Abstract
Purpose
To review the short- and long-term results in patients who underwent removal of infected or exposed mesh and reconstruction of the abdominal wall with simultaneous mesh replacement.
Methods
Patients undergoing removal of an infected or exposed mesh and single-staged reconstruction of the abdominal wall with synthetic mesh replacement over a 16-year period were retrospectively reviewed from a prospectively maintained database. Patients were operated and followed by a single surgeon. Outcome measures included wound complications and hernia recurrence.
Results
From 1996 until 2012, 41 patients (23 F, 18 M), with a mean age of 53.4 years and mean BMI of 31.2 ± 8 kg/m2, were treated for chronic mesh infection (CMI). A suppurative infection was present in 27 patients, and 14 had an exposed mesh. The need for recurrent incisional hernia repair was observed in 25 patients; bowel resections or other potentially contaminated procedures were associated in 15 patients. The short-term results showed an uneventful post-operative course after mesh replacement in 27 patients; 6 (14.6 %) patients developed a minor wound infection and were treated with dressings and antibiotics; 5 (12 %) patients had wound infections requiring debridement and one required complete mesh removal. On the long-term follow-up, there were three hernia recurrences, one of which demanded a reoperation for enterocutaneous fistula; 95 % of the patients submitted to mesh replacement were considered cured of CMI after a mean follow-up of 74 months.
Conclusions
CMI can be treated by removal of infected mesh; simultaneous mesh replacement prevents hernia recurrence and has an acceptable incidence of post-operative acute infection. Standard polypropylene mesh is a suitable material to be used in the infected surgical field as an onlay graft.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A great step towards the cure of hernia has been achieved with the introduction of biomaterials for the reinforcement of the abdominal wall. Nowadays it is worldwide accepted that the best results in hernia surgery are obtained with the use of mesh. This is especially true for the treatment of incisional and recurrent hernias [1]. Hernia repair is usually safe on the hands of experienced surgeons [2], but misuse of mesh can lead to consequences that are worse than the hernia itself.
Although the safety of synthetic materials has been exhaustively evaluated, many mesh-related complications were reported. Most of the unfavorable events associated with prosthetic repair where later explained, as knowledge with mesh interaction with the host tissues and the principles of their applications became available [3, 4]. The main failures of mesh repair are hernia recurrences, mesh migration and mesh infection. The common presentations of mesh infection are draining sinuses, mesh extrusion and enteric fistulas caused by mesh erosion into hollow viscera.
Most surgeons avoid treating mesh complications because the standards for the treatment of mesh morbidity have not been set. Despite the extensive literature about hernia care and the several reports on mesh-related complications, there is a lack of information about how to deal with this complex scenario [5]. Besides, there is a wide and persistent reluctance about using synthetic mesh in the setting of contamination or infection, and most authors consider it as an absolute contraindication [6].
Chronic mesh infection (CMI) is one of the most challenging clinical conditions in the abdominal wall surgery. Whether an infected mesh must be totally removed or not, the role of antibiotics, the right timing for a repair and guidelines on how to treat the abdominal wall must be discussed.
In this study, we reviewed our experience and analyzed the short- and long-term results in patients who underwent removal of infected or exposed mesh and simultaneous reconstruction of the abdominal wall with polypropylene mesh replacement. The incidence of acute post-operative infection, the recurrence of CMI, hernia recurrence, and the microbiology of mesh infection were evaluated.
Methods
During a 16-year period, all our patients undergoing removal of infected or exposed mesh and single-stage reconstruction of the abdominal wall with simultaneous synthetic mesh replacement were retrospectively reviewed from a prospectively maintained database. All patients presented either with suppurative mesh infection characterized by draining sinuses or non-suppurative mesh infection, herein described as exposed mesh. Patients with mesh erosion into the bowel or mesh-related enteric fistulas and patients with mesh infection after inguinal hernia repair were not included in this series.
