. It has been shown that infiltration of waters from upper formations increases with increasing aquifer depth in the western part of the Molasse Basin (Prestel et al. 1991). Here, the concentration of total dissolved solids (TDS) shows an increasing trend from north (meteoric water) to south (meteoric water with local supply of higher mineralized waters). An increase of TDS has also been postulated for the central basin. Furthermore, based on the hydrochemical characterization of the Malm groundwater close to the River Danube and in the Eastern Molasse Basin, the following hypotheses about the hydrochemical characteristics have been postulated:
-
Sulfate concentrations decrease from the basin margins to the central basin (Prestel et al. 1991).
-
The Malm groundwater is a mix of meteoric water and formation water seeping from the overlying sediments (Hesse and Schmidt-Thomé 1975; Prestel et al. 1991; Udluft 1975; Villinger 1977).
-
Waters with the hydrochemical character of the Malm groundwater in the central basin have also been observed at the northern basin margin (Prestel et al. 1991). This process is explained as an inflow of groundwater at faults and fissures from the central part (Baumann and Quentin 1981).
-
The general flow regime relevant for the study area is from the recharge areas in the northwest to the central basin near Munich and from there to the southeast (Wagner et al. 2009).
The first analyses from the geothermal wells in the central basin south of Munich in this study showed a hydrochemical composition which did not only contradict some of these hypotheses, but also revealed a high gas loading up to 0.5 L gas per L of groundwater. The main components of the gas phase are methane, carbon dioxide, and nitrogen. The concentrations of H2S in the gas phase reached a surprisingly high level in the percent range. Not only is H2S a severe risk to human health but also a critical component with respect to technical safety as it causes corrosion in steel pipes, heat exchangers and other materials (Macdonald et al. 1978; Morris et al. 1980). Initially, given that H2S concentrations in this range were considered very unlikely for groundwater, impregnation of the thermal water with sour gas from a gas reservoir was assumed to be the cause for the high concentrations of H2S in the gas phase. Consequently, additional safety measures, like preventers, would have been required.
However, impregnation with sour gas, regardless of its origin, is only one possible mechanism leading to high concentrations of H2S in the gas phase. The key question raised at the outset of this study was, whether the geothermal wells are in contact with a small gas reservoir containing sour gas or if the high concentrations of H2S in the gas phase are the result of sulfate reduction in the aquifer, leading to high H2S concentrations in the aqueous phase, followed by degassing and stripping of H2S from the thermal water during production.
The processes which have to be considered as a source for H2S in the Malm aquifer are thermal decomposition of organic sulfur, dissolution of pyrite, bacterial sulfate reduction (BSR), and thermochemical sulfate reduction (TSR).
During acidic stimulation of the wells, pyrite can be dissolved from the Malm limestones. As the pyrite in limestones is known to have δ34S values < 0 (Clark and Fritz 1997), H2S generated by dissolution of pyrite is characterized by depleted δ34S values in the range of −10 to −25 ‰ (Schulte et al. 1997). Thermal decomposition of organic sulfur compounds in kerogen or oil requires reservoir temperatures greater than 200 °C (Krouse et al. 1987). The temperatures in the study area are well below 200 °C; therefore, this explanation was not pursued.
BSR can be described by a simplified net reaction (Nöth 1997):

Here CH3O is representative of organic matter like humic substances, organic acids, hydrocarbons, or solid organic matter. Sources of dissolved sulfate can be connate waters, anhydrite dissolution, buried seawater or evaporitic brines, decomposition of organosulfur compounds, and/or dissolution of sulfur-bearing mineral deposits. BSR is an instantaneous reaction, mass limited with respect to sulfate and organic matter, and, under typical reservoir conditions, mainly transport limited with respect to the sulfate transport to the bacteria (Berner 1980). Furthermore, bacteria can reduce sulfate only as long as the H2S concentration is below the toxicity level for bacterial metabolism (Reis et al. 1992). BSR is a common process at lower reservoir temperatures and produces H2S concentrations in the gas phase in the range of 3–10 %. While most sulfate-reducing bacteria prefer temperatures up to 80 °C (Machel 2001; Orr 1977), some hyperthermophilic sulfate-reducing bacteria even exist at temperatures up to 110 °C (Jörgensen et al. 1992); however, presumably with limited activity. The isotopic composition of H2S produced by BSR is strongly dependent on the precursor sulfate and yields a wide range of δ34S–H2S values between +20 and −40 ‰ (Emery and Robinson 1993). At the northern basin margin Desulfovibrio desulfuricans was detected in groundwaters with temperatures in the range from 10 to 60 °C (Baumann and Quentin 1981). A recent study in the central basin revealed the presence of Desulfococcus, Thermodesulforudis, and Desulfotomaculum. The latter was detected even at groundwater temperatures above 100 °C (Alawi et al. 2011); however, the presence of both, sulfate and sulfide, at this site points to a greatly reduced activity of the bacteria.
