Abstract
This study aimed at characterizing the groundwater flow pattern in a semi-arid agricultural area in northern India crossed by an intermittent monsoon-controlled watercourse, the Najafgarh drain. More specifically, it focused on studying the impact of groundwater recharge from the riverbed to the regional aquifer using hydrogeochemical and isotopic data. Significant hydrogeochemical zonation was observed between the northern, central and southern sides of the drain, linked to different mineralization processes and mixings. Northward from the drain, groundwater was mainly brackish (4.1–23.4 mS/cm), due to dissolution of evaporites (halite and anhydrite). Southward from the drain, mostly fresh groundwater was found (from 0.5 to 2.3 mS/cm), revealing notable cation exchange processes. In the vicinity of the drain (central area), mineralization was intermediate (0.7–4 mS/cm) and groundwater showed low geochemical evolution, supposing a distinct origin. Stable isotopes of water (δ18O, δ2H) confirmed that central groundwater was not a simple mixing between northern and southern groundwater masses, but had a significant component of infiltrated surface water from the drain. Potentiometric data supported these findings and confirmed the contribution of the drain to the recharge of the aquifer, setting up a hydraulic barrier between north and south, despite surface water availability limited to the monsoon season and low hydraulic conductivity of the riverbed. This study demonstrates the value of the geochemical and isotopic analysis of groundwater to characterize groundwater flow pattern in peri-urban agricultural areas, especially surface water–groundwater interactions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Population growth and the development of irrigation-based agriculture, together with uneven distribution of seasonal rainfall, have put severe pressure on water resources, leading in many places worldwide to the overexploitation of groundwater (e.g., Custodio 2002; Massuel et al. 2013; Baudron et al. 2013, 2014). In India, this resource is of critical importance (Massuel et al. 2009; Lorenzen et al. 2010; Perrin et al. 2011; Brindha and Kavitha 2014; Katpatal et al. 2014; Murkute 2014; Dwivedi et al. 2015). In the southwestern district of the Indian capital Delhi and its neighboring state Haryana, water supply management faces two major problems: limited groundwater resources affected by salinity ingress and erratic availability of surface water.
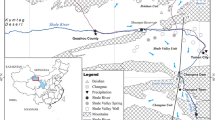
The Najafgarh drain (Fig. 1), an intermittent water channel flowing eastward until reaching the Yamuna River in Delhi, is not integrated as a water source because of the uneven annual distribution of water, linked to the monsoon. In the end, the fresh water it carries during the annual monsoon discharge flows towards the city of Delhi where it is polluted by raw sewage. Why not make this discharge usable before it is polluted? Well injection techniques (e.g., aquifer storage and recovery) or in-channel modifications (e.g., check dams) could be promising ways to enhance recharge for groundwater replenishment and quality improvement.
However, no specific infrastructure was available for long-term surface and groundwater sampling and monitoring, and the intermittent regime of the Najafgarh drain appeared to be a serious limitation for a consistent recharge from the drain to the aquifer. In addition, the riverbed and the banks were shown to have a limited hydraulic conductivity, which restricts the infiltration potential (Baudron 2008). Therefore, to understand the dynamics of groundwater flow in the area and assess whether the drain has a significant impact on recharge, it was decided to perform a study of the regional hydrogeochemistry, with a special focus on mineralization and mixing processes. To this end, 19 tubewells and hand pumps were sampled.
Study area
Geomorphology and climate
The study area is a plain terrain, with an average elevation of 213 meters above sea level (m asl). The Najafgarh drain flows from west to east across the central part of the study area. A long continuous chain of rocky ridges, locally called Delhi Ridge, rises up to 320 m asl, 20 km southeastward of the study area (Fig. 1). The climate is of semi-arid nature; 80 % of annual rainfall occurs during monsoon (July–September). Annual precipitation is between 720 mm (Delhi), 510 mm (Badli) and 400 mm (Najafgarh) (CGWB 2006). In Delhi, the seasonal average temperature varies from 18.7 to 30.5 °C (Kumar et al. 2006).
