Abstract
Introduction
The surgical treatment of large ventral hernias with accompanying contamination is challenging. We have reviewed our institution's experience with single-staged repair of complex ventral hernias in the setting of contamination.
Methods
We retrospectively reviewed the medical records of all patients who underwent ventral hernia repairs in the setting of a contaminated field. Pertinent details included baseline demographics, reason for contamination, operative technique and details, postoperative morbidity, mortality and recurrence rates.
Results
Between December 1999 and January 2006, 19 patients were identified with ventral hernia repairs performed in contaminated fields. There were 6 males and 13 females with a mean age of 61 years (40–82), ASA 3.2 (2–4), and BMI of 34 kg/m2 (20–65). Fourteen patients had prior mesh: prolene (9), composix (3), goretex (1), and alloderm (1). Reasons for contamination included: mesh infection (14), enterocutaneous fistula (7), concomitant bowel resection (8), chronic non-healing wound (2), and necrotizing fasciitis (1). Operative approaches included primary repair (3), component separation without reinforcement (2), and with prosthetic reinforcement (9). In five patients the fascia could not be reapproximated in the midline and the defect was bridged with surgisis (1), Marlex (1), lightweight polypropylene (1) placed in the retrorectus space, and alloderm (2). Mean operative time was 260 min (90–600). Twelve postoperative complications occurred in nine (47%) patients and included wound infection (6), respiratory failure (1), ileus (2), postoperative hemorrhage (1), renal failure (1), and atrial fibrillation (1). One patient died in this series. During routine follow-up two recurrences were identified by physical exam.
Conclusions
This study shows that single-stage treatment of ventral hernias in contaminated fields can be accomplished with a low recurrence rate and acceptable morbidity in these extremely challenging patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventral hernias occur in up to 20% of laparotomy incisions [1]. This results in up to 150,000 ventral hernias repaired annually in the US, making it one of the most common procedures performed by general surgeons [1]. Based on the principles of tension-free repair, prosthetic devices have revolutionized the surgical treatment of this disease, reducing recurrence rates by 50% [2]. These procedures are now being performed using minimally invasive techniques, in which a large piece of prosthetic material can be placed in an intraperitoneal position with excellent results [3].
The treatment of ventral hernias in the setting of gross contamination has not met with the same encouraging results. In the presence of gross contamination most surgeons believe that permanent prosthetic materials are contraindicated. These contaminated hernias are often the product of multiple recurrences, contain enterocutaneous fistulas, or have infected prosthetic material that must be addressed. After removal of the infected material and takedown of the various fistulae, the surgeon is often left with a large fascial defect that cannot be closed primarily. There is little published literature evaluating the outcome of various surgical approaches to these incredibly challenging patients. Some authors advocate a multi-staged procedure due to the perceived prohibitively high infectious complication risks and subsequent morbidity of one-stage procedures. These multi-staged procedures can leave the patients with massive ventral hernias and can take up to six months to one year to be completed [4, 5]. This may result in increased patient morbidity and health cost before a definitive closure can be attained. With the introduction of biologic meshes and the techniques of abdominal-wall component separation some authors have recently examined the outcomes of single-staged repairs for these patients [6, 7]. The use of a single-staged definitive approach might result in decreased morbidity from multiple re-operations. In this study, we reviewed our institution's experience with single-staged repair of complex ventral hernias in the setting of surgical field contamination.
Patients and methods
After obtaining approval from the Institutional Review Board at University Hospitals of Cleveland, all patients who underwent ventral hernia repairs in the setting of a contaminated surgical field between December 1999 and January 2006 were identified using current procedural terminology (CPT) and the international classification of diseases, ninth revision, clinical modification (ICD9CM) codes from billing records. The CPT/ICD9CM codes for ventral hernia utilized were CPT codes 49560, 49561, 49565, 49566, and 49568, ICD9CM code 5351 and presence of infection.
The charts were reviewed and data collected including patient gender, age, body mass index (BMI), American society of anesthesiologist (ASA) score, reason for contamination, and presence of synthetic mesh at time of repair, method of repair, operative time, and postoperative complications and follow-up. Postoperative follow-up was achieved by review of recent clinic visits or telephone call interviews. Patients under 18 years of age, patients with hernias other than ventral hernias, and patients in whom a multi-staged procedure was performed were excluded from this study.