Thermochemical reduction of sulfate (TSR) starts at temperatures in the range of 100–140 °C. The reaction for TSR is outlined in Eq. (2) (Goldhaber and Kaplan 1974; Machel 1987; Machel et al. 1995; Nöth 1997; Orr 1977):

where R– stands for high molecular weight alkanes, alkenes, and methane. TSR reaction rates are controlled by the temperature, the concentration and type of reactive organic material (gaseous, oil, solid), and the concentration of sulfate. As Liu et al. (2013) point out based on their previous work, minimum temperatures are higher (> 140 °C) for TSR by gas compared to > 110 °C for TSR by oil. High H2S concentrations are often associated with methane-rich gases (Orr 1982). The sources for sulfate are the same as for BSR. Reactive organic material can be sedimentary organic matter, dissolved organic carbon, crude oil, microbial methane, thermogenic gas and/or gas condensate. Furthermore, the composition of the total gases, catalysts, sulfate availability and migration/diffusion rates of the major reactants play a role for the rate of TSR (Machel 2001). As the porosity of the reservoir indirectly controls the TSR rates dolomite reservoirs (e.g. Sichuan and Ordos Basin, China) usually show higher H2S concentrations than limestone reservoirs because of a higher matrix porosity in dolomites. In addition, Mg2+ can catalyze TSR (Zhu et al. 2007a).
The products of TSR according to Eq. (2) are H2O, H2S/HS−, HCO3 −/CO2, and organic sulfur compounds. The relative proportions of H2S/HS− and HCO3 −/CO2 highly depend on pH. The dissociation reactions and dissociation constants (Ka) at 25 °C are given in Eq. (3) (Perrin 1982):
TSR produces H2S concentrations in the gas phase up to 90 %. Data from deep buried carbonate reservoirs in the Alberta Basin in Canada show a δ34S–H2S of 20.1 ± 1.5 ‰, whereas the corresponding anhydrite showed a range of 22.7–33 ‰ (Emery and Robinson 1993). In laboratory experiments the observed kinetic isotope fractionation by TSR was close to zero; consequently, the isotopic composition of produced H2S is similar or a few per mil lighter than precursor sulfate (Krouse et al. 1988; Orr 1977). Orr (1974) indicated that as a result of TSR the H2S content increases with increasing temperature, after examining isotopic measurements of Big Horn paleozoic oils in Wyoming, USA. Furthermore, the sulfur isotopic composition of reduced organic and inorganic sulfur changed towards that of reservoir sulfate. In other field studies, higher fractionation factors were observed. Zhu et al. (2007b) determined in the Weiyuan Gas Field, Sichuan Basin, China typical isotope fractionation values for TSR of 8–12 ‰, which are comparable to the fractionation values in the Alberta Basin. Wynn et al. (2010) recorded isotopic fractionation values up to 53 ‰ in Cerna Valley, Romania, which are much higher than expected for TSR. This was attributed to incomplete TSR a unique hydrogeologic setting, and a contribution of BSR along the flow path.
Finally, kinetic isotope fractionation, although variable, yields ratios of −25 to −50 ‰ (Goldhaber and Kaplan 1974); however, the rates for isotopic exchange between dissolved sulfide and sulfate is slow at temperatures below 200 °C (Emery and Robinson 1993). Despite all differences, both the isotopic ratio and isotope fractionation depend on the precursor sulfate and change along the reaction path in a closed system; therefore, an interpretation based on isotopic data alone carries some ambiguity.
This study connects hydrochemical data, gas analyses and isotope analyses to bring new insights in the generation processes of H2S in the Malm aquifer. It also introduces new data on the hydrochemical characteristics, especially for the central part of the Molasse Basin where no groundwater wells had been previously available, to reduce the uncertainty for geothermal exploration.
Materials and methods
Water sampling
Samples were taken at the well head. When the temperatures were above 98 °C, the samples were taken using a cooler, or after the heat exchanger if available. The water temperature, pH, redox potential (Eh), and electrical conductivity (EC) were measured with pH 330 (WTW), Redox SenTix ORP chain (Ag/AgCl 3.5 mmol KCl), and LF 92 (WTW) on-site. Chloride, iodide, and bromide were measured using ion chromatography with EC detector (IC 25, IonPac AS9-HC column, Dionex). Sodium and potassium were measured using atomic emission spectrometry (ELEX 6361, Eppendorf). Calcium was titrated with EDTA, and magnesium was measured using flame atomic absorption spectrometry (AAS 3300, Perkin Elmer). Dissolved sulfides in water (S2−, HS– and H2S) were precipitated on-site with 10 mL zinc acetate (2 %). The concentration of the precipitated zinc sulfide was measured with the methylene blue colorimetric method (Deutsches Institut für Normung 1995) using a spectrophotometer (Double Beam UV-190, Shimadzu). Analytical errors were determined using six samples taken at site 4 collected within 20 min. For cations and anions the analytical error is between 2 and 11 %.