Najafgarh drain
The Najafgarh drain was dug at the beginning of the nineteenth century along an old course of the Sahibi River, an intermittent river emanating from more than 150 km southwest, in the State of Rajasthan (Mohan 2007). It is connected to several other drains and rivers that belong to the Northern Indian Storm Water and Irrigation canal Network. It is interrupted by a weir at the interstate border between Haryana and Delhi. Although the upper segment in Haryana State (study area) falls dry in summer (Fig. 2), it receives surface runoff and storm water from the Sahibi catchment in the Aravalli range during monsoon (AGC 2001). Downstream in Delhi, it was canalized to drain a morphological depression in western Delhi (Najafgarh Jheel) and integrated into the storm water and sewer drainage system of the city (Nema and Agarwal 2003). The discharge of the Najafgarh drain is controlled by monsoon (Flood Control Wing 1976).
Geology and aquifer characteristics
The study area is located in the Ganga Plain within the world’s largest terrestrial foreland basin, the Himalayan molasse basin. The main lithostratigraphic units are described below and summarized in Table 1. The Delhi Ridge, from the Alwar group, is the only outcrop of the bedrock, composed of Precambrian quartzitic rocks with thin bands of mica-schist. Overlying the Delhi Ridge and reaching thicknesses up to 300 m (Shekhar and Sarkar 2013), the Quaternary formation is composed of Pleistocene to Holocene medium to fine-grained sand with varying content of silt and clay, together with local aeolian deposits (see Table 1). It forms fairly thick aquifers, generally semi-confined. The presence of calcrete nodules (also called “kankar”) in these sediments is a classical feature of the Ganga Plain. Little information on the hydrodynamic properties of the Quaternary formation is available. Nonetheless, Shekhar and Sarkar (2013) mentioned, at some locations, hydraulic conductivity below and adjacent to the Najafgarh drain ranging from 3 to 28 m/day and transmissivity ranging from 63 to 928 m2/day.
Methods
A hydrogeological survey was carried out to obtain an overview of the main mineralization and mixing processes in the area as potential indicators of geochemical areas and groundwater flow patterns. In April 2008, 19 groundwater samples (hand pumps and tubewells) were collected and in situ parameters (pH, T, EC, DO) were measured with Eutech devices. Alkalinity was determined by HCl titration in the field using a Merck field kit. All water samples were stored in 20 ml polypropylene (PP) bottles. All samples for ion determination were filtered on site with 0.2 mm acetate cellulose filters. The sample for cation measurements was acidified to pH 2 with pure HNO3 and one bottle of each sample (not acidified) was kept for anion determination. Additionally, one 20-ml PP bottle was taken for stable isotopes of water (δ2H and δ18O) measurement. Isotope analysis was performed at the Freie Universitaet Berlin on a Thermo Finnegan Mat 253 isotope ratio spectrometer using a Gas Bench II peripheral unit with autosampler-assisted loop injection.
For δ18O and δ2H measurements, a sample volume of 1000 μl was flushed with 0.5 % CO2 in helium (He) environment and then equipped with platinum catalyst to accelerate equilibrium. Measurement was performed after an equilibration time of 40 min. Precision was ±0.6 (‰ VSMOW) for δ2H and ±0.02 for δ18O (‰ VSMOW). Analytical values were standardized to the international reference, Vienna Standard Mean Ocean Water (VSMOW). Water samples were analyzed for K, Na, Ca and Mg by the ICP Optima 2100 Perkin Elmer with a detection limit of 0.02 mg/l and for Br, Cl, SO4 by ICS 1100 with a detection limit of 0.1 mg/l. A reliability check based on electrical balance was made and only samples with ion balance (IBE) ≤10 %, calculated from Eq. 1 were used for further interpretation:
Table 2 presents the hydrochemistry, ion balance error and several saturation indexes. Whenever possible, water table elevation was measured manually with a dip meter. Although at certain places it was possible to dismantle hand pumps and measure the depth to water table from the borehole directly, some measurements were made in abandoned dug wells. Due to the declining groundwater table, many of the dry dug wells are equipped with suction pumps standing on the bottom of the dug well, a few meters below ground level. Under normal atmospheric pressure, suction pumps can draw water up to a maximum height of approximately 10 m. This basic principle makes it possible to estimate the depth to water table at dug wells equipped with suction pumps to be a maximum 10 m below the bottom of the dry dug well. Figure 3 illustrates estimated depth to water table as dotted circles. At each of the sampling points, ground level elevation was measured by a Garmin Legend-C GPS, and checked by topographical maps and a digital surface model (Fig. 1) was derived from SRTM data.
