Abstract
Introduction
Open tension-free hernioplasty using prosthetic meshes dramatically reduced recurrence rates after hernia or incisional hernia repair and has become the rule. Mesh infections (MI) are the major complication of prosthetic material. The aim of this study was to assess the efficacy of partial removal of mesh (PRM) therapy in the treatment of MI.
Materials and methods
From January 2000 to April 2010, from a prospective database, we retrospectively selected patients who underwent surgery for MI. We studied the epidemiological data (sex, age, obesity, diabetes, smoking), the operating time of the initial intervention, the presence of intestinal injuries during the first intervention, the average interval between initial surgical procedure and MI, the location of the hernia, the average size of the hernia, type of mesh used, the position of the mesh, type of surgery performed, the number through interventions required to achieve a cure, the cumulative duration of hospital stay and hernia recurrence rates.
Results
Twenty-five patients were supported for a MI in our institution. There were 9 women (36 %) and 16 men (64 %). The median age was 59 years (range 37–78). There were 4 inguinal hernias (16 %), 15 incisional hernias (60 %) and 6 multirecurrent incisional hernias (24 %). It was performed a PRM in 92 % of cases (n = 23), a total excision of the prosthesis in 4 % of cases (n = 1) and no removal of prosthesis in 4 % of cases (n = 1). The average number of reoperations before healing was 1 (range 1–5). The mean cumulative duration of hospitalization until healing was 9.5 days (range 2–43). No visceral resection was performed.
Conclusion
PRM is feasible in most cases allowing first to spare the capital parietal patients and secondly to avoid major surgery. In case of failure, total removal of the mesh can be discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Open tension-free hernioplasty using meshes dramatically reduced recurrence rates after hernia or incisional hernia repair and has become the rule [1]. This foreign material causes an inflammatory reaction gradually replaced by fibrosis [2]. The initial inflammatory reaction can cause the attachment of bacteria and thus be the bed of mesh infections (MI).
MI are thus the major complication of prosthetic material. In a meta-analysis on antibiotic prophylaxis for hernia repair, Manuel Sanchez et al. [3] estimated MI at 1.4 % when prophylactic antibiotics were used and 2.9 % in the absence of antibiotics, and the French Association of anaesthesiology and reanimation (SFAR) does not recommend any prophylactic antibiotherapy in this setting of wound surgery [4].
When a MI occurs, most authors propose a complete removal of the prosthesis [5–7]. A low level of scientific evidence supports this therapeutic approach, and it is associated with 36.3 % postoperative complication rate [8]. An alternative to total removal of the prosthesis is the partial removal of meshes (PRM). PRM means the excision of the non-integrated mesh. The aim of this study was to assess the efficacy of PRM therapy in the treatment of MI.
Patients and methods
From January 2000 to April 2010, from a prospective database, we retrospectively selected patients who underwent surgery for MI.
MI is a deep infection and is defined as involving the skin, the subcutaneous tissues, the fascia or muscle, but not an organ space wound infection [9].
All patients with local or general signs of MI, after hernia or incisional hernia repair, requiring reoperation under general anaesthesia, were included in this study. Local signs of infection were an inflammatory abdominal wall near to the scar with a flow in which it was found germs. We studied the epidemiological data (sex, age, obesity, BMI > 25 kg/m²), diabetes (maintained-fasting plasma glucose ≥ 7.0 mmol/l), smoking, the operating time of the initial intervention, intestinal injuries during the first intervention, the average interval between initial surgical procedure and MI, the location of the hernia or incisional hernia, the average size of the incisional hernia, type of mesh used, the position of the mesh, the number through interventions required to achieve a cure, postoperative major morbidities (reoperations or organ failure), the cumulative duration of hospital stay and hernia recurrence rates. We analysed whether the outcomes were different between hernias and incisional hernias. We analysed bacteriological results.
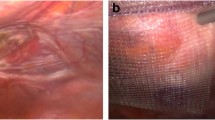
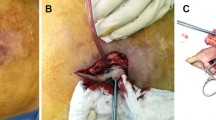
PRM was always performed under general anaesthesia. When the mode of diagnosis was an abscess, incision of the abscess under general or local anaesthesia was performed. In this case, the goal of the procedure was to realize a guided healing management. A module of irrigation wash (with a saline solution), 50 cc three times a day, was realized and when there were no more clinical and biological signs of sepsis, the patient was discharged from the hospital with a guided healing management using alginate meshes until formation of a fistula. The technique of irrigation was always the same. Guided healing management means that the skin defect was progressively closed from the depth to the skin. When the mode of diagnosis was a cutaneous fistula, excision of the fistula was performed immediately. The fistula was identified by methylene blue and was excised, and the path to the exposed mesh was excised to. The unincorporated portion of the mesh near to the fistula orifice was also excised (Table 1; Fig. 1). When the unincorporated portion of the mesh was excised, a herniorrhaphy was performed. In case of MI, intravenous antibiotic therapy was systematic for 7 days. First, a probabilist antibiotherapy was introduced. This probabilist antibiotherapy had to be active on staphylococcus. In our department, this antibiotherapy was ofloxacin and metronidazole until 2008, and on decision of infectiologists, this antibiotherapy was changed for vancomycine in 2008. A bacteriological analysis of the liquid of the abscess and of the mesh was systematically performed, and the antibiotherapy was adapted to the results of this analysis.