Gas sampling
Gas samples were collected with two different methods. For the first method, a constant flow of thermal water was established through an on site gas stripping column made of polypropylene. The pressure inside the stripping column was established at 10 hPa above atmospheric pressure. The gas loading was calculated by dividing the volume of gas inside the semi-transparent column by the measured volume of thermal water which passed through the column. The separated gas phase was transferred into two glass gas sampling tubes (Witeg). Samples for analysis were taken out of the gas sampling tubes.
Additionally, and at lower gas loadings, the thermal water was transferred into 0.4-L double-ended stainless steel containers at a pressure of about 0.4 MPa. Here, the thermal water was first flushed through the container, then the outlet valve was closed first, followed by closing the inlet valve. For analysis, this container was connected to an evacuated glass bottle. The pressure after connecting the two containers was recorded to calculate the gas loading. Samples for analyses were taken out of this glass bottle.
A full analysis of the gas phase was performed at five sites in the study area. The gas analysis was performed by IGU Dr. Salvamoser, Wörthsee, Germany, using gas chromatography with a thermal conductivity detector (N2, O2, CO2) and a flame ionization detector (CH4, C2H4, C2H6, C3H8, C4H10). As interactions of H2S with the stainless steel container are very likely, no H2S was determined for the samples from the stainless steel containers. Partial pressures were calculated using PHREEQC (Parkhurst and Appelo 1999) based on the full gas analyses.
For the quantification of H2S, the gas sampling tubes were flushed with nitrogen (≥ 99.9 % N2) and passed into three gas washing bottles (NS 45/40, Schott/Duran), which were filled with 60 mL zinc acetate (2 %) each. The concentration of sulfides was measured as described in the preceding.
The error of the relative composition of the gases with GC is on the order of 2 % and below 15 % for the determination of H2S. The variation in gas volume was below 15 % for the on-site stripping column and less than 10 % for the analyses out of the stainless steel containers.
Stable isotope measurements
Stable isotope measurements were performed by IGU Dr. Salvamoser, Wörthsee, Germany, at the five sites with complete gas analyses. For measuring 34S in sulfate, barium chloride was added to the water samples to precipitate dissolved sulfate as barium sulfate. Barium sulfate was then decomposed at high temperature to form SO2 for isotope analysis in a mass spectrometer (Bailey and Smith 1972). To determine 34S in sulfides, the water samples were prepared with zinc acetate immediately after sampling. The dissolved hydrogen sulfide was precipitated as ZnS. For analysis, the samples were acidified to produce H2S, which was transferred into a AgNO3 solution to form Ag2S, which later was oxidized at high temperatures to form SO2 for mass spectrometry analysis. 18O in water was determined with mass spectrometry (Epstein and Mayeda 1953). To measure 2H, the water sample was reduced in a uranium furnace at 800 °C (Coleman et al. 1982; Friedman 1953) and measured using a mass spectrometer. Results of the isotopic analyses are given relative to the Diablo Canyon Iron Meteorite scale (CDT, 34S) and the Vienna Standard Mean Ocean Water (V-SMOW, 18O and 2H). Analytical errors provided by the laboratory were below 5 % of the given values.
Results and discussion
General hydrochemical characteristics
The data presented in this study include 429 groundwater samples obtained at 37 wells, with 1–70 samples per well, and taken over a time period of 7 years. An overview of the hydrochemical characteristics is given in Table 1. The classification into regions follows the idea of Birner et al. (2011) who divided the Bavarian Molasse Basin into six different hydrochemical provinces: the central basin (region I, sites 1–8), the Lower Bavarian Basin, (II; sites 9–11), the northern basin margin (III, sites 12–13), north-eastern basin (IV, site 14), the Landshut-Neuötting High (V, site 15) and the south-western basin (VI, site 16). For each region the median value and the interquartile range (IQR; 50 % of the data around the median falls within these values) are given. Not all of the regions have an equal number of wells and samples. Therefore, an unconditional statistical evaluation would be biased. In this study, the data of region I mainly reflect the spatial heterogeneity, while data from region VI nicely show the temporal variability of the hydrochemical conditions at one single well.
Samples taken during the initial pumping tests of freshly installed geothermal wells turned out to be heavily influenced by the drilling process and acidic stimulation. These samples are characterized by a pH-value below the average and an increase of the concentrations of calcium, magnesium and chloride. In this study, data affected by stimulation are not included in Table 1 or any other evaluation except Fig. 3. However, it is noted that some of these data, especially for those wells which are used as reinjection wells later on, are the only data available.
The temporal development of the hydrochemical conditions is shown in Fig. 3 for site 4. From the first pumping test of the well (a) to the second test (b) to the long-term equilibrium after 6 years of operation there is a decrease in the concentrations of calcium and magnesium by almost two orders of magnitude. The concentrations of chloride decrease by a factor of 10 and the concentrations of sulfate decrease by a factor of 5. The concentrations of bicarbonate are almost constant over the complete history of this well. This effect can be explained by the chemical stimulation of the well. After reaching the final depth, and an initial lift test to remove the drilling mud, HCl was injected into the well to remove traces of the drilling mud and to open the fractures. It dissolved the carbonate rock causing an increase in the concentrations of calcium, magnesium, and CO2. At site 4, an equilibrium was reached with a standard deviation of less than 3 % only after 2 years of full operation.