Results
Water table elevation
Figure 3 shows the elevation of groundwater table and ground surface in m asl, derived from SRTM data and from the GPS measurements of water level. Given the limited number of water table measurements, only local equipotential lines were drawn. Two trends appear in Fig. 3: (1) the Delhi Ridge (and Aravalli Hills), rising up 20 km southwards from the drain, is the most important source of groundwater recharge for the area, as water table levels are higher in the south than in the north, an observation also confirmed by Kumar et al. (2011) and (2) water table is higher along the drain than in the northern and the southern areas, suggesting a flow from the drain to the north, and from the drain to the south.
Although no data are available about the decrease of the groundwater level in the area over the last decades, field observations and information from locals clearly indicate the depleted groundwater state, as well as the progressive salinization of the groundwater. Overexploitation of the alluvial aquifer, mainly for irrigation purposes, seems to be the main cause.
Hydrogeochemistry
Groundwater presents a large variability of hydrogeochemical water types: Ca-HCO3, Na-HCO3, Ca-Cl, Mg-HCO3, Mg-Cl and Na-Cl. Electrical conductivity (EC) varies from 0.5 to 23.4 mS/cm (Table 2). Based on these geochemical characteristics, groundwater samples can be divided in three major groups, as shown in the Piper diagram (Fig. 4). It is important to note that these geochemical signatures correspond to specific geographic areas, as illustrated by the Stiff diagrams represented on the map of the area (Fig. 5). Indeed, the first area (“south”) corresponds primarily to samples taken southward from the drain where groundwater is partly fed from the Ridge, in accordance to the regional groundwater flow pattern. The water from the corresponding five samples was found to be mainly Ca-HCO3 and Na-HCO3 type, with EC between 0.5 and 2.3 mS/cm. The second area (“center”) corresponds to groundwater sampled close to the drain (except no. 17). It contains seven samples, mostly with HCO3-Cl to Na-Cl water types and EC values between 0.7 and 4 mS/cm. The third area (“north”) corresponds to groundwater samples taken northward from the drain. It consists of seven waters of Mg-Cl and NaCl water types with high salinities (EC from 4.1 to 23.4 mS/cm).
Stable isotopes of water
All samples plot below the Global Meteoric Water Line (GMWL) and, except for one, below the Local Meteoric Water Line (LMWL) for New Delhi (Datta et al. 1991) (Fig. 6). The most enriched signatures (δ18O mostly between −5.5 and −4 ‰ VSMOW) include all samples from the north and south areas. The most depleted signatures correspond to the central area and are aligned between the September monsoon rainfall composition in Delhi and the enriched samples from the north and the south area. Mean monsoon isotope compositions were taken from data of the Global Network of Isotopes in Precipitation (GNIP) from the New Delhi station. According to this data, the monsoonal compositions of rainfall in July are relatively enriched in heavy isotopes and become more and more depleted during monsoon. The range of mean monsoonal rain isotope composition is clearly reflected in the samples from the study area. This finding confirms that groundwater recharge mainly takes place during monsoon, but undergoes substantial changes due to large deviations from the LMWL.
Discussion
As the hydrochemical data indicates, the three groundwater types reflect three distinct geographical areas: southward from the drain, northward from the drain, and along the drain. They reveal different geochemical processes and provide insights for characterizing the regional groundwater flow pattern, especially regarding the role of the drain. In addition, the origin of groundwater salinity is investigated.
Stable isotopes of water
The deviation of samples from the LMWL is likely to result from non-equilibrium fractionation during evaporation and mixing between isotope end-members. This effect is well illustrated by groundwater samples from the central area that link groundwater from northern and southern areas with an independent source whose composition is close to that of monsoon rainfall in September.