A single surgeon (CB) operated and followed the patients, and all except one were operated at the University of São Paulo School of Medicine teaching hospital (Hospital das Clínicas), currently a tertiary referral center for abdominal wall repair. The remaining patient was operated at a private institution. Patients were followed on an outpatient basis during the follow-up period. A telephone call follow-up interview performed by July 2013 provided updated information about 29 (over 41) patients.
Patients were operated through the previous surgical incision. The infected or exposed mesh was completely removed as well as the foreign body granulomas, stitches, tacks and fibrotic tissue. Whenever needed, the abdominal cavity was opened for associated procedures such as cholecystectomy, enterectomy, appendectomy and/or colostomy take down. Fluids taken from a sinus and/or samples of the infected mesh were sent for culture.
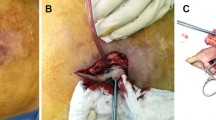
The abdominal wall was reconstructed as anatomically as possible, with primary closure and restoration of the midline. Adjunctive maneuvers to increase the abdominal circumference, such as relaxing incisions along the rectus sheath were used when needed, to relieve the tension. When it was not possible to perform a primary closure, the defect was partially bridged using the hernia sac to isolate the abdominal cavity. A wide standard (heavyweight, large pore) onlay polypropylene mesh (Bard™ Mesh, Davol, Warwick, RI or Intracorp®, Venkuri, São Paulo, SP) was used to reinforce the abdominal wall, overlapping the defect and the relaxing incisions by at least 5 cm (Fig. 1). One patient with a lumbar hernia had his mesh positioned in the pre-peritoneal space. All meshes were fixed in place with running sutures of absorbable polyglactin and no dead space was left between the mesh and the underlying tissues. We did not use antiseptic solutions, or mesh soaking in antibiotics before implanting the mesh. The subcutaneous space was drained with suction drains, removed when they had less than 50 ml/day of output. An abdominal binder was used during the first post-operative month.
We used individual criteria to determine the type and duration of the antimicrobial therapy and broad-spectrum antibiotics were used in most of the patients. All the known staphylococcal infections were treated with methicillin or vancomycin. For the patients who developed post-operative acute wound infection after mesh replacement, the antibiotic scheme was revised in accordance with culture results. A surgical site infection was classified as minor, when there was wound erythema or skin breakdown with purulent discharge, controlled with antibiotics and wound dressings. A major wound infection was considered in those patients who required surgical debridement, drainage of an infected seroma, excision of exposed mesh or when readmission to the hospital was required. A minor skin breakdown was not considered a surgical site infection. Outcome measures included early and late wound morbidity, and long-term hernia recurrence.
Data analysis was performed with Epi-Info™ statistical software. T test and Chi square were used when appropriate. The study protocol was analyzed and approved by the institutional ethics committee.
Results
From January 1996 until April 2012, 41 patients with a mean age of 53.4 (18–82) years were treated for CMI after ventral hernia repair. There were 18 males and 23 females. The number of previous abdominal operations ranged between 1 and 13; 73 % of the patients were classified as ASA 2 or 3, and 24.4 % had a BMI higher than 35 kg/m2 (mean BMI 31.2). The onset of CMI symptoms ranged from 1 month to 29 years, and the most common clinical presentation was a chronic sinus, verified in 65.8 % of the patients while 34.2 % presented with an exposed mesh; 61 % of the patients had an associated recurrent hernia (Table 1). We had originally placed the mesh in six of these patients.
The characteristics of mesh infection, as well as the clinical presentation, onset of symptoms, explanted mesh type and location, and the possible causes for mesh infection or extrusion are presented in Table 2. The most common explanted mesh was polypropylene, a product widely employed in our country. The most frequent location was onlay or bridged onlay, accounting for 68.3 % of the mesh explants. The main cause for extrusion was a second-intention healing wound (Fig. 2) while mesh fixation with polypropylene or multifilament sutures, was the commonest finding in patients with a chronic sinus (Fig. 3). Other causes for mesh infection included mesh over mesh fixation and unincorporated PTFE or bovine pericardium mesh. In two patients, methicillin-resistant Staphylococcus aureus (MRSA) infection was the only identified cause for the chronic infection (Figs. 4, 5).