Statistical analysis
An ANOVA test was performed to compare qualitative variables. A p < 0.05 was considered as significantly different. All tests were performed using the SPSS statistical software (SPSS 15.0 for Windows, SPSS Inc., Chicago, IL).
Results
From January 2000 to April 2010, the rate of MI was 1.1 % (n = 9). Twenty-five patients were treated for a MI in our institution. Among these patients, on 795 patients operated on for a hernia or an incisional hernia with a mesh, 9 had initially been treated in our institution while 16 had hernia or incisional hernia repair in other hospitals (Table 2). Among these patients, 604 patients have an operation for hernia (76 %) and 191 for an incisional hernia (24 %). The rate of laparoscopic procedure during the study period was 2.6 % (n = 21). All infections appear in open procedures.
Among the 25 patients, there were 9 women (36 %) and 16 men (64 %). The median age was 59 years (range 37–78). There were 20 % of diabetics (n = 5), 16 % of smokers (n = 4) and 44 % of obeses (n = 11). The median operating time of the first procedure was 185 min (range: 35–220). The interventions were inguinal hernias in 16 % of cases (n = 4), incisional hernias in 60 % of cases (n = 15) and multirecurrent incisional hernias in 24 % of cases (n = 6). There were no umbilical hernia and no Stoppa procedure. The average size of incisional hernias was 71 mm (range 8–150). The type of mesh was noted in 18 cases. It was a polyester prosthesis in 88.8 % (n = 16) of cases, and a polypropylene prosthetic in 11.2 % (n = 2) cases. The position of the prosthesis was identified in 18 cases. In 61.1 % of cases, it was retrorectus (n = 11), and in 16.6 % of cases, it was intraperitoneal (n = 3), and it was a Lichtenstein’s procedure in 16.6 % of cases (n = 3) and a plug in 5.5 % of cases (n = 1). Bowel perforation during the first intervention was stated in the report surgery in 4 % of cases (n = 1).
The median interval between first intervention and diagnosis of MI was 10 months (range 2–72). It was performed a PRM in 92 % of cases (n = 23), a total excision of the prosthesis in 4 % of cases (n = 1) and no removal of prosthesis in 4 % of cases (n = 1). After PRM, the median number of reoperations before healing was 1 (range 1–5). The mean number of reoperation before healing was in the incisional hernia repair of 1.33, in the multirecurrence incisional hernia repair 1.7 and in the inguinal hernia repair: 1.5 (p = 0.7). The median cumulative duration of hospitalization until healing was 5.5 days (range 2–43). The hernia recurrence rate was 20 % with a follow-up duration of 40 months (range: 1–120) (Table 3). On bacteriological analysis, germs found were in 15 patients, staphylococcus aureus, in 8 patients Streptococcus, in 8 patients Enterococcus, in 1 patient Escherichia coli, in 1 patient Proteus mirabilis and in 1 patient Cynetobacterium.
Discussion
MI induces a real problem of management, and its prevention is a real challenge [10]. Its incidence is influenced by patient’s factors such as diabetes, obesity, smoking, chronic obstructive pulmonary disease and immunosuppressive therapy. These patients are more likely to develop a hernia recurrence if a mesh is not implemented [11]. Risk factors related to the intervention are less clearly identified. Leber et al. [12] have identified the use of polyester prostheses as being significantly associated with MI. Petersen et al. [13] have identified the operative time as an independent risk factor for MI. The influence of peroperative antibiotic prophylaxis on the rate of MI is less clear as evidenced by the results of the Cochrane Database. In this meta-analysis, the rate of MI in the group of patients with the establishment of a parietal prosthesis was 1.4 % in the antibiotic group and 2.9 % in the control group (OR 0.49, 95 % CI 0.29–0.86). The authors of this meta-analysis concluded that we could not recommend routine antibiotic prophylaxis to reduce the infection rate in the cure of hernia [3].
The curative treatment of MI induces many problems. Most authors recommend a total removal of the prosthesis combined with antibiotic therapy [11, 12]. The surgical excision of the entire prosthesis associate or not with myofascial-advanced flap can lead to major wound defects and high complications rate, until 36 % (Table 3) [13]. As a result, techniques of conservation prosthetic have been developed to limit the parietal defect. Trunzo et al. and Aguilar et al. [11, 14] so proposed drainage of the abscess percutaneously associated with intravenous antibiotics for 15 days followed by the instillation of percutaneous drain gentamycin 80 mg three times daily for 4 weeks. Stremitzer et al. [15] have proposed the use of the vacuum-assisted closure (VAC, KCI Inc; San Antonio, TX) when there was a cavity of more than two centimetres. PRM has been introduced in our department by Stoppa et al. [16] in 1980 to limit the parietal defect of a total removal of the prosthesis. Our series showed that a conservative approach by PRM was associated with a healing in every case with a median number of reoperations of 1 (range 1–5) with a postoperative major morbidity rate of 0 % (including reoperations and organ failures) and could be considerate as the first procedure to perform to avoid the complications of more aggressive procedures as complete mesh removal associated with myofascial-advanced flaps as described by Szczerba et al. [8] (Table 3). Procedures could be performed as long as the healing is not obtained. Reoperations are necessary to remove the unincorporated mesh that would not have been removed during the former procedures. PRM is also associated with a high rate of recurrences. In our series, the rate of recurrence was associated with a recurrence rate of 20 % with a mean follow-up of 40 months. This recurrence rate is superior to that of complete removal of mesh that is from 0 to 14.3 % [5, 6]. This recurrence rate could be explained by the method of closure of the abdominal wall after removing the mesh. An herniorrhaphy is performed when possible, and as shown by Luijendijk et al., the recurrence rate is higher when an herniorrhaphy is performed.