Groundwater samples from the southern area are closer to the LMWL and more uniformly distributed than samples from the north area. These samples, which are assumed to represent isotope signatures from the recharge zone in the Aravalli hills, show a certain degree of evaporative enrichment. A conceptual interpretation may include water recycling by abstraction for irrigation, evaporative losses on the paddy fields, partial recharge (irrigation return flow) and subsequent re-abstraction further down gradient (towards the drain). This cycle of abstraction–evaporation–recharge may explain the isotope composition of this group. It is not possible to derive clear evaporation lines because the initial isotope composition for each groundwater sample seems to be different.
Groundwater samples from the northern area are largely scattered and sometimes far below the LMWL (max. 2 ‰ deviation in δ18O). This composition cannot be explained by water recycling through irrigation and recharge and it seems likely that mixing with other end-members occurs. Lorenzen et al. (2012) have characterized the deep, brackish to saline groundwater by isotopic composition of <−4.5 ‰ for δ18O. The authors conclude that dissolution of salts from deep geological formations (e.g., salt clays) may explain the elevated mineralization. While this brine end-member was not sampled in the study area, it seems likely that a highly mineralized and isotopically depleted groundwater mixes in the northern area.
Carbonate geochemistry
Except for sample 3, most samples are saturated to oversaturated with respect to calcite and dolomite (Table 2). This could be explained by: (1) the dissolution of primary carbonate minerals from the sediments, or (2) the presence of nodules of “kankar” in the Older Alluvium, a typical feature of the Ganga Plain (Singh and Singh 1972; Thussu 2006; Chandra et al. 2007). Also called caliche or calcrete, kankar is made of secondary calcium carbonates that can be formed close to the surface in the sediments by two processes: alluvial concentration of carbonates by pedogenetic processes in the “B” horizon of soil, and capillary rise and evaporation of CaCO3-charged groundwater during the hot and dry period. Kulkarni (1989) suggested that the development of intense kankar deposits took place mainly under an interpluvial climate in the Pleistocene. In the study area, where semi-arid climate limits pedogenesis, the water table increases after monsoon and decreases during the dry season. Thus, contemporaneous formation of Kankar, linked to precipitation/dissolution processes, could be more logically explained by the second option.
Ion exchange processes
Ion exchange is a common process during re-freshening or salinization of aquifers (Appelo and Postma 2005). Most samples from the southern area present depletion in Ca and Mg compared to HCO3 − and SO4 2− and an excess of Na compared to Cl (Fig. 7). These characteristics are explained by a re-freshening through cation exchange reactions between Ca2+ and Na+, catching calcium ions on the exchanger and releasing sodium ions to the solution, as expressed by Eq. 2:
Ion exchange diagram. For simplification, the value of sample 14, around −60/60 is not represented (see Table 2)
where X indicates the soil exchanger. This process typically occurs when freshwater (dominated by Ca2+ and HCO3 − ions) flushes a salt water aquifer, where sediments in contact with salt water have adsorbed Na+ for a large part, resulting in Na-HCO3 type groundwater. This type of water is found predominantly in the southern part of the study area. When saline water intrudes in a fresh water aquifer, ion exchange process can be described by Eq. 3:
where Na is taken up by the binding sites of the exchanger and Ca is released into the water in return. The water type changes here from NaCl to CaCl2. This type of water is found predominantly in the northern part of the study area.
Here, as shown by Kumar et al. (2011), we can safely suppose that fresh water comes from the Ridge, located southward from the study area. The alteration of the plagioclases of the quartzitic rocks from the Ridge could be an additional source of dissolved sodium.
The high amount of Mg compared to Ca (Table 2) could be linked to the presence of Mg-calcite in the sediments. When a solution is in equilibrium with a mineral, permanent dissolution and precipitation reactions occur. Here, all samples are saturated with respect to calcite, which means that when Mg-calcite is dissolved, pure calcite precipitates, and Mg2+ is released into the solution, as expressed by Eq. 4:
Dissolution of evaporites (north)
Samples from the northern area have a very similar HCO3 − content (between 230 and 420 mg/l), and present an important excess of (Ca2+ + Mg2+) compared to HCO3 − and SO4 2− (Fig. 7), showing that the dissolution of calcite and dolomite cannot be the only source for these cations. Indeed, as Table 2 indicates, the highest saturation indexes for anhydrite (CaSO4) are found in the northern area, reaching up to 0.5 (sample 14). Accordingly, the above-mentioned excess in the northern area is partly caused by anhydrite, as a substantial additional source of Ca2+. In addition, the (K + Na) -Cl relation (Fig. 7) shows a strong excess in Cl versus Na, indicating that groundwater from the northern area also dissolved halite.