The reservoir temperatures are dominated by the geothermal gradient and the flow regime in the aquifer. It has to be pointed out that most of the temperatures measured in this study were recorded at the well head. Due to significant heat loss in the well, especially during the initial pumping tests when the area surrounding of the borehole is still at ambient temperatures (Tilley and Baumann 2012), the reservoir temperatures are expected to be up to 10–15 °C higher.
In the central basin and in the south-western basin, reservoir temperatures of 90 to 140 °C are expected with increasing temperatures to the south. At the Lower Bavarian Basin and the Landshut-Neuötting High the temperatures are in a range of 50–70 °C. Reservoir temperatures of 20–30 °C are characteristic at the northern basin margin and at the north-eastern basin margin.
The pH values of the groundwater in the central basin are around 7.0 (IQR 6.4–7.5). Lower pH values were measured in the south-western basin (6.8) and higher pH values were measured in the Lower Bavarian Basin, northern basin margin and north-eastern basin (7.3/7.4). IQRs for all regions except the central part are on the order of 0.2 pH units. The EC values in the central basin show a median of 1,500 μS/cm which is comparable to the north-eastern basin and the Lower Bavarian Basin. The IQR of 830–3,100 indicates a large spatial variation. Groundwater at the northern margin shows significantly lower EC. Site 16 in the south-western basin shows an EC value of 8,100 μS/cm, suggesting an influence of saline waters. The decreasing redox potential towards the central basin has been postulated and is consistent with hypotheses of Prestel et al. (1991). Groundwaters with low EC are generally dominated by calcium, magnesium and bicarbonate (Ca−Mg−HCO3-type). This composition reflects the limestones and dolomites forming the Malm aquifer. Increasing EC is caused by an increase of the concentrations of sodium, chloride, and sulfate. However, only chloride reaches relative concentrations above 20 %, thus characterizing these waters as Ca−Mg−Cl−HCO3-type. This indicates the infiltration of highly mineralized waters from higher strata as suggested by Lemcke (1976) and reflects a downward oriented flow in the lower Tertiary sediments (Andres and Frisch 1981).
The hydrochemical characteristics of the groundwater in the different regions are summarized in a Piper diagram in Fig. 4. The groundwater type shows little variation within most parts of the study area, indicating homogeneous lithostratigraphic groundwater flow conditions. As most of these wells have been in operation at constant pumping rates for more than 20 years, an equilibrium between the different sources contributing to the well has been established.
Waters from the Lower Bavarian Basin are characterized by higher sodium and potassium concentrations originating from the exchange of calcium and magnesium with sodium at clay mineral exchange sites in the overlying Tertiary sediments (Na−HCO3−Cl type) as numerous analyses from the Tertiary aquifers in this area show. Samples from the northern basin margin show two different hydrochemical types. Samples from wells 12a, 12c, 13a, 13b, 13d, 13e and 13g show related characteristics to wells 9, 10 and 11 in the Lower Bavarian Basin and can be classified as Na−HCO3−Cl waters. The other hydrochemical group (well 12b, 13c and 13f) is characterized by lower potassium and chloride concentrations as well as higher bicarbonate concentrations. These wells characterize a Ca−Na−Mg−HCO3 water type. The general water type of site 14 in the north-eastern basin is Na−Cl−HCO3/Na−Ca−Mg−HCO3. Waters from well 15 at the Landshut-Neuötting High show a Na−Ca−HCO3−Cl type. Site 16 in the south-western basin shows an extraordinary composition with a TDS of 5.8 g/L and high concentrations of natural gas and hydrocarbons. This is unusual for Malm groundwater and cannot be explained with an interaction with the limestone alone. As this well is close to an oil and gas field, the groundwater is possibly affected by oilfield waters.
The situation in central basin, however, is more complicated. A high spatial heterogeneity was observed. In general, groundwaters of Ca−Mg−HCO3 type dominate. Infiltrating waters are richer in sodium and chloride (Carlé 1975; Lemcke 1988) and might change the groundwater type. Lower sodium, chloride and sulfate concentrations were observed north of Munich which suggests less infiltration compared to the deeper groundwater south and south–southeast of Munich.
The main constituents of the Malm aquifer are limestone and dolomite, which are reflected in the calcium-to-magnesium ratio. The Ca/Mg ratios in the groundwater in the central Malm aquifer are in the range of 0.8 to 8.5 with a median of 3.8. Ca/Mg > 2.2 represents water in contact with limestone, while Ca/Mg < 2.2 indicates water in contact with dolomites (Langmuir 1971). Lower ratios are observed at the northern basin margin and high ratios in the central basin and in the Lower Bavarian Basin.