It was possible to retrieve the microbiological data from 20 patients. There was no bacterial growth in three cases and 17 positive cultures. In 11 patients, the cultures tested positive for Staphylococcus aureus. Other agents included Klebsiella pneumoniae, Escherichia coli, Acinetobacter baumannii and Enterococci spp. The microbiological findings and the post-operative antibiotic regimens are shown, respectively, in Tables 3 and 4.
The results of mesh replacement are presented in Table 5. The overall post-operative incidence of wound infection was 26.8 % (11 cases); 6 patients had a minor wound infection without the need for further treatment, except wound dressings and antibiotics. One patient in this group presented pulmonary thromboembolism and was treated with systemic anticoagulation. Among the major complications in five patients (12.2 %), one patient developed post-operative MRSA and Acinetobacter baumannii wound infection during her stay in the ICU, after suffering an intra-operative ischemic stroke. After 2 weeks, her mesh was not incorporated at all, and it had to be removed. Other complications included three non-infected seromas and/or minor wound breakdown; 27 patients (65.9 %) had an uneventful post-operative course. There was no mortality.
On the long-term follow-up, there were two major complications. One patient developed a hernia recurrence, whose treatment caused an enteric fistula due to mesh over mesh fixation with polypropylene sutures (a perforation of the small bowel was caused by a polypropylene suture knot). As a consequence, he required a third operation to replace the mesh once again. Unfortunately, he died 3 months after the last operation due to myocardial infarction. Another case presented with a draining sinus, and upon reoperation this sinus was found to be caused by an intra-peritoneal granuloma (cotton sutures used to tie the mesenterium vessels) unrelated to the mesh.
There were two other hernia recurrences in the late follow-up. Both patients were not re-operated due to impaired medical conditions. One died 13 years later, due to progression of obstructive lung disease. The other (the one who required complete removal of the mesh) developed a bulge in her right flank; she is being followed for 7 years. After a minimum follow-up of 1 year, 39 patients with mesh replacement were considered cured of CMI (Table 5).
The follow-up period ranged between 12 and 174 months, with a mean of 74 months; 25 patients (61 %) are still being followed and until July 2013, 4 of our patients had died due to unrelated diseases (myocardial infarction, chronic obstructive lung disease, prostate cancer and natural causes). We were unable to obtain updated information about 11 patients operated between 1996 and 2001 (mean follow-up of 62.4 months), and about one patient operated in 2005 (follow-up of 51 months) and for these patients we considered the information retrieved from their records when they were last seen, or discharged from the outpatient facility.
There was no statistical difference in demographics between patients who developed wound infection and those who did not. There were no significant predictors of wound morbidity, including age, gender, ASA score, diabetes mellitus, recent smoking history, BMI >30 kg/m2, association with a recurrent hernia, CMI presentation (sinus or exposed mesh), longer operative or anesthesia time, associated procedures, MRSA infection, or a positive culture.
Discussion
There is a common agreement that infected mesh must be removed [7] and that a primary repair with prosthetic mesh in any level of contamination is contraindicated [6]. The recommended treatments include the partial removal of the infected mesh [8], the complete removal with primary reconstruction using absorbable meshes [9] and single or staged operations (with a minimum gap of 6 months) using open or laparoscopic component separation techniques [10–12] with recurrence rates of up to 52 % [13]. Besides, an increasing number of authors are recommending the use of biological prosthesis to repair these complex defects [14, 15]. Considering the results of up to 66 % surgical site occurrences and 30 % to 50 % hernia recurrences in the long-term follow-up [16–18], the use of biologic mesh seems to be an expensive step-back in the treatment of the complex abdominal wall. The poor long-term outcomes of biological mesh repair in contaminated abdominal wall defects were recently compared to the results of absorbable synthetic products, at much higher costs [18].