The chloride/bromide relation can provide additional insights on the origin of salinity. Rainfall Cl/Br ratios are close to that of seawater with a weight ratio of 293 (Alcalá and Custodio 2008), despite some small deviations caused mainly by distance and pollution. When a solution dissolves halite, the Cl/Br becomes enriched, with weight ratio up to thousands (Alcalá and Custodio 2008). As Table 2 illustrates, samples from the northern area (except no. 17) have Cl/Br values from 415 to 517, with an average value of 428, which is notably higher than seawater. These waters correspond therefore to a mixing between saline water, having dissolved halite and fresh water deriving from infiltration. Groundwater from the central area shows an important scatter, with Cl/Br ratios between 160 and 450 (average value of 322). Two points show some possible influence of halite (samples 5 and 6). Even though it is located next to sand dunes, sample 3, has a low value of 160 that does not correlate with the presence of halite suggested by Subramayian and Saxena (1983) in such environment. Samples from the south (except sample 12) are mostly below the seawater value, with an average Cl/Br value of 198. These ratios are typical of inland fresh groundwater (Alcalá and Custodio 2008), due to enrichment in Br in rain-water compared to seawater. As Br is easily linked to organic matter, an additional explanation could be a change in the soil use, such as deforestation that may oxidize the organic matter, release Br in solution, and thus reduce the Cl/Br ratio to the observed values.
Infiltration of surface water and mixing (drain)
Samples from the central area are less mineralized than those from the southern and northern areas. They have a low-evolved geochemistry, as illustrated by Fig. 7. Groundwater in this area seems to interrupt the geochemical processes of the freshening of saline groundwater from the south. Groundwater recharge from the drain could therefore act as a hydraulic barrier for groundwater originating from the southern area.
This interpretation is strengthened by two independent approaches. As Fig. 6 shows, groundwater from the central area has an isotopic signature distributed along a mixing line between the ambient groundwater (i.e., northern + southern areas) and another source whose composition is close to that of the September monsoon. Therefore, groundwater from the central area is not a simple mixing between northern and southern groundwater, but receives a significant contribution of geochemically low-evolved water located at the Najafgarh drain. Secondly, the groundwater table elevation map (Fig. 3) confirms: (1) the presence of a regional groundwater flow from the Delhi Ridge, and (2) loosing conditions of the drain, even during the dry season. Thus, an active and consistent recharge from the Najafgarh drain to the aquifer is occurring in this eastern part of the Haryana region despite the low availability of surface water and the low hydraulic conductivity of the riverbed. Only further east, downstream the weir at the Delhi border, surface water–groundwater interaction is dominated by drainage of the land and gaining river conditions most of the year (Lorenzen et al. 2010).
Conclusions
The aim of this investigation was to characterize the groundwater flow pattern in a rural area of Haryana, India, with a special focus on groundwater recharge from an intermittent monsoon-controlled watercourse, the Najafgarh drain, using geochemical, isotopic and potentiometric data.
An important hydrogeochemical zonation between the southern and the northern parts of the area was observed. Southward from the drain, fresh groundwater was found (electrical conductivity between 0.5 and 2.3 mS/cm) with a dominant Na-HCO3 water type. Mineralization revealed strong cation exchange processes, primarily explained by the flushing of saline groundwater by fresh groundwater originating from the Aravalli hills. Northward from the drain, groundwater was moderately to highly saline (electrical conductivity from 4.1 to 23.4 mS/cm) and the dominant water type was Na-Cl. Mineralization indicated the presence of water having dissolved evaporates, mostly halite and gypsum. In the vicinity of the drain, salinity was intermediate (0.7–4 mS/cm) and groundwater geochemistry was much less evolved, leading to the hypothesis of a significant recharge from the drain. Isotopic data correlated this assumption by revealing the critical input at the drain of water having the isotopic composition of monsoon rainfall (September). Finally, potentiometric data provided additional evidence and led to the conclusion that the drain acts as a hydraulic barrier for groundwater between the southern and the northern parts of the area. Therefore, in this specific area, the Najafgarh drain significantly contributes to the recharge of the aquifer despite the limited recharge potential, restricted by the availability of monsoonal flood-water and the low hydraulic conductivity of the banks.