Isotopic signature of the Malm groundwater
Figure 5 shows the ratio of δ 2H to δ18O, and the global (GWML; Craig 1961) and local meteoric water line (LMWL). The LMWL is slightly shifted to lower δ2H values reflecting both, the latitude and continental effects. Most of the groundwaters are situated around the LMWL for Middle Europe, indicating meteoric waters as primary source. In the central basin the values of δ18O are between −10.4 and −11.9 ‰ and the values of δ2H are from −79.9 to −86.7 ‰. The δ18O and δ2H values reflect the isotopic fractionation during evaporation and precipitation. Low values indicate low ambient temperatures and are usually assigned to the last ice age (Pleistocene). For instance the groundwater in the Styrian basin (Austria) shows δ2H values of < −70 ‰ and was assigned to generation temperatures of 4.6 °C (Goldbrunner 1997). Another study puts temperatures of 5 °C to waters with isotopic signatures of δ2H between −95 and −75 ‰ and δ18O between −12.8 and −10.6 ‰ (Stichler et al. 1987). Consequently, the Malm groundwater can be assigned to the same climatic conditions and be considered of Pleistocene Age with a minimum age of 8,000–12,000 years.
δ2H/δ18O ratio at five sites in the central basin (base graph from Domenico and Schwartz 1998)
The isotopic signature of the groundwater at site 16 clearly deviates from the LMWL with δ18O of values of −1.9 ‰ and δ2H of −58 ‰, indicating an influence of formation waters. Together with the high gas loading, the hydrochemical characteristics and high concentrations of hydrocarbons, a connection to an oil reservoir seems likely, although the nearest oil field is at 20 km distance and located in the Tertiary sediments. In the Munich area, site 4 shows slightly higher δ18O values than the rest (10.9 ‰), supporting the assumption of an influence of higher mineralized waters from the superstructure.
Gas composition in the Malm aquifer
The results of the screening of the gas composition at eight geothermal wells at five sites in the central basin are given in Table 2. Here, gas samples were taken after acidic stimulation, during initial and long-term pumping tests, and during operation.
The total gas volume per liter of thermal water in the central basin is in the range of 4.0–900 NmL/L with a median of 70 NmL/L and an IQR from 41 to 113 NmL/L. Under reservoir conditions, all gases are dissolved; however, with partial pressures up to 1.5 MPa in the central basin, degassing during production is likely to occur. At low gas concentrations, CO2 dominates the gas phase. The concentration of CO2 matches the carbonate equilibrium; excess CO2 was detected only after acidic stimulation. With increasing gas concentrations, the fraction of methane increases, while the fraction of N2 is rather constant with an IQR of 11–27 vol-%. There is a general trend to lower concentrations of H2S with increasing gas loading. This suggests a mass limited process as source of H2S in the gas phase which is consistent with the hypotheses of H2S being stripped from the thermal water during production. On the other hand, this contradicts an impregnation with sour gas from a gas reservoir, which would yield of a constant concentration of H2S in the gas phase.
The average concentrations of all sulfur species in gas phase and water are given in Fig. 6 for each of the 16 geothermal sites in the study area. With pH values between 6.8 and 7.8 the ratio between HS– and H2S is between 67:33 and 95:5 at 120 °C. However, only H2S will be stripped from the thermal water, thus causing the sulfur species to reequilibrate and eventually leading to a complete stripping of S(−II) from the thermal water.
The highest sulfur concentrations were measured in the south-western basin at site 16, where sulfate dominates with 5.6 mmol/L. Waters from the Lower Bavarian Basin, the north-eastern basin and the Landshut-Neuötting High generally show lower sulfur concentrations. Total H2S concentrations are highest in the central basin, followed by the northern basin margin, an area where several springs contain dissolved H2S and which is known for its Roman spas. The measured total H2S concentrations and especially the H2S concentrations in the gas phase show a high variance. The highest concentrations of H2S in the gas phase were observed in central basin with up to 170 μmol/L or 8.7 vol %. Outside the central basin, H2S concentrations in the gas phase are low to negligible, reflecting the lower temperatures and low total gas concentrations.
An assessment of the short and long-term variations of the H2S concentrations in the gas phase was performed at site 4 in 2009 and 2010. This site has been in full production at an average production rate of 32 L/s since 2005. There was a decrease of the H2S concentrations from 885 μmol/L in 2006/2007, to an average of 80 μmol/L in 2010 (see Fig. 7a). During this time, the gas concentrations remained in the same range at an average value of 190 ±80 NmL/L. Also the sulfur speciation in water shows little variability. The H2S concentration is above average in 2006/2007 (see Fig. 7b).
While the monitoring in 2009 indicates that the H2S concentrations might depend on the flow rate, the 2010 monitoring campaign did not support this assumption. Rather it seems that the H2S concentration at this site is mainly controlled by natural short-term variations in the gas loading.
The effect of the geological facies on the H2S concentration is shown in Fig. 8, where the calcium/magnesium ratio is plotted against the H2S concentration, if both values were measured. Although magnesium catalyzes TSR and a higher porosity in dolomites might support the production of H2S by TSR (Zhu et al. 2007a), there is no clear trend in the data for the central basin.
Figure 9 shows the relation between the ratio of S(−II) to total sulfur and the partial pressure of methane in MPa. With increasing partial pressure of methane there is an increasing fraction of sulfides. This supports TSR as outlined in Eq. (2), but also indicates that a thermodynamic equilibrium has not been reached. This might indicate that infiltration is faster than TSR under the given conditions.