The current publications on the treatment of ventral hernias in contaminated surgical fields evaluate a series of miscellaneous conditions, including all together, mesh infection, enterocutaneous fistula, concomitant bowel resection, colostomy take down and incidental procedures such as cholecystectomies or enterotomies. For every publication, there is also a wide range of operative approaches, including primary repair, open or laparoscopic component separation with or without prosthetic reinforcement and mesh bridging or reinforcement with synthetic or biological meshes [19]. Also, emergency and elective surgical outcomes must be analyzed separately, considering the wide difference between these two groups of patients. All these variables make it hard to conclude on what is the best way to approach these complex cases since taking care of a mesh infection only, is entirely different from operating an enterocutaneous fistula caused by mesh erosion into the bowel or performing an elective colostomy take down in a patient with a ventral hernia. In fact, there are no guidelines, and there is no consensus on the use of mesh in the contaminated settings.
About the treatment of infected and exposed mesh, a few recent studies propose very different approaches. Szerba and Dumanian [10] recommend the removal of the infected mesh and autogenous flap reconstruction as a safe and reliable solution to the problem, with a 9 % hernia recurrence and 18 % readmission for post-operative stitch abscesses in 11 patients with a mean follow-up of 24 months. Sabbagh et al. [8] state that partial removal of infected meshes is a safe procedure, and it could be considered as the first procedure to perform, thus avoiding the complications of more aggressive procedures such as complete mesh removal. In their series of 25 patients, they reported a hernia recurrence rate of 20 % with a mean follow-up of 40 months.
Other studies advocate the conservative management of early mesh-site infection using wound dressings and negative pressure therapy (NPT). Meagher [20] successfully treated both absorbable and non-absorbable infected mesh cases conservatively with a median time to heal of 199 days (range 82–456 days) at a median cost of € 4,650 for the VAC® machine rental plus €1,939 for additional consumables per patient, after a median of 103 days. Berrevoet [21] reported on the use of NPT after early mesh infection for ventral and incisional hernia repair. They managed to salvage the mesh in 100 % of cases using polypropylene while infected polyester meshes had to be removed.
To make it easier to understand the outcomes, we included in this series, only patients presenting with suppurative (sinus) or non-suppurative (exposed mesh) infection, operated electively. The surgical strategy was similar in all patients, including the complete removal of the infected mesh and a primary closure of the abdominal wall with simultaneous mesh replacement, an approach that is usually considered not only controversial, but mainly contraindicated.
Considering the mesh positioning, the onlay repair is easier and promotes less tissue dissection and mobilization than the other repairs, especially when compared to component separation techniques, which sometimes may cause a total dismantling of the abdominal wall. When using the onlay repair, the occurrence of seromas and skin necrosis is easily avoided if the large lateral perforating vessels are spared and passed through lateral openings in the mesh margins. General measures to prevent seromas include the routine association of dermolipectomy and the use of suction drains and abdominal binders. Another advantage of onlay placement of mesh is that all tissue repairs remain under the mesh, creating a healthy isolation layer between the mesh and the abdominal cavity. If infection occurs, it is always superficial and easily handled by opening a few stitches for seroma drainage or wound debridement. When the polypropylene mesh is placed over well-irrigated tissues and fixed in place with absorbable sutures, it will surely incorporate, no matter if it becomes partially exposed. It is mandatory to eliminate the dead space between mesh and tissues. Standard, large pore polypropylene is absolutely safe under this condition.
In this series, we noted a wound infection rate of 26.8 %, similar to the 32 % reported in Alaedeen [19] series, slightly higher than the 21.2 % rate reported by Choi [6] in clean-contaminated settings and much lower than the 47.7 % incidence reported by Rosen [18] in a recent retrospective series utilizing biologic mesh. This wound infection rate is reasonable, considering that all our patients had ongoing infection, including 11 patients with active Staphylococcus aureus infection. Most of the post-operative infections in this series were superficial and could be treated with wound dressings or local debridement, except for the two patients requiring de-roofing of a seroma under general anesthesia (1) or complete mesh excision (1).