Results
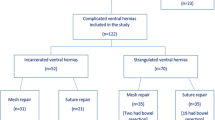
A total of 19 patients were identified who underwent planned single-stage repair for ventral hernias in the setting of gross contamination at our institution. There were six men and thirteen women with a mean age of 61 years (range 40–82 years), ASA score of 3.21 (range 2–4) and BMI of 34.3 kg/m2 (range 20–65 kg/m2). The reasons for surgical field contamination are summarized in Table 1. Seventy-four percent (N = 14) of the patients had infected prosthetic mesh.
In 16% of the patients (N = 3) the fascia was closed primarily without reinforcement. In 26% of the patients (N = 5) the fascia could not be approximated and the defect was bridged with either surgisis (Cook surgical, Bloomington, IN) (N = 1), Marlex (N = 1), lightweight polypropylene (N = 1) placed in the retrorectus space, or AlloDerm (LifeCell Corp., Branchburg, NJ) (N = 2). The remainder of the patients, 58% (N = 11), were repaired using the component separation method. The myofascial flaps were approximated and the fascia repaired primarily in all 11 patients. The fascia was approximated without mesh reinforcement in two patients. Component separation with mesh reinforcement was utilized in nine patients using AlloDerm as an underlay in three patients, AlloDerm as an onlay in three patients, AlloDerm sandwich (onlay and underlay) in two patients, and prolene onlay in one patient. Seventy-four percent (N = 14) of the patients were operated on within the last three years of the study (2004–2006), and 10 out of the 11 patients in whom the component separation method was utilized were included in this group.
The mean operative time was 260 min (range 90–600 min). Concomitant bowel resection was necessary in eight patients; four with colonic resection and four with small bowel resection. Inadvertent enterotomies occurred in two patients.
Twelve postoperative complications occurred in nine (47%) patients. Superficial wound infection occurred in six patients; wound infection was defined as any wound that required the prescription of antibiotics and/or skin opening and dressing application with or without debridement. Three patients were treated with local wound-care measures and application of vacuum-assisted closure, and three patients eventually required operative debridement, but mesh removal or fascial violation was not necessary in any of these patients. None of the patients with wound infections developed hernia recurrence. Prolonged ileus was manifested in two patients. Postoperative hemorrhage occurred in one patient after administration of therapeutic intravenous heparin for a remote history of a deep venous thrombosis on the sixth postoperative day. After cessation of the heparin the bleeding resolved and the patient did not require operative drainage. One patient developed transient renal failure, one patient had new onset atrial fibrillation, and one patient with transient respiratory failure required a brief dependence on mechanical ventilation.
One patient (5%) died in this series. This patient presented with necrotizing fasciitis, had a BMI of 65 kg/m2, and had severe preoperative cardiac and respiratory comorbidities and expired four months after her hernia repair. The mean length of stay was 16 days (average 2–105 days) for all patients, and nine days (average 2–35 days) when adjusted for survivors only.
Although several attempts were made to contact the patients by phone, six patients were lost to follow-up. Follow-up was done through a physical exam at patients’ visits to our clinic or through a phone interview. The average follow-up for the remaining 12 survivors was 14 months (range 2–68 months). Number of patients, follow-up, and recurrence by repair technique are summarized in Table 2. All three patients with primary fascial repair were lost to follow-up. Two out of the five patients with bridged fascia were lost to follow-up, and only one out of the eleven patients with component separation method was lost to follow-up. There were two hernia recurrences identified by physical exam and computed tomography. These were in two male patients who had their hernias repaired utilizing AlloDerm to bridge their fascial defect. One recurred within five months of his initial operation, the other within seven months of the initial operation. Both of these patients have been re-explored laparoscopically and repaired definitively in a non-contaminated field. Follow-up records were available for ten out of 11 patients repaired utilizing the component separation method. There were no hernia recurrences in this group of patients after an average follow-up of 16 months.
Discussion
The principles of ventral hernia repair in the setting of surgical field contamination involve the removal of the source of contamination, resection of infected prosthetic material or takedown of enterocutaneous fistulas, and the reconstruction of the abdominal wall. These operations are challenging and often result in both surgeon and patient frustration. Literature regarding various surgical approaches is sparse but can be broadly categorized into single- or multi-staged repairs. Multi-staged repair involves the placement of mesh and skin grafting, followed by subsequent mesh excision, leaving the abdominal wall with a massive defect in need for additional definitive repair [4, 5]. Using this technique several authors have reported recurrence rates of 5–10% and fistula rates of up to 10%. Our study shows that various approaches using single-stage treatment of ventral hernias in contaminated fields can be accomplished with a low recurrence rate and acceptable morbidity and mortality in these extremely challenging patients.