By providing a special insight on the impact of an intermittent watercourse on groundwater recharge, this study demonstrates the value of hydrogeochemical and isotopic data for characterizing groundwater flow pattern in anthropized agricultural areas with scarce discharge. Further investigation on the quantification of recharge from the drain to the aquifer should focus on the evolution of the system before, during and after monsoon. More specifically, a notable state forward regarding the quantification of the spatiotemporal variability of recharge would be attained by using environmental time tracers.
References
AGC (2001) Report on evaluation study of soil conservation in seven watersheds of Sahibi catchment in Haryana and Rajasthan. Report. Agricultural Finance Corporation, Sponsored by the Ministry of Agriculture, India, New Delhi
Alcalá FJ, Custodio E (2008) Using the Cl/Br ratio as a tracer to identify the origin of salinity in aquifers in Spain and in Portugal. J Hydrol 359(1–2):89–207
Appelo CAJ and Postma D (2005) Geochemistry, groundwater and pollution, 2nd edn. AA Balkema Publishers, Rotterdam, The Netherlands
Baudron P (2008) Hydrochemical characterization of groundwater and estimation of recharge from the Najafgarh drain, South-West of Delhi, India. Unpublished Dissertation. Université Paris Sud, Orsay, France
Baudron P, Alonso-Sarría F, García-Aróstegui JL, Cánovas-García F, Martínez-Vicente D, Moreno-Brotóns J (2013) Identifying the origin of groundwater samples in a multi-layer aquifer system with random forest classification. J Hydrol 499:303–315. doi:10.1016/j.jhydrol.2013.07.009
Baudron P, Barbecot F, Aróstegui JLG, Leduc C, Travi Y, Martinez-Vicente D (2014) Impacts of human activities on recharge in a multilayered semiarid aquifer (Campo de Cartagena, SE Spain). Hydrol Process 28:2223–2236. doi:10.1002/hyp.9771
Brindha K, Kavitha R (2014) Hydrochemical assessment of surface water and groundwater quality along Uyyakondan channel, south India. Environ Earth Sci. doi:10.1007/s12665-014-3793-5
CGWB—Central Ground Water Board of India (2006) Ground water year book 2005–2006, National Capital Territory, Delhi. Government of India, Ministry of Water Resources, New Delhi
Chandra S, Rhodes E, Richards K (2007) Luminescence dating of late Quaternary fluvial sediments in the Rapti Basin, north-central Gangetic Plains. Quatern Int 159(1):47–56
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
Custodio E (2002) Aquifer overexploitation: what does it mean? Hydrogeol J 10:254–277. doi:10.1007/s10040-002-0188-6
Datta PS, Tyagi SK, Chandrasekharan H (1991) Factors controlling stable isotopic composition of rainfall in New Delhi. J Hydrol 128:223–236
Dwivedi AK, Vankar PS, Sahu RS (2015) Geochemical trends of heavy metal in aquifer system of Kanpur Industrial Zone, Uttar Pradesh (India): a case study. Environ Earth Sci. doi:10.1007/s12665-015-4017-3
Flood Control Wing (1976) Master plan for drainage of storm water drainage of the Najafgarh basin in Union Territory of Delhi. Master Plan Organization, Delhi Administration
Katpatal YB, Pophare AM, Lamsoge BR (2014) A groundwater flow model for overexploited basaltic aquifer and Bazada formation in India. Environ Earth Sci 72:4413–4425. doi:10.1007/s12665-014-3342-2
Kulkarni KM et al (1989) “Origin of saline groundwaters of Haryana State, India”, Regional Characterization of Water Quality (Proceedings of the Baltimore Symposium, May 1989). IAHS Publication no. 182, Wallingford, pp 125–129
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. Environ Geol 50(7):1025–1039