There might be some criticism about replacing a mesh in a patient with mesh infection, but no hernia recurrence. In this series, 16 (39 %) of the patients did not present with an associated hernia. As a matter of fact, removing an infected mesh will almost always cause more damage on an already weakened abdominal wall and this will possibly cause up to 20 % hernia recurrences as observed by Sabbagh [8] in patients submitted to partial removal of meshes. In this setting, we prefer to replace the mesh rather than facing the risk of hernia recurrence for one who has usually suffered several surgical procedures. In fact, our only patient requiring a complete removal of the mesh developed a recurrence.
Although the associated procedures are not the focus in this paper, the need for such interventions is extremely common in this group. Since using a mesh will cause a certain shielding on the abdominal wall and based on the fact that most general surgeons do not feel comfortable operating on a patient who has gone through a mesh repair, we adopted a complete work-up on the abdominal cavity. This approach includes a complete adhesion lysis and the incidental removal of the appendix and the gallbladder, whenever the abdominal cavity is entered. This will prevent the need for further elective or emergency operations in such patients. Other associated procedures, such as colostomy take down, bowel resection and uro-gynecological procedures will be performed as required.
A common factor for all causes of CMI is the occurrence of dead space between the mesh and the host tissues, a condition that prevents the incorporation of the mesh. Among the common findings in mesh infection are mesh wrinkles, the use of microporous mesh, the fixation of mesh with non-absorbable multifilament sutures and the fixation of mesh over mesh to treat recurrences. The chronic infection by Staphylococcus aureus also difficult mesh incorporation, possibly because of its biofilm. This seems to be the only condition when antibiotics play a major effect in the treatment of mesh infection, when replacing a mesh. The role of MRSA in mesh infection is being evaluated both experimentally and clinically. It has already been demonstrated that monofilament polypropylene mesh can clear a large percentage of MRSA contaminants [22] and that a history of MRSA infection in previous operations is not a contraindication for the use of mesh in subsequent operations [23]. The intra-operative care of mesh infection must include complete removal of the infected mesh and other foreign material, where bacteria can hide. It is mandatory to remove all the scars and fibrosis, and place the new mesh over healthy and well-vascularized tissues.
Finally, a long follow-up is required to evaluate the results of hernia surgery; 71 % of the patients in this study were followed for more than 3 years, and 61 % are still being followed as outpatients and were clinically evaluated along 2013. Eleven patients operated between 1996 and 2001 were discharged or lost to follow-up after a mean period of 62.4 months, thus a number of recurrences might have been missed in this group. We suggest a minimum follow-up of 2 years to evaluate the results of mesh replacement in the infected setting and of 5 years to evaluate hernia recurrences.
Conclusion
We concluded that the removal of an infected or exposed mesh with simultaneous reconstruction of the abdominal wall using polypropylene mesh cured 95 % of patients with CMI, even in the setting of gross contamination and ongoing infection, with reasonable rates of wound infection and hernia recurrence.