The values of δ34S−H2S in the central basin are in a range of +3.2 to +9.7 ‰ and the values of δ34S−SO4 2− range from +8.5 to +20.8 ‰. Isotopic fractionation values for δ34S−SO4 2− – δ34S−H2S were calculated and are between 3.4 and 17.6 ‰. Thus, both processes, TSR and BSR, are possible and the temperature regime seems to control the sulfate reduction process. Production of H2S by dissolution of pyrite during acidic stimulation can be ruled out because the values of δ34S−H2S from sedimentary pyrite in limestone are expected to be < 0.
Conclusions
The hydrochemical conditions in the Malm aquifer in the Bavarain Molasse Basin can be mainly characterized as the result of meteoric waters interacting with the carbonates that make up the Malm aquifer. The hydrochemical analyses, although showing a great degree of variation, generally mirror the lithostratigraphic setting. This implies slow groundwater movement in the Malm aquifer in order to reach equilibrium conditions. In the central basin, mixing with groundwater from higher strata leads to an increase of the concentrations of sodium and chloride. Stable isotopic analyses for the central basin suggest a traveltime from the recharge areas to the central basin around Munich of at least about 10,000 years. There are two exceptions, one located in the central basin south of Munich and one in the western part of the basin. The site south of Munich is influenced by waters with higher mineralization, infiltrating from the superstructure. The site in the south-western basin is most likely influenced by oil field waters. It has to be understood that the hydrochemistry of a geothermal well cannot be determined from the first pumping tests if acidic stimulation has previously taken place. Data also suggests that it might take up to 2 years of full operation to establish a hydrochemical equilibrium. Gas loadings seem to decrease during operation, but monitoring data are not yet sufficient to predict short-term and medium-term variation.
The highest sulfide concentrations in water and H2S concentrations in the gas phase were measured in the central basin. Prestel et al. (1991) assumed a trend of decreasing sulfate concentrations to the central basin with increasing sulfide concentrations. This hypothesis has been generally supported by the data obtained in this study. However, infiltrating waters may lead to a coexistence of sulfate and sulfide. The fractionation values for δ34S suggest incomplete reduction of sulfate to sulfide in the southern central part, because the isotopic signatures of δ34S−H2S and δ34S−SO4 2− differ. TSR and BSR occur in two mutually exclusive thermal regimes (Machel 2001; Nöth 1997; Orr 1977). Based on the results of this study, TSR is expected to be the H2S generating process in the southern part of the Bavarian Molasse Basin, because the reservoir temperatures exceed the possible range for BSR of almost 110 °C. TSR is also known for the generation of higher H2S concentrations compared to BSR. Indications for TSR in the central basin can also be detected in the relation between the methane partial pressure and the H2S concentrations. For low temperature settings like the Lower Bavarian Basin, northern basin margin, north-eastern basin, Landshut-Neuötting High and the south-western basin BSR is supposed to be the H2S producing mechanism. This is in agreement with results at the northern basin margin, where reduction of sulfate to sulfide by bacteria was confirmed by the analysis of pyrite-marcasite-geodes (Baumann and Quentin 1981). Production of H2S by degradation of organic sulfur compounds to sulfides is not likely in the Malm aquifer, because the required temperatures of 200–300 °C are not reached even in the deepest parts of the Malm aquifer close to the Alps. Finally, the results of this study rule out the impregnation of the waters in the Malm aquifer with sour gas from a natural gas reservoir. Instead, the results indicate that the production of H2S by TSR in the central basin is limited by the availability of methane and the rate of infiltrating sulfate from higher strata relative to the production rate. Both parameters will decrease during production when the system shifts from an equilibrium state to a kinetically controlled state. The decreasing salinity, gas loadings and H2S concentrations during production support this conceptual model.