For some authors, the possibility of conservative treatment was related to the type of prosthesis used. Petersen et al. [13] in their study showed that MI on prosthetic implants made of polyester or polypropylene could be treated conservatively while infections on PTFE prostheses required a total removal of the prosthesis. These conclusions were different from conclusions of Paton et al. who did a conservative treatment of infected PTFE when infection was limited, and Greenberg et al. conserved 64 % of PTFE prothesis according to a long conservative treatment [17, 18]. In our series, we showed that a conservative approach was feasible for polyester or polypropylene meshes with a healing response obtained after 1.5 procedures. In our series, there was no need for complete removal of the mesh, and it may be due to the absence of PTFE meshes in this series. The excision of the fistula as it is performed in our series requires low durations of hospitalization and induces no morbidities.
Conclusion
MI are rare situations in which care is difficult. A more conservative attitude is feasible in most cases allowing one hand to spare the capital parietal patients and secondly to avoid major surgery.
References
Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN, Boelhouwer RU, de Vries BC, Salu MK, Wereldsma JC, Bruijninckx CM, Jeekel J (2000) A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 10; 343:392–8
Bellon JM, Bujan I, Contreras L, Juranto F (1994) Macrophage response to experimental implantation of polypropylene prostheses. Eur Surg Res 26:46–53
Sanchez-Manuel FJ, Seco-Gil JL (2009) Antibiotic prophylaxis for hernia repair. Cochrane Database Syst Rev
SFAR (2010) Antibioprophylaxie en chirurgie et médecine interventionnelle (patients adultes). Recommendations
Delikoukos S, Tzovaras G, Liakou P, Mantzos F, HatzitheoWlou C (2007) Late-onset deep mesh infection after inguinal hernia repair. Hernia 11:15–17
Fawole AS, Chaparala RP, Ambrose NS (2005) Fate of the inguinal hernia following removal of infected prosthetic mesh. Hernia 10:58–61
Johanet H, Contival N (2011) Deep mesh infection and cure of inguinal hernia. J Visc Surg (in press)
Szczerba SR, Dumanian GA (2003) Definitive surgical treatment of infected or exposed ventral hernia mesh. Ann Surg 237:437–441
Larochelle M, Hyman N, Gruppi L, Osler T (2011) Diminishing surgical site infections after colorectal surgery with surgical care improvement project: is it time to move on? Dis Colon Rectum 54:394–400
Stoppa R (2005) About prosthetic repair and contaminated areas. Hernia 9:107
Aguilar B, Chapital AB, Harold KL (2010) Conservative management of mesh-site infection in hernia repair. J Laparoendosc Adv Surg Tech A 20:249–252
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Petersen S, Henke G, Freitag M, Faulhaber A, Ludwig K (2001) Deep prosthesis infection in incisional hernia repair: predictive factors and clinical outcome. Eur J Surg 167:453–457
Trunzo JA, Ponsky JL, Jin J, Williams CP, Rosen MJ (2009) A novel approach for salvaging infected prosthetic mesh after ventral hernia repair. Hernia 13:545–549
Stremitzer S, Bachleitner-Hofmann T, Gradl B, Gruenbeck M, Bachleitner-Hofmann B, Mittlboeck M, Bergmann M (2010) Mesh graft infection following abdominal hernia repair: risk factor evaluation and strategies of mesh graft preservation. A retrospective analysis of 476 operations. World J Surg 34:1702–1709
Stoppa R, Henry X, Odimba E, Verhaeghe P, Largueche S, Myon Y (1980) Dacron tulle prothesis and biological glue in the surgical treatment of incisional hernias. Nouv Presse Med 9:3541–3545
Paton BL, Novitsky YW, Zerey M, Sing RF, Kercher KW, Heniford BT (2007) Management of infections of polytetrafluoroethylene-based mesh. Surg Inf 8:337–341
Greenberg JJ (2010) Can infected composite mesh be salvaged? Hernia 14:589–592
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sabbagh, C., Verhaeghe, P., Brehant, O. et al. Partial removal of infected parietal meshes is a safe procedure. Hernia 16, 445–449 (2012). https://doi.org/10.1007/s10029-012-0931-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-012-0931-4