Kumar M, Rao MS, Kumar B, Ramanathan A (2011) Identification of aquifer-recharge zones and sources in an urban development area (Delhi, India), by correlating isotopic tracers with hydrological features. Hydrogeol J 19:463–474. doi:10.1007/s10040-010-0692-z
Lorenzen G, Sprenger C, Taute T, Pekdeger A, Mittal A, Massmann G (2010) Assessment of the potential for bank filtration in a water-stressed megacity (Delhi, India). Environ Earth Sci 61:1419–1434. doi:10.1007/s12665-010-0458-x
Lorenzen G, Sprenger C, Baudron P, Gupta D, Pekdeger A (2012) Origin and dynamics of groundwater salinity in the alluvial plains of western Delhi and adjacent territories of Haryana State, India. Hydrol Processes 26:2333–2345
Massuel S, Perrin J, Wajid M, Mascre C, Dewandel B (2009) A Simple. Low-cost method to monitor duration of ground water pumping, ground water 47:141–145. doi:10.1111/j.1745-6584.2008.00511.x
Massuel S, George BA, Venot JP, Bharati L, Acharya S (2013) Improving assessment of groundwater-resource sustainability with deterministic modelling: a case study of the semi-arid Musi sub-basin, South India. Hydrogeol J 21:1567–1580. doi:10.1007/s10040-013-1030-z
Mohan I (2007) Environment education. APH Publishing Corporation, Daryaganj, India
Murkute YA (2014) Hydrogeochemical characterization and quality assessment of groundwater around Umrer coal mine area Nagpur District, Maharashtra, India. Environ Earth Sci 72:4059–4073. doi:10.1007/s12665-014-3295-5
Nema A, Agarwal L (2003) Wastewater management in Najafgarh drainage basin: a key to water quality improvement in river Yamuna. Indian Association of Environment Management, Annual Conference, New Delhi. http://www.greenensys.org/site/publications/Wastewater%20management%20in%20Najafgarh%20drainage%20basin.pdf. Accessed 28 Dec 2014
Perrin J, Ahmed S, Hunkeler D (2011) The effects of geological heterogeneities and piezometric fluctuations on groundwater flow and chemistry in a hard-rock aquifer, southern India. Hydrogeol J 19:1189–1201. doi:10.1007/s10040-011-0745-y
Shekhar S, Sarkar A (2013) Hydrogeological characterization and assessment of groundwater quality in shallow aquifers in vicinity of Najafgarh drain of NCT Delhi. J Earth Syst Sci 122:43–54. doi:10.1007/s12040-012-0256-9
Singh L, Singh S (1972) Chemical and morphological composition of kankar nodules in soils of the Vindahyan region of Mirzapur, India. Geoderma 7(3–4):269–276
Subramayian V, Saxena KK (1983) Hydrogeochemistry of groundwater in the Delhi region of India, Relation of Groundwater Quantity and Quality (Proceedings of the Hamburg Symposium, August 1983. IAHS Publication no. 146
Thussu JL (2006) Geology of Haryana and Delhi. Geological Society of India, Bangalore, India
Acknowledgments
This study was carried out within the framework of the EU integrated project TECHNEAU (work package 5.2) with scientific mentoring by Prof. A. Pekdeger (†) at Freie Universität Berlin. Thanks to Yann Moreau-Le Golvan, Janek Greskowiak and Gesche Grützmacher from Kompetenzzentrum Wasser Berlin for their support in project management and logistics. This study is a result of collaboration between Kompetenzzentrum Wasser Berlin, the Indian Institute of Technology and Freie Universität Berlin.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baudron, P., Sprenger, C., Lorenzen, G. et al. Hydrogeochemical and isotopic insights into mineralization processes and groundwater recharge from an intermittent monsoon channel to an overexploited aquifer in eastern Haryana (India). Environ Earth Sci 75, 434 (2016). https://doi.org/10.1007/s12665-015-4911-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12665-015-4911-8