References
Alawi M, Lerm S, Vetter A, Wolfgramm M, Seibt A, Würdemann H (2011) Diversity of sulfate reducing bacteria in a plant using deep geothermal energy. Grundwasser 16:105–112
Andres G, Frisch H (1981) Hydrogeologie und Hydraulik im Malmkarst des Molassebeckens und der angrenzenden Fränkisch-Schwäbischen Alb [Hydrogeology and hydraulics in the Malm karst in the Molasse basin and the Swabian-Frankonian Alb]. Schriftenr Bayer Landesamt Wasserwirtsch 15:108–117
Bailey AA, Smith JW (1972) Improved method for the preparation of sulfur dioxide from barium sulfate for isotope ratio studies. Anal Chem 44(8):1542–1543
Baumann M, Quentin KE (1981) Hydrochemische Ergebnisse und Beurteilung des Thermal- und Schwefelwassers [Hydrochemical results and evaluation of the thermal and sulfuric waters]. Schriftenr Bayer Landesamt Wasserwirtsch 15:117–125
Bayer Geol Landesamt (ed) (1996) Erläuterungen zur Geologischen Karte von Bayern 1:500000 [Explanation to the geological map of Bavaria 1:500000]. Bayer. Geol. Landesamt, Munich, Germany
Berner RA (1980) Early diagenesis. Princeton University Press, Princeton, NJ
Birner J, Mayr C, Thomas L, Schneider M, Baumann T, Winkler A (2011) Hydrochemie und Genese der tiefen Grundwässer des Malmaquifers im bayerischen Teil des süddeutschen Molassebeckens [Hydrochemistry and evolution of deep groundwaters in the Malm aquifer in the Bavarian part of the South German Molasse Basin]. Z Geol Wiss 39:291–308
Carlé W (1975) Die Mineral- und Thermalwässer von Mitteleuropa [Mineral and thermal waters of Central Europe]. Wiss. Verlagsges., Stuttgart, Germany
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC, New York
Coleman ML, Shephard TJ, Durham JJ, Rouse JE, Moore GR (1982) Reduction of water with zinc for hydrogen isotope analysis. Anal Chem 54:993–995
Craig H (1961) Isotopic variation in meteoric waters. Science 133:1702–1703
Deutsches Institut für Normung (1995) DIN 51855–4: Prüfung von gasförmigen Brennstoffen und sonstigen Gasen - Bestimmung des Gehaltes an Schwefelverbindungen - Teil 4: Gehalt an Schwefelwasserstoff, Zinkacetat-Verfahren [Testing of gaseous fuels and oother gases: determination of sulphur compounds content, part 4—content of hydrogen sulfide, zinc acetate method]. Beuth, Berlin
Domenico PA, Schwartz W (1998) Physical and chemical hydrogeology. Wiley, New York
Emery D, Robinson A (1993) Inorganic geochemistry: applications to petroleum geology. Blackwell, Oxford
Epstein S, Mayeda T (1953) Variation of 18O content of waters from natural sources. Geochim Cosmochim Acta 4:213–224
Friedman I (1953) Deuterium content of natural waters and other substances. Geochim Cosmochim Acta 4:89–103
Goldbrunner JE (1997) Vergleich von Isotopenuntersuchungen an Tiefengrundwässern des Steirischen Beckens und des Oberösterreichischen Molassebeckens [Comparison of the isotopic data of deep groundwater in the Styrian Basin, Austria, and the Upper Austrian Molasse Basin]. Mitt Österr Geol Ges 88:31–39
Goldhaber MB, Kaplan IR (1974) The sea. Wiley, Chichester, UK, pp 569–665
Hesse R, Schmidt-Thomé P (1975) Neue Jodwasservorkommen im Bereich der bayerischen Alpenrand-Strukturen bei Bad Tölz [New iodine groundwater resources at the alpine structures near Bad Tölz, Bavaria, Germany]. Geol Jahrb C 11:31–66
Jörgensen BB, Isaksen MF, Jannash HW (1992) Bacterial sulfate reduction above 100 °C in deep sea hydrothermal vent sediments. Science 258:703–704
Krouse HR, Ritchie RGS, Roche RS (1987) Sulphur isotope composition of H2S evolved during the non-isothermal pyrolysis of sulphur-containing materials. J Anal Appl Pyrol 12:19–29
Krouse HR, Viau AA, Eliuk LS, Ueda A, Halas S (1988) Chemical and isotopic evidence of thermochemical sulphate reduction by light hydrocarbon gases in deep carbonate reservoirs. Nature 333:415–419
Langmuir D (1971) Geochemistry of some carbonate ground waters in central Pennsylvania. Geochim Cosmochim Acta 35:1023–1045
Lemcke K (1976) Übertiefe Grundwässer im süddeutschen Alpenvorland [Excess depth groundwater in Wouth Germany]. Bull Ver Schweiz Petroleum-Geol Ing 42:9–18
Lemcke K (1988) Geologie von Bayern, I: Das bayerische Alpenvorland vor der Eiszeit [Geology of Bavaria, I: the Bavarian Alpine foothills before the Quaternary]. Schweizerbart, Stuttgart, Germany
Liu QY, Worden RH, Jin ZJ, Liu WH, Li J, Gao B, Zhang DW, Hu AP, Yang C (2013) TSR versus non-TSR processes and their impact on gas geochemistry and carbon stable isotopes in Carboniferous, Permian and Lower Triassic marine carbonate gas reservoirs in the Eastern Sichuan Basin, China. Geochim Cosmochim Acta 100:96–115
Macdonald DD, Roberts B, Hyne JB (1978) Corrosion of carbon steel during cyclical exposure to wet elemental sulphur and the atmosphere. Corros Sci 18(5):499–501