References
Burger JWA, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240:578–585
Deysine M, Soroff HS (1990) Must we specialize herniorrhaphy for better results? Am J Surg 160:239–240
Amid PK, Shulman AG, Lichtenstein IL, Hakakha M (1994) Biomaterials for abdominal wall hernia surgery and principles of their applications. Langenbecks Arch Chir 379:168–171
Amid P (1997) Classification of biomaterials and their related complications in abdominal wall surgery. Hernia 1:15–21
Seker D, Kulacoglu H (2011) Long-term complications of mesh repair for abdominal wall hernias. J Long Term Eff Med Implants 21:205–218
Choi JJ, Palaniappa NC, Dallas KB, Rudich TB, Colon MJ, Divino CM (2012) Use of mesh during ventral hernia repair in clean-contaminated and contaminated cases: outcomes of 33,832 cases. Ann Surg 255:176–180
Tolino MJ, Tripoloni DE, Ratto R, Garcia MI (2009) Infections associated with prosthetic repairs of abdominal wall hernias: pathology, management and results. Hernia 13:631–637
Sabbagh C, Verhaeghe P, Brehant O, Browet F, Garriot B, Regimbeau JM (2012) Partial removal of infected parietal meshes is a safe procedure. Hernia 16:445–449
Dayton MT, Buchele BA, Shirazi SS, Hunt LB (1986) Use of an absorbable mesh to repair contaminated abdominal wall defects. Arch Surg 121:954–960
Szczerba SR, Dumanian GA (2003) Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg 237:437–441
Van Geffen HJ, Simmermacher RK, van Vroonhoven TJ, van der Werken C (2005) Surgical treatment of large contaminated abdominal wall defects. J Am Coll Surg 201:206–212
Rosen MJ, Jin J, McGee MF, Williams C, Marks J, Ponsky JL (2007) Laparoscopic component separation in the single-stage treatment of infected abdominal wall prosthetic removal. Hernia 11:435–440
Johnson EK, Tushoski PL (2010) Abdominal wall reconstruction in patients with digestive tract fistulas. Clin Colon Rectal Surg 23:195–208
Franklin ME Jr, Treviño JM, Portillo G, Vela I, Glass JL, González JJ (2008) The use of porcine small intestinal submucosa as a prosthetic material for laparoscopic hernia repair in infected and potentially contaminated fields: long-term follow-up. Surg Endosc 22:1941–1946
Miserez M, Fitzgibbons RJ Jr, Schumpelick V (2013) Hernia surgery and contamination: biological mesh and nothing else? Hernia 17:1
Itani KM, Rosen M, Vargo D, Awad SS, Denoto G III, Butler CE, RICH Study Group (2012) Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 152:498–505
Rosen MJ, DeNoto G, Itani KMF, Butler C, vargo D, Smiell J, Rutan R (2013) Evaluation of surgical outcomes of retro-rectus versus intraperitoneal reinforcement with bio-prosthetic mesh in the repair of contaminated ventral hernias. Hernia 17:31–35
Rosen MJ, Krpata DM, Ermlich B, Blatnik JA (2013) A 5-year clinical experience with single-staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 257:991–996
Alaedeen DI, Lipman J, Medalie D, Rosen MJ (2007) The single-staged approach to the surgical management of abdominal wall hernias in contaminated fields. Hernia 11:435–440
Meagher H, Clarke Moloney M, Grace PA (2013) Conservative management of mesh-site infection in hernia repair surgery: a case series. Hernia. doi:10.1007/s10029-013-1069-8
Berrevoet F, Vanlander A, Sainz-Barriga M, Rogiers X, Troisi R (2013) Infected large pore meshes may be salvaged by topical negative pressure therapy. Hernia 17:67–73
Blatnik JA, Krpata DM, Jacobs MR, Gao Y, Novitsky YW, Rosen MJ (2012) In vivo analysis of the morphologic characteristics of synthetic mesh to resist MRSA adherence. J Gastrointest Surg 16:2139–2144
Hicks CW, Blatnik JA, Krpata DM, Novitsky YW, Rosen MJ (2013) History of methicillin-resistant Staphylococcus aureus (MRSA) surgical site infection may not be a contraindication to ventral hernia repair with synthetic mesh: a preliminary report. Hernia. doi:10.1007/s10029-012-1035-x
Conflict of interest
CB declares no conflict of interests.
JSM declares no conflict of interests.
EMU declares no conflict of interests.
SR declares no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Birolini, C., de Miranda, J.S., Utiyama, E.M. et al. A retrospective review and observations over a 16-year clinical experience on the surgical treatment of chronic mesh infection. What about replacing a synthetic mesh on the infected surgical field?. Hernia 19, 239–246 (2015). https://doi.org/10.1007/s10029-014-1225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-014-1225-9