In reviewing our institution's experience it is apparent that surgeons approach the closure of complicated abdominal wall defects in a multitude of ways. The retrospective nature of this study limits the conclusions that can be drawn regarding the rationale for which procedure was applied to each individual patient. Likely, the degree of contamination, patient factors, surgical judgment, and personal experience play a major role in each of the decisions for these very complex patients. Although, this study does include several different surgeons, almost two thirds of the procedures were performed by the senior authors (MJR, DM).
Three patients in our study underwent primary repair of their defects without mesh reinforcement. None of these patients were available for follow-up and we cannot comment on their rate of hernia recurrence. However, the results of a randomized prospective multi-center trial show that the recurrence rate of 6 cm ventral hernias without the presence of contamination, repaired primarily is rather high at 43% [2]. Interestingly, all three of these patients underwent repair prior to the availability of biologic mesh prosthesis at our institution. With the introduction of biologic mesh several years ago, must surgeons prefer some additional support provided by these collagen based mesh products. To date, little long-term follow-up is available to guide the surgeon in their applicability in the setting of contamination despite their widespread use by many surgeons. These products are extremely expensive and should be validated in carefully controlled studies.
When placing a biologic prosthetic several options are available. The defect can be bridged with the mesh, it can be used to reinforce a primary repair in conjunction with lateral releasing incisions, and it can be placed above, below, or sandwiched on both sides of the fascia. No consensus exists to the best approach as evidenced by the varied techniques used in the present series. However, both patients in whom AlloDerm mesh was used to bridge a fascial defect recurred within several months. On re-exploration of these patients, the AlloDerm was found to be extremely lax and had pulled away from the edges of the defect, leading to a recurrent hernia. Based on this experience, we no longer bridge the fascial defect with biologic mesh. In cases where the fascial edges cannot be reapproximated despite adequate component separation we plan for a multi-staged procedure.
The majority of patients in our series were treated using the component separation method (CSM) with or without prosthetic reinforcement. In this group of patients there were no hernia recurrences after an average follow-up of 16 months. In order to perform adequate separation of the anterior abdominal-wall musculature, large subcutaneous flaps are required. In the setting of contamination one would expect a high wound complication rate. In our series 32% of patients developed a wound infection. However, with the use of biologic mesh prosthesis, all of these wound infections were treated with local measures without recurrence. It is our contention that the high rate of wound infection in these patients justifies the use of a biologic prosthesis to augment the closure provided by the component separation. It is a well-accepted concept that prior wound infection increases the likelihood of hernia recurrence, and therefore we feel that any additional measures to reduce this likelihood are justified. In a recent series, van Geffen et al. reported an 8% recurrence rate in 26 patients treated primarily by CSM without reinforcement in the setting of gross contamination [6]. Similar to our series, wound complications including infections and seromas occurred in 31% of their patients. Despite these seemingly positive results, this group did use non-absorbable mesh augmentation in several patients after component separation. In those patients treated with mesh augmentation, none developed a recurrence during follow-up. Shestak et al. recently, reported a series of 22 patients undergoing component separation without mesh reinforcement, in which nine patients had active infection [8]. They did not sub-analyze the data based on those patients with active infection, however, wound infections only occurred in two patients in the study and only one patient had a recurrent hernia. Dibello et al. performed component separation for large ventral hernias in 35 patients [9]. The majority of patients in their series had active infection. Due to attenuated fascia and tension on the closure after adequate component separation, they reinforced their closure with either non-absorbable mesh or vicryl mesh in almost half of their patients. While the duration of follow-up was not reported, these authors noted an 8.5% recurrence rate. Another group of investigators recently reported 43 cases of component separation without reinforcement for large ventral hernias in which almost half were in the setting of contamination [10]. With a mean follow-up of 16 months they noted a recurrence rate of 32%. The majority of patients in our study had additional collagen-based mesh to reinforce the fascial closure. In our experience the majority of these patients often have attenuated fascia and substantial defects after the resection of infected mesh or takedown of enterocutaneous fistulas, and despite bilateral component separations the midline fascial closure is often under some tension. Additionally, the biologic mesh provides the specific advantage of resistance to superficial wound infections as none of the cases in our series that developed wound infections had a recurrence. However, these biologic mesh implants are fairly costly and their effectiveness should be studied carefully. To date no prospective randomized trial has addressed this issue. Conclusions from our results regarding the use of mesh reinforcement when utilizing the CSM technique are difficult to draw since we only had two patients without mesh reinforcement. However, we can conclude that the CSM, with or without mesh reinforcement, provided an excellent repair for these ventral hernias in the setting of surgical field contamination.