Machel HG (1987) Some aspects of diagenetic sulphate-hydrocarbon redox-reactions. In: Marshall J (ed) Diagenesis of sedimentary sequences. Geol Soc Spec Publ 36, pp 15–28
Machel HG (2001) Bacterial and thermochemical sulfate reduction in diagenetic settings: old and new insights. Sediment Geol 140:143–175
Machel HG, Krouse HR, Sassen R (1995) Products and distinguishing criteria of bacterial and thermochemical sulfate reduction. Appl Geochem 10:373–389
Morris DR, Sampaleanu LP, Veysey DN (1980) The corrosion of steel by aqueous-solutions of hydrogen-sulfide. J Electrochem Soc 127(6):1228–1235
Nöth S (1997) High H2S contents and other effects of thermochemical sulfate reduction in deeply burried carbonate reservoirs: a review. Geol Rundsch 86:275–287
Orr WL (1974) Changes in sulfur content and isotope ratios of sulfur during petroleum maturation study of Big Horn basin Paleozoic oils. AAPG Bull 58(11):2295–2318
Orr WL (1977) Geologic and geochemical controls on the distribution of hydrogen sulfide in natural gas. In: Advances in Organic Geochemistry. Enadisma, Madrid, pp 571–597
Orr WL (1982) Rate and mechanism of non-microbial sulfate reduction. GSA, Boulder, CO, 580 pp
Parkhurst DL, Appelo CAJ (1999) User’s guide to PHREEQC (version 2): a computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. US Geol Surv Water Resour Invest Rep 99–4259
Perrin DD (1982) Ionization constants of inorganic acids and bases in aqueous solution. Pergamon, Oxford
Prestel R, Stier P, Jobmann M, Schulz R, Strayle G, Wendebourg J, Werner J, Wolf M, Eichinger L, Salvamoser J, Weise S, Fritz P (1991) Hydrogeothermische Energiebilanz und Grundwasserhaushalt des Malmkarstes im süddeutschen Molassebecken [Hydrogeothermal energy balance and groundwater balance of the Malm karst in the South German Molasse Basin]. Bayer. LfW und LGRB, Munich, Germany
Reis MAM, Almeida JS, Lemos PC, Carrondo MJT (1992) Effect of hydrogen sulfide on growth of sulfate reducing bacteria. Biotechnol Bioeng 40:593–600
Schulte U, Strauß H, Bergmann A, Obermann P (1997) Isotopenverhältnisse der Schwefel- und Kohlenstoffspezies aus Sedimenten und tiefen Grundwässern der Niederrheinischen Bucht [Isotopic ratios of sulfur and carbon species from sediments and deep groundwater in the lower Rhine Bay]. Grundwasser 2:103–110
Stichler W, Rauert W, Weise S, Wolf M, Koschel G, Stier P, Prestel R, Hedin K (1987) Isotopenhydrologische und hydrochemische Untersuchungen zur Erkundung des Fließsystems im Malmkarstaquifer des süddeutschen Alpenvorlandes [Isotope hydrological and hydrochemical investigations of the flow regime in the Malm karst of the South German Alpine foothills]. Z Dtsch Geol Ges 138:387–398
Tilley B, Baumann T (2012) On temperature attenuation in open-loop wells. Renew Energy 48:416–423
Udluft P (1975) Das tiefere Grundwasser zwischen Vindelicischem Rücken und Alpenrand [The deep groundwater between Vindelician Crest and the Alps]. Geol Jahrb C 11:3–29
Villinger E (1977) Über Potentialverteilung und Strömungssysteme im Karstwasser der Schwäbischen Alb [Hydraulic potential and flow regime of the groundwater in the karst of the Swabian Alb]. Geol Jahrb C 18:9–93
Wagner B, Kus G, Kainzmaier B, Spörlein T, Wilferth T, Veit W, Fritsch P, Wrobel M, Lindenthal W, Neumann J, Sprenger W (2009) Erläuterungen zur Hydrogeologischen Karte von Bayern 1:500000 [Explanation to the hydrogeological map of Bavaria 1:500000]. Bayer. Landesamt f. Umwelt, Munich, Germany
Wynn JG, Sumrall JB, Onac BP (2010) Sulfur isotopic composition and the source of dissolved sulfur species in thermo-mineral springs of the Cerna Valley, Romania. Chem Geol 271:31–43
Zhu GY, Zhang SC, Liang YB (2007a) The controlling factors and distribution prediction of H2S formation in marine carbonate gas reservoir, China. Chin Sci Bull 52:150–163
Zhu GY, Zhang SC, Liang YB, Li QR (2007b) The genesis of H2S in the Weiyuan Gas Field, Sichuan Basin and its evidence. Chinese Sci Bull 52(10):1394–1404
Acknowledgements
Financial support by the Federal Ministry for the Environment, Nature Conservation and Nuclear Safety is gratefully acknowledged. We also would like to thank our project partners FU Berlin, Germany (coordinator), Erdwerk GmbH, Munich, Germany, and LfU Bayern, Munich, Germany for their support and D. Kirste for his very helpful suggestions during the review process.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the theme issue “Hydrogeology of Shallow Thermal Systems”
Rights and permissions
About this article
Cite this article
Mayrhofer, C., Niessner, R. & Baumann, T. Hydrochemistry and hydrogen sulfide generating processes in the Malm aquifer, Bavarian Molasse Basin, Germany. Hydrogeol J 22, 151–162 (2014). https://doi.org/10.1007/s10040-013-1064-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10040-013-1064-2