If one acknowledges the potential advantages of mesh reinforcement in the setting of component separation the ideal mesh remains controversial. Despite a somewhat universal fear of placing permanent mesh in the setting of a contaminated field, Birolini et al. recently, reported a series of 20 patients undergoing simultaneous elective colon resections and closure of abdominal wall defects using an onlay nonabsorbable mesh to reinforce the primary fascial defect (repaired with relaxing lateral incisions when necessary) [11]. Despite a 20% wound infection rate, no recurrences were reported with a follow-up of between one and seven years. While these are certainly impressive results, it is important to consider that these were elective colon resections, in which a complete bowel preparation was likely performed and the contaminated area could be isolated from the operative field. This is often not the case during resection of infected mesh or takedown of an enterocutaneous fistula occurring through a piece of mesh. Three patients in our series had nonabsorbable mesh placed at the time of hernia repair. One patient had lightweight polypropylene mesh placed in the retrorectus space, one had Marlex mesh bridging the fascial defect, and the remaining patient had prolene mesh placed in an onlay position after primary fascial closure. Only one patient was available for follow-up and no infection was present at seven months. Despite these results, we do not advocate the placement of permanent mesh except in highly selected cases, in which the contamination is well controlled.
In summary, this study exemplifies the need for future prospective comparative trials evaluating the various methods of repairing these challenging hernias. Likely, this will require a multi-institutional series in order to obtain adequate power. Based on our current experience, we approach these patients in the following manner. The initial phase involves local control of the contamination and correcting nutritional deficits. Once the patients are stabilized, they are explored and all of the infected material is resected. The resultant abdominal-wall defect is then reapproximated using component separation and biologic mesh augmentation. Based on our experience using this technique, no patients have recurred and the procedure can be performed with acceptable morbidity.
References
Cobb WS, Kercher KW, Heniford BT (2005) Laparoscopic repair of incisional hernias. Surg Clin North Am 85(1):91–103
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, JN IJ, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 343(6):392–398
Heniford BT, Park A, Ramshaw BJ, Voeller G (2003) Laparoscopic repair of ventral hernias: nine years’ experience with 850 consecutive hernias. Ann Surg 238(3):391–399, discussion 399–400
Fabian TC, Croce MA, Pritchard FE, Minard G, Hickerson WL, Howell RL, Schurr MJ, Kudsk KA (1994) Planned ventral hernia. Staged management for acute abdominal wall defects. Ann Surg 219(6):643–650, discussion 651–653
Jernigan TW, Fabian TC, Croce MA, Moore N, Pritchard FE, Minard G, Bee TK (2003) Staged management of giant abdominal wall defects: acute and long-term results. Ann Surg 238(3):349–355, discussion 355–357
van Geffen HJ, Simmermacher RK, van Vroonhoven TJ, van der Werken C (2005) Surgical treatment of large contaminated abdominal wall defects. J Am Coll Surg 201(2):206–212
Ramirez OM, Ruas E, Dellon AL (1990) “Components separation” method for closure of abdominal-wall defects: an anatomic and clinical study. Plast Reconstr Surg 86(3):519–526
Shestak KC, Edington HJ, and Johnson RR (2000) The separation of anatomic components technique for the reconstruction of massive midline abdominal wall defects: anatomy, surgical technique, applications, and limitations revisited. Plast Reconstr Surg 105(2):731–738, quiz 739
DiBello JN Jr, Moore JH Jr (1996) Sliding myofascial flap of the rectus abdominus muscles for the closure of recurrent ventral hernias. Plast Reconstr Surg 98(3):464–469
de Vries Reilingh TS, van Goor H, Rosman C, Bemelmans MH, de Jong D, van Nieuwenhoven EJ, van Engeland MI, Bleichrodt RP (2003) “Components separation technique” for the repair of large abdominal wall hernias. J Am Coll Surg 196(1):32–37
Birolini C, Utiyama EM, Rodrigues AJ Jr, Birolini D (2000) Elective colonic operation and prosthetic repair of incisional hernia: does contamination contraindicate abdominal wall prosthesis use? J Am Coll Surg 191(4):366–372
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alaedeen, D.I., Lipman, J., Medalie, D. et al. The single-staged approach to the surgical management of abdominal wall hernias in contaminated fields. Hernia 11, 41–45 (2007). https://doi.org/10.1007/s10029-006-0164-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-006-